Abstract
Thoc1 encodes an essential component of the mammalian TREX protein complex. TREX is an evolutionary conserved complex that couples elongating RNA polymerase II with RNA processing factors. Depletion of Thoc1 protein (pThoc1) compromises transcriptional elongation and nuclear export of some RNAs. Loss of Thoc1 causes periimplantation embryonic lethality in the mouse. Early embryonic lethality precludes analysis of the physiological requirements for Thoc1 in the developing embryo or adult. To circumvent this limitation, we have generated mice containing hypomorphic or conditional alleles of Thoc1. Mice homozygous for the conditional allele appear normal. Mice containing Cre recombined conditional alleles phenocopy the previously characterized Thoc1 null allele. Mice homozygous for the hypomorphic allele are viable and born at a frequency that is not significantly different from the expected Mendelian ratio. However, these mice express less pThoc1 than wild type mice and exhibit a dwarf phenotype. The dwarf phenotype can be detected in mid- gestation embryos, suggesting that Thoc1 is also required later in embryonic and postnatal development.
Keywords: gene expression, development, RNA processing, mRNP
Co-transcriptional loading of RNA processing and export factors onto nascent RNA supports efficient and regulated gene expression(Moore, 2005; Reed, 2003). Assembly of the TREX complex is one molecular mechanism utilized by cells to physically couple transcription with factors involved in RNA processing and export. TREX has been best characterized in S. cerevisiae where it is composed of the THO sub-complex containing four proteins (Hpr1p, Tho2p, Mft1p, and Thp2p) that are essential for normal transcriptional elongation and genome stability(Chavez et al., 2000). The THO sub-complex recruits two additional proteins, Sub2p and Yra1p, that are involved in nuclear RNA export and splicing(Luo et al., 2001; Strasser et al., 2002). Hpr1p genetically and physically interacts with RNA PolII(Chang et al., 1999; Chavez et al., 2000; Fan et al., 1996; Strasser et al., 2002). Hpr1p is an essential component of TREX since its loss impairs both transcriptional elongation and nuclear RNA export(Chavez and Aguilera, 1997; Chavez et al., 2001; Fan et al., 1996; Huertas and Aguilera, 2003; Rondon et al., 2003; Strasser et al., 2002; Zenklusen et al., 2002). Despite the defects in gene expression associated with HPR1 loss, it is not essential for yeast viability(Aguilera and Klein, 1990).
Metazoan structural homologues are apparent for the yeast TREX proteins Tho2p (Thoc2), Sub2p (UAP56), and Yra1p (Aly)(Luo et al., 2001; Strasser and Hurt, 2000; Stutz et al., 2000; West et al., 2000). Although lacking statistically significant similarity at the primary amino acid level, functional orthologues of yeast HPR1 have been identified in both human and insect cells (alternatively known as Thoc1, Hpr1, or p84)(Li et al., 2005; Masuda et al., 2005; Rehwinkel et al., 2004). Depletion of Thoc1 protein (pThoc1) from human or insect cells compromises transcriptional elongation and nuclear RNA export of some genes. These observations suggest that metazoan species with long, intron-containing genes utilize an evolutionary conserved TREX complex to regulate gene expression. However, the subunit composition of yeast and metazoan TREX complexes are not identical, suggesting possible functional differences.
Thoc1 is expressed widely during mouse embryonic development and mice lacking Thoc1 fail to develop past the late blastocyst stage(Wang et al., 2006). Early embryonic lethality is associated with loss of cell viability, particularly among the pluripotent stem cells that make up the inner cell mass. Thoc1 is also expressed widely in adult tissue, but early embryonic lethality of Thoc1 null mice has precluded characterization of the physiological requirements for Thoc1 during later stages of embryonic development or in the adult mouse. To address this limitation, we have generated hypomorphic and conditional alleles of the murine Thoc1 gene.
