Abstract
Relaxin stimulates cAMP production and activation of ERK and PI3K in THP-1 cells. Relaxin also stimulates protein kinase C zeta (PKCζ) translocation to the plasma membrane in a PI3K-dependent manner in THP-1 and MCF-7 cells. However, relaxin did not increase cAMP production in MCF-7 cells. We overexpressed different adenylyl cyclase (AC) isoforms in MCF-7 cells to examine coupling of endogenous relaxin receptors to cAMP production. Overexpression of types II and IV AC had no effect on cAMP production by relaxin. However, overexpression of type V AC, which is activated by PKCζ, showed synergistic stimulation of cAMP by relaxin and forskolin.
Keywords: adenylyl cyclase, PI3K, PKCζ, relaxin, cyclic AMP, ACV, ACII
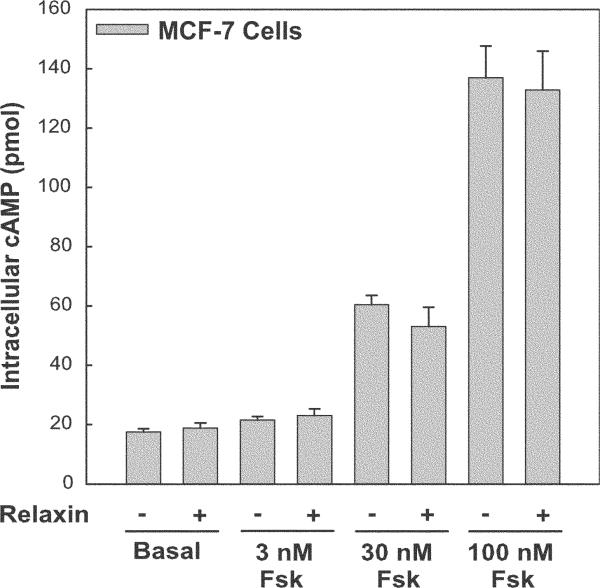
Relaxin is a polypeptide hormone which stimulates the leucine-rich repeat containing G-protein–coupled receptors (LGR7 and LGR8).1 Studies to date have shown that relaxin is capable of activating multiple signaling pathways (reviewed in Sherwood2). In the human monocytic cell line (THP-1), relaxin elicited a robust cAMP response with strong synergy between relaxin and forskolin in stimulating the activity of adenylyl cyclase.3–5 Relaxin also activated p42/44 mitogen–activated protein kinase (MAPK)6 and phosphoinositide-3 kinase (PI3K)5 and stimulated protein kinase C zeta (PKCζ) translocation in THP-1 cells.7 In the human breast cancer cell line (MCF-7), relaxin inhibited proliferation, promoted differentiation, and enhanced expression of the surface molecule E-cadherin.8 In MCF-7 cells, relaxin also stimulated PKCζ translocation in a PI3K-dependent manner, but independent of cAMP production.7 However, relaxin failed to elicit a detectable increase in cAMP production in MCF-7 cells even upon addition of a wide range of forskolin concentrations (Fig. 1).
FIGURE 1.
Relaxin fails to increase cAMP in MCF-7 cells. Duplicate samples (5 × 104 cells per treatment) were pretreated in the presence of phosphodiesterase inhibitor (50 μM isobutyl-1-methylxanthine, IBMX) for 15 minutes and then treated either with vehicle or with 0.1 μg/mL relaxin in the presence or absence of 3, 30, or 100 nM forskolin (Fsk) for 20 minutes. Treatments were stopped by removal of media and addition of 0.1 N HCl. Intracellular cAMP was detected by enzyme immunoassay. Data are expressed as the mean ± SE from a single experiment and are representative of three different experiments.
Based on the observation that relaxin stimulated PI3K and PKCζ in both THP-1 and MCF-7 cells, we hypothesized that relaxin could stimulate cAMP production in MCF-7 cells upon overexpression of adenylyl cyclase (AC) to drive coupling to the endogenous receptors. Currently, nine mammalian transmembrane isoforms of AC have been identified with diverse and complex regulation.9 Gsα activates all membrane-bound AC isoforms, whereas forskolin stimulates all isoforms, except ACIX. Protein kinase C (PKC) is a large family of serine/threonine kinases that is divided into three groups: conventional PKC (α, βI, βII, and γ), novel PKC (δ, ε, η, θ), and atypical PKC (ι/λ and ζ). Several isoforms of PKC have shown to stimulate the activity of AC, including PKCα (ACII and ACV), PKCδ (ACVII), and PKCζ (ACV and ACII).10
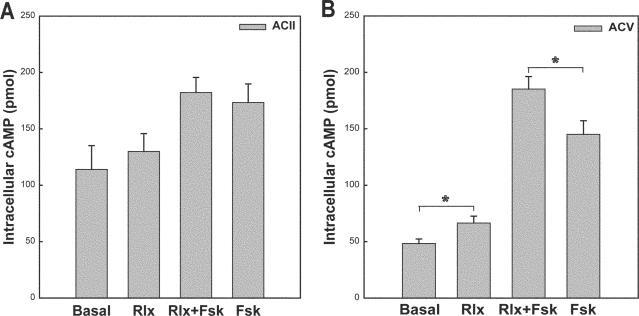
To test our hypothesis, we transfected MCF-7 cells with ACII, ACIV, or ACV and treated AC-transfected cells with vehicle, relaxin, relaxin plus forskolin, or forskolin alone. Relaxin had no effect on cAMP levels in ACII or ACIV-transfected cells in the presence or absence of forskolin (Fig. 2A and data not shown). The lack of cAMP stimulation by relaxin was not caused by insufficient AC activity. MCF-7 cells have a robust cAMP response to forskolin or isoproterenol and express at least five different AC isoforms (Fig. 1 and data not shown). In addition, AC-transfected cells produced a greater increase in forskolin-stimulated cAMP production than those transfected with empty vector (4.8- and 1.7-fold increase for AC II and ACV, respectively). However, in MCF-7 cells transfected with ACV, relaxin produced a small but significant increase in cAMP production and displayed synergy with forskolin (Fig. 2B). These results suggest that overexpression of AC can drive the coupling of endogenous LGR7/8 receptors in MCF-7 cells with transfected ACV, but not ACII or ACIV. It is unlikely that a particular isoform of PDE is absent from MCF-7 cells and thus responsible for relaxin stimulation as previously suggested,11 because all isoforms of AC increased basal cAMP levels, whereas only ACV was capable of producing cAMP stimulation by relaxin.