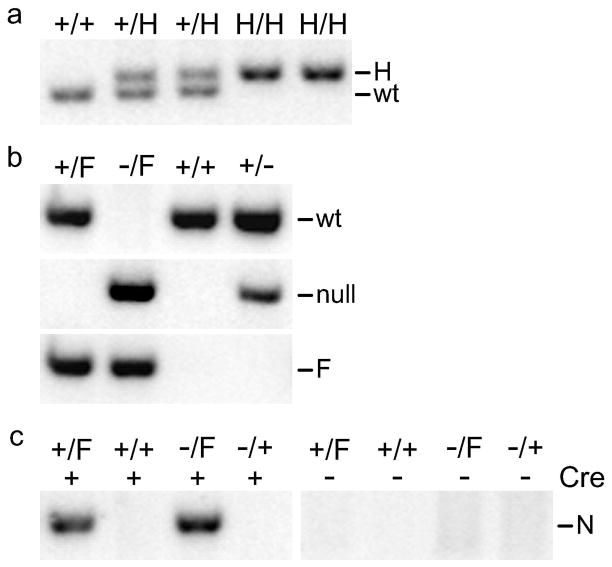
The mutant Thoc1 alleles were engineered by targeted homologous recombination in mouse ES cells. The targeting vector contained approximately 7 kilobase pairs (kbp) of Thoc1 from intron 4 to intron 7 (Fig. 1A). A PGK-neo selection cassette flanked by frt sites was inserted within intron 5 in reverse orientation relative to Thoc1. A loxP site was situated within intron 5, 3′ of the PGK-neo cassette. A second loxP site was inserted in intron 7. Eighty-two ES cell clones that resisted drugselection were screened by Southern blotting. Three of these clones gave the expected fragment size for the targeted allele using a 5′ flanking probe (Fig. 1B). With a 3′ flanking probe, two of these three gave bands of the size expected for a correctly targeted allele. The 3′ recombination junction in the remaining clone was between the loxP sites, thus removing the 3′ loxP site from the targeted allele. Correctly targeted ES cells of the 129/SVJae background were used to generate chimeric mice. The chimeric mice were crossed to C57BL/6J mice thus giving rise to a founder strain of mice carrying theThoc1 H allele on a mixed 129/SvJae X C57BL/6J genetic background (Fig. 1A). This strain was subsequently mated to a Flpe recombinase expressing mouse strain to remove the drug selection cassette. Successful excision of the neomycin selection cassette was demonstrated by Southern blotting that distinguishes the wild type, H, and F alleles (Fig. 1C). PCR assays have been designed for routine genotyping of the H, F, and the previously described Thoc1 null alleles (Fig. 2A-B)(Wang et al., 2006). Cre expression correctly excised exons 6 and 7 from the F allele to generate a new, presumed null allele (N) as indicated by the appearance of a PCR amplified fragment of expected size (Fig. 2C).
Figure 1. Construction of mutant Thoc1 alleles in the mouse.
A) A representation of the exon/intron structure of the targeted region of the Thoc1 gene (not to scale), the targeting vector, and the expected structure of the successfully targeted alleles. Exons are numbered and shown as solid boxes. The position of XhoI (X), BglI (B), NcoI (N), and BamHI (Ba) restriction enzyme sites, the flanking hybridization probes, and the expected sizes of fragments detected by Southern blotting are indicated. The positions of the loxP and frt sites as well as the PGK-neo drug selection cassette, in reverse orientation to the Thoc1 gene, are also indicated. B) Southern blot analysis of XhoI+BglI restricted genomic DNA from the three successfully targeted ES cell lines. The left panel shows Southern blotting results using the 5′ flanking probe while the right panel displays the results with the 3′ flanking probe. Sizes of the bands are indicated based on comparison to molecular weight markers run on the agarose gel. The band marked with an * indicates a successfully targeted clone where the 3′ recombination junction occurs between the loxP sites of the 3′ homology arm. C) Southern blot analysis of BamHI+NcoI restricted genomic DNA from representative mice of the indicated genotypes using the same 3′ flanking probe used in B. As shown by the indicated expected band positions in A, this analysis distinguishes the wild type (wt), H, and Flp recombined (F) Thoc1 alleles.
Figure 2. PCR assays for genotyping the H, F, and N Thoc1 alleles.
A) A representative ethidium bromide stained agarose gel resolving PCR fragments amplified from genomic DNA of mice with the indicated genotypes. The bands amplified from the wild type (wt) or H alleles can be resolved on the gel and serve as an assay for routine genotyping. The pixels of the image have been inverted for improved legibility. B) Representative ethidium bromide stained agarose gels resolving PCR fragments amplified from MEF genomic DNA with the indicated genotypes. The PCR reactions utilize primers specific for the wild type (wt), F, or previously characterized null alleles of Thoc1. C) A representative agarose gel resolving PCR fragments specific for the Thoc1 N, generated by Cre mediated excision of the F allele. The MEFs were treated with a Cre expressing recombinant adenovirus (+) or mock treated (−) 72 hours prior to genomic DNA isolation.
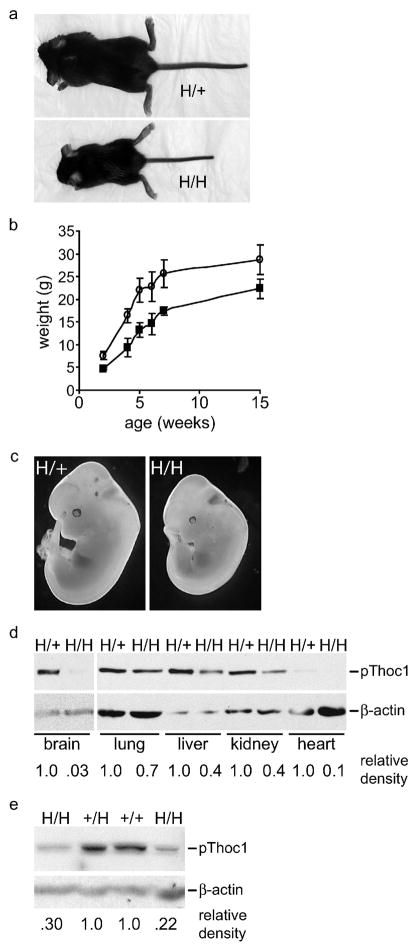
Mice heterozygous or homozygous for the H allele were born at a frequency not significantly different from the expected Mendelian ratio (p>.05 by Chi squared test)(Table 1). Thoc1 H/+ mice were indistinguishable from wild type, but Thoc1 H/H mice exhibited a dwarf phenotype with 100% penetrance (Fig. 3A). The weight of newborn Thoc1 H/H mice was less than mice containing at least one wild type allele (1.1g±0.1 versus 1.6±0.1, P<0.01 by Student’s T test). Reduced size persisted throughout adulthood (Fig. 3B). By four months of age, total body weight of H/H mice was approximately 80% of wild type and most individual organ weights (thymus, heart, lung, liver, kidney, spleen, and urogenital tract) were also proportionally less. The relatively small size of H/H mice was detectable as early as embryonic day 12.5 (E12.5) (Fig. 3C), suggesting the dwarf phenotype was not solely due to changes in the activity of growth factors and hormones that function during postnatal development. Viable neonates hemizygous for the Thoc1 H allele (H/−) were not recovered (Table 1). However, hemizygous embryos were detected at E13.5, thus reaching a stage of embryonic development significantly more advanced than Thoc1 null embryos(Wang et al., 2006). As with H/H embryos, H/- embryos were considerably smaller than their wild type littermates (data not shown). To ascertain whether the phenotypes observed were due to reduced pThoc1 expression, we isolated tissueor embryonic fibroblasts (MEF) from mice of informative genotypes and assessed pThoc1 levels by western blotting. In multiple tissues pThoc1 levels were significantly lower in Thoc1 H/H mice than in wild type or H/+ mice (Fig. 3D–E). The relative decrease in pThoc1 levels ranged from more than 30-fold in brain tissue, to approximately 30% in lung tissue. Most H/H tissues and MEFs show a 2–4 fold decrease in pThoc1 levels relative to samples from wild type or heterozygous mice.
Table 1.
Viable neonates from intermating of Thoc1 mutant mice
| Mating | Genotype | Number | Expected |
|---|---|---|---|
| H/+ X H/+ | +/+ | 51 | 42.5 |
| H/+ | 83 | 85 | |
| H/H | 36 | 42.5 | |
| H/+ X +/− | +/+ | 29 | 21 |
| +/− | 25 | 21 | |
| H/+ | 30 | 21 | |
| H/− | 0* | 21 | |
| F/+ X F/+ | +/+ | 14 | 15.25 |
| F/+ | 30 | 30.5 | |
| F/F | 17 | 15.25 | |
| F/+ X +/− | +/+ | 6 | 7.75 |
| +/− | 4 | 7.75 | |
| F/+ | 12 | 7.75 | |
| F/− | 9 | 7.75 |
Embryos from Thoc1+/− heterozygote intercrosses were collected on day 14 post-partum (P14) and genotyped by PCR.
Statistically significant difference from expectation by Chi squared test (p<.001).
Figure 3. The Thoc1 H allele is hypomorphic.
A) Representative three-week-old mice of the indicated genotype demonstrating a dwarf phenotype in mice homozygous for the H allele. B) Male mice of the indicated genotype were weighed at the indicated ages in weeks. The data points represent the mean weight and standard deviation from at least three mice for each datapoint. C) Representative E12.5 mice of the indicated genotype photographed under dark field microscopy indicating a detectable size difference prior to birth. D) A representative western blot indicating pThoc1 levels in various tissues isolated from adult mice of the indicated genotypes. β-actin serves as a protein loading control. Relative signal intensities are shown at bottom after normalization against the loading control. E) MEFs isolated from mice of the indicated genotype were analyzed for pThoc1 levels by western blotting as above. Each lane represents an independent MEF isolate.
Mice homozygous, heterozygous, or hemizygous for the F allele were born at the expected Mendelian ratio (Table 1). Mice with these genotypes exhibited no phenotype distinguishable from wild type mice to at least nine months of age. These observations suggested that the F Thoc1 allele functioned equivalently to the wild type allele. Cre mediated excision of exons 6–7 was accomplished by mating heterozygous Thoc1 F mice to the Meox2-Cre strain(Tallquist and Soriano, 2000). Offspring heterozygous for the Cre excised N allele were then intermated, or mated with heterozygous Thoc1 null mice. No viable neonates homozygous or hemizygous for the N allele were recovered. We also assayed the genotype of embryos at different stages of development resulting from intermating of Thoc1 N/+ mice. Homozygous Thoc1 N embryos were not recovered at any stage tested that was past E3.5. Homozygous Thoc1 N E3.5 blastocysts were recovered at the expected Mendelian ratio and appeared normal. However, they were unable to form blastocyst outgrowths upon in vitro culture (Fig. 4A), phenocopying previously characterized homozygous Thoc1 null blastocysts(Wang et al., 2006). Treatment of MEFs hemizygous for the F allele with a recombinant adenovirus designed to express Cre significantly depleted pThoc1 levels relative to control MEFs containing a wild type allele (Fig. 4B). These observations indicated that Cre mediated excision of exons 6–7 precluded normal pThoc1 expression thus generating a functionally null allele.
Figure 4. The F allele is conditionally null for Thoc1.
A) Blastocysts isolated at E3.5 from intermating of mice heterozygous for the N allele were cultured in vitro for 0 or 48 hours. Representative blastocysts were photographed under phase contrast microscopy. Wild type blastocysts generate typical outgrowths consisting of flat trophoblast cells and rounded cells of the inner cell mass. Homozygous Thoc1 N blastocysts typically fail to hatch from the zona pellucidae and do not generate viable blastocyst outgrowths. B) Individual MEF isolates of the indicated genotypes are treated with the Cre expressing adenovirus (+) or mock treated (−) and total proteins extracted 72 hours after treatment. A representative western blot showing pThoc1 levels is shown. The bottom panel shows the same blot immunostained for β-actin to verify protein loading. Relative signal intensities are shown at bottom after normalization against the loading control. Since the efficiency of adenoviral infection under the conditions used is less than 100%, residual pThoc1 detected in hemizygous MEFs likely reflects pThoc1 expression from cells that have escaped infection.
We describe the construction and initial characterization of three new alleles of the murine Thoc1 gene. The H allele is hypomorphic as mice homozygous or hemizygous for the allele display developmental phenotypes that are considerably less severe than those observed in Thoc1 null mice. The hypomorphic phenotype is likely caused by diminished pThoc1 expressed from this allele sincethe protein coding sequence is unaltered. Intron 5 insertion of the drug selection cassette causes decreased expression from the H allele since its removal to generate the F allele restores normal pThoc1 expression (compare pThoc1 levels F/−, H/H, and F/+ MEFs in Figs. 3 and 4). Thoc1 null mice demonstrate a requirement for Thoc1 early in embryonic development. The dwarf phenotype of the hypomorphic Thoc1 mice, detectable as early as midgestation, indicate that Thoc1 is also required for later stages of embryonic and postnatal development. Consistent with restoration of pThoc1 expression by removal of the drug selection cassette, the F allele functions normally since mice homozygous, heterozygous, or hemizygous for the allele do not display detectable phenotypes distinguishable from wild type. Cre mediated recombination of the F allele removes exons 6 and 7 deleting codons 126–173 and causing a reading frame shift that generates a premature stop codon. Consistent with Cre mediated generation of a premature stop codon, pThoc1 levels detected by a carboxy terminal specific antibody f are significantly reduced in F/− MEFs exposed to Cre recombinase. The Cre recombined N allele phenocopies the previously characterized null allele since N/N embryos fail to develop beyond the blastocyst stage(Wang et al., 2006). Due to the perimplantation embryonic lethality of Thoc1 null mice, these two new alleles will be useful in studying Thoc1 function during later stages of development and in adult mice.
Methods
Generation of mutant Thoc1 alleles in the mouse
A BAC clone spanning the Thoc1 gene was isolated from a 129/SV mouse strain genomic library and used as a template to PCR amplify the homology arms for construction of the targeting vector. The 2.1 kbp 5′ homology arm spanning exon 5 was subcloned into the KpnI and BamHI sites of pPNT4(Conrad et al., 2003). The split 3′ homology arm was subcloned in two pieces, a 1.2 kbp fragment spanning exons 6 and 7 subcloned into the NotI and SalI sites and a 4.0 kbp piece of intron 7 subcloned into the SfiI and BlnI sites. All exons, flankingintron regions, frt sites, and loxP sites were verified by DNA sequencing. The targeting construct was electroporated into a 129/SV derived embryonic stem cell line and G418 resistant colonies screened by Southern blotting of XhoI+BglI restricted genomic DNA using 5′ and 3′ flanking probes. Correctly targeted ES cells were used to construct germ line transmitting chimeras by blastocyst injection. Transmission of the targeted alleles was confirmed by Southern blotting of genomic DNA extracted from tail biopsies.
Mice were mated with the FLPeR 129/SVJae strain to delete the PGK-neo drug selection cassette from the H Thoc1 allele to generate the F allele(Farley et al., 2000). Cre mediated recombination to generate the N allele was accomplished by mating Thoc1 F/+ mice with the Meox2-Cre C57BL/6 mouse strain(Tallquist and Soriano, 2000). Both strains were obtained from the Jackson Laboratory (Bar Harbor, Maine). Southern blotting of BamHI+NcoI restricted genomic DNA using the 3′ flanking probe was used to confirm the wild type, H, F, and N Thoc1 alleles.
Genotyping and protein analyses
Routine genotyping of genomic DNA extracted from tail biopsy specimens or MEFs was performed by PCR assays. All PCR reactions were performed in Taq buffer (Fermentas Inc., Hanover, MD). PCR was initiated with a 3 minute denaturation cycle at 94°C, followed by 20–30 cycles of 30 seconds denaturation, 30 seconds annealing at 61°C, and 30 seconds extension at 72°C. After a final 7 minute extension at 72C, PCR products were resolved by agarose gel electrophoresis and stained with ethidium bromide. Primers 5′-AAAGTACATGCTTTCAAGCAGGCA-3′ and 5′-CAGAAAATGGAAACAGAATAGGCTT-3′ amplified a 325bp PCR fragment from the N allele. Primers 5′-CAGTAAGTGCCTTGGTTTCCT-3′ and 5′-GGAAATCAAACCCAGGTTCTCT-3′ amplified a 390bp PCR fragment from the wild-type allele. Primers 5′-AGGTCGACGGTATCGATAAGCTTGATA-3′ and 5′-CTGTTACTGATTTCAAGCATTTCCA-3′ amplified a 350bp PCR fragment from the F allele. The primers 5′-CTCTTTTTGGCCAGGCTTT-3′ and 5′CAGAAAATGGAAACAGAATAGGCT3′ amplified a 390bp PCR fragment from the H allele and a 326bp PCR fragment from the wildtype allele.
Genotyping of preimplantation embryos was performed using a nested PCR assay. Individual embryos or cell outgrowths were lysed by incubation at 55°C for 4 hr in 20 μl of PCR lysis buffer [10mM Tris-HCl pH 8.8, 50mM KCl, 0.08% NP40, 0.2mM dNTP, 2.5 mM MgCl2] containing proteinase K (60 μg/ml). After boiling, an aliquot of the lysates was subjected to nested PCR amplification using first round primers: 5′AAAGTACATGCTTTCAAGCAGGCA3′ and 5′CAGAAAATGGAAACAGAATAGGCTT3′ (N allele); 5′CAGTAAGTGCCTTGGTTTCCT3′ and 5′GGAAATCAAACCCAGGTTCTCT3′ (wild type). Second round primers were: 5′TGTAAAGTTATGAGGTATAGGTAGGAATGC3′ and 5′TCTGAAATTGAATACAGACAGAATAGTT3′ (N allele); 5′TAATTCTAATACTGGTGGTTGCCA3′ and 5′CTTTGTTTGTGACAGGTTCTCC3′ (wild type). The N allele generates a 250bp PCR fragment while the wild type allele produces a 229bp fragment.
Isolation of protein extracts and western blot analysis of pThoc1 was performed as previously described(Li et al., 2005).
Embryo and cell culture
Timed pregnancies in superovulated Thoc1 heterozygous females were used to generate preimplantation embryos (E3.5) for analysis as previously described(Wang et al., 2006). Embryos were cultured in M16 medium without leukemia inhibitory factor. Mouse embryo fibroblasts(MEFs) were isolated from E13.5 embryos by standard procedures. Cells are cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and 0.5% penicillin and streptomycin and grown at 37°C in a humidified atmosphere of 5% CO2. Cells were cultured according to the standard 3T3 protocol.
Adenovirus
The Ad-Cre adenoviral stock was purchased from the Gene Transfer Vector Core of the University of Iowa. The early promoter of cytomegalovirus drives expression of the Cre gene in the recombinant adenovirus. Infections are performed at a multiplicity of infection of 100 infectious units per cell for two consecutive passages.
Acknowledgments
We thank Andrea Fleskin-Nikitin (Cornell University) for assistance with in vitro culture of mouse blastocysts. We also thank Yanqing Wang for technical assistance and helpful discussions. This work was supported by grants from the National Institutes of Health (CA70292, CA125665) to D.W.G. We acknowledge Aimee Stablewski of the RPCI Gene Targeting Core, supported by an NIH Cancer Center Support Grant (CA016056), for helpful advice and assistance in the construction of the mice.
References
- Aguilera A, Klein HL. HPR1, a novel yeast gene that prevents intrachromosomal excision recombination, shows carboxy-terminal homology to the Saccharomyces cerevisiae TOP1 gene. Mol Cell Biol. 1990;10:1439–1451. doi: 10.1128/mcb.10.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, French-Cornay D, Fan HY, Klein H, Denis CL, Jaehning JA. A complex containing RNA polymerase II, Paf1p, Cdc73p, Hpr1p, and Ccr4p plays a role in protein kinase C signaling. Mol Cell Biol. 1999;19:1056–1067. doi: 10.1128/mcb.19.2.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez S, Aguilera A. The yeast HPR1 gene has a functional role in transcriptional elongation that uncovers a novel source of genome instability. Genes Dev. 1997;11:3459–3470. doi: 10.1101/gad.11.24.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez S, Beilharz T, Rondon AG, Erdjument-Bromage H, Tempst P, Svejstrup JQ, Lithgow T, Aguilera A. A protein complex containing Tho2, Hpr1, Mft1 and a novel protein, Thp2, connects transcription elongation with mitotic recombination in Saccharomyces cerevisiae. EMBO J. 2000;19:5824–5834. doi: 10.1093/emboj/19.21.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez S, Garcia-Rubio M, Prado F, Aguilera A. Hpr1 is preferentially required for transcription of either long or G+C-rich DNA sequences in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:7054–7064. doi: 10.1128/MCB.21.20.7054-7064.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M, Brielmeier M, Wurst W, Bornkamm GW. Optimized vector for conditional gene targeting in mouse embryonic stem cells. Biotechniques. 2003;34:1136–1138. doi: 10.2144/03346bm03. [DOI] [PubMed] [Google Scholar]
- Fan HY, Cheng KK, Klein HL. Mutations in the RNA polymerase II transcription machinery suppress the hyperrecombination mutant hpr1 delta of Saccharomyces cerevisiae. Genetics. 1996;142:749–759. doi: 10.1093/genetics/142.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- Huertas P, Aguilera A. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol Cell. 2003;12:711–721. doi: 10.1016/j.molcel.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang X, Zhang X, Goodrich DW. Human hHpr1/p84/Thoc1 Regulates Transcriptional Elongation and Physically Links RNA Polymerase II and RNA Processing Factors. Mol Cell Biol. 2005;25:4023–4033. doi: 10.1128/MCB.25.10.4023-4033.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ML, Zhou Z, Magni K, Christoforides C, Rappsilber J, Mann M, Reed R. Pre-mRNA splicing and mRNA export linked by direct interactions between UAP56 and Aly. Nature. 2001;413:644–647. doi: 10.1038/35098106. [DOI] [PubMed] [Google Scholar]
- Masuda S, Das R, Cheng H, Hurt E, Dorman N, Reed R. Recruitment of the human TREX complex to mRNA during splicing. Genes Dev. 2005;19:1512–1517. doi: 10.1101/gad.1302205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- Reed R. Coupling transcription, splicing and mRNA export. Curr Opin Cell Biol. 2003;15:326–331. doi: 10.1016/s0955-0674(03)00048-6. [DOI] [PubMed] [Google Scholar]
- Rehwinkel J, Herold A, Gari K, Kocher T, Rode M, Ciccarelli FL, Wilm M, Izaurralde E. Genome-wide analysis of mRNAs regulated by the THO complex in Drosophila melanogaster. Nature Structural & Molecular Biology. 2004;11:558–566. doi: 10.1038/nsmb759. [DOI] [PubMed] [Google Scholar]
- Rondon AG, Jimeno S, Garcia-Rubio M, Aguilera A. Molecular evidence that the eukaryotic THO/TREX complex is required for efficient transcription elongation. J Biol Chem. 2003;278:39037–39043. doi: 10.1074/jbc.M305718200. [DOI] [PubMed] [Google Scholar]
- Strasser K, Hurt E. Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export. EMBO J. 2000;19:410–420. doi: 10.1093/emboj/19.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, Rondon AG, Aguilera A, Struhl K, Reed R, Hurt E. TREX is a conserved complex coupling transcription with messenger RNA export. Nature. 2002;417:304–308. doi: 10.1038/nature746. [DOI] [PubMed] [Google Scholar]
- Stutz F, Bachi A, Doerks T, Braun IC, Seraphin B, Wilm M, Bork P, Izaurralde E. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA. 2000;6:638–650. doi: 10.1017/s1355838200000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist MD, Soriano P. Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis. 2000;26:113–115. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Wang X, Chang Y, Li Y, Zhang X, Goodrich DW. Thoc1/Hpr1/p84 is essential for early embryonic development in the mouse. Mol Cell Biol. 2006;26:4362–4367. doi: 10.1128/MCB.02163-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RW, Jr, Kruger B, Thomas S, Ma J, Milgrom E. RLR1 (THO2), required for expressing lacZ fusions in yeast, is conserved from yeast to humans and is a suppressor of SIN4. Gene. 2000;243:195–205. doi: 10.1016/s0378-1119(99)00510-7. [DOI] [PubMed] [Google Scholar]
- Zenklusen D, Vinciguerra P, Wyss JC, Stutz F. Stable mRNP formation and export require cotranscriptional recruitment of the mRNA export factors Yra1p and Sub2p by Hpr1p. Mol Cell Biol. 2002;22:8241–8253. doi: 10.1128/MCB.22.23.8241-8253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]