FIGURE 2.
Overexpression of ACV but not ACII in MCF-7 cells leads to relaxin stimulation of cAMP. MCF-7 cells (4 × 105 cells per treatment) were transfected with 0.3 μg pcDNA3-ACII (A) or 0.3 μg pcDNA3-ACV (B) for 36 h using Fugene 6 transfectant reagent and starved overnight in medium without fetal bovine serum. Transfected cells were pretreated in the presence of phosphodiesterase inhibitor (500 μM IBMX) for 15 minutes and then treated with vehicle, 0.1 μg/ml relaxin (rlx), 0.1 μg/ml relaxin + 30 nM forskolin (Fsk), or 30 nM forskolin for 20 min. Treatments were stopped by removal of media and addition of 0.1 N HCl. Intracellular cAMP was detected by enzyme immunoassay. (A) Data are expressed as the mean ± SE from a single experiment and are representative of two different experiments. (B) Data are expressed as the mean ± SE from three different experiments each performed in duplicate. Significant differences (*P <.05) between groups are designated (t test).
CONCLUSION
With a broad range of biological activity, relaxin is likely to stimulate multiple signaling pathways upon activation of its receptors (LGR7/8).1 The relaxin signaling pathways observed in THP-1 and MCF-7 cells support this observation. Relaxin activated ERK and PI3K in THP-1 cells.5,6 Currently, we report that relaxin stimulated PKCζ translocation which is dependent on PI3K in both THP-1 and MCF-7 cells.7 On the contrary, relaxin elicited a strong cAMP response in THP-1 cells5 but failed to show a detectable increase in MCF-7 cells (Fig. 1). However, relaxin produced a significant increase in cAMP production in MCF-7 cells transfected with ACV (Fig. 2B). These data lead to several possibilities. First, relaxin stimulation of cAMP may require expression of an AC isoform capable of synergistic activation by Gsα and PKCζ which may be missing in MCF-7 cells. However, stimulation of cAMP in THP-1 cells was still observed, albeit reduced, even upon inhibition of PI3K/PKCζ.5 Second, relaxin may produce only local increases in cAMP production, perhaps in lipid rafts where ACV and ACVI are often localized.12–14 Thus, a method that measures a global increase in cellular cAMP production may not detect small local increases in cAMP. Finally, an adapter protein/scaffolding protein may be needed for efficient coupling of the system which could be missing in MCF-7 cells. Taken together, relaxin stimulates a number of signaling pathways, but one pathway may be more prevalent than another depending on the cell type and collection of signaling molecules expressed.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grant GM60419 and the Texas Advanced Research Program No. 011618-00590-1999. We thank Drs. Barbara Sanborn and Chun-Ying Ku for all their help and support.
REFERENCES
- 1.Hsu SY, Nakabayashi K, Nishi S, et al. Activation of orphan receptors by the hormone relaxin. Science. 2002;295:671–674. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- 2.Sherwood OD. Relaxin's physiological roles and other diverse actions. Endocrinol. Rev. 2004;25:205–234. doi: 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- 3.Parsell DA, Mak JY, Amento EP, Unemori EN. Relaxin binds to and elicits a response from cells of the human monocytic cell line, THP-1. J. Biol. Chem. 1996;271:27936–27941. doi: 10.1074/jbc.271.44.27936. [DOI] [PubMed] [Google Scholar]
- 4.Bartsch O, Bartlick B, Ivell R. Relaxin signalling links tyrosine phosphorylation to phosphodiesterase and adenylyl cyclase activity. Mol. Hum. Reprod. 2001;7:799–809. doi: 10.1093/molehr/7.9.799. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen BT, Yang L, Sanborn BM, Dessauer CW. Phosphoinositide 3-kinase activity is required for biphasic stimulation of cyclic adenosine 3′,5′-monophosphate by relaxin. Mol. Endocrinol. 2003;17:1075–1084. doi: 10.1210/me.2002-0284. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Liu SH, Erikson M, et al. Relaxin activates the MAP kinase pathway in human endometrial stromal cells. J. Cell Biochem. 2002;85:536–544. doi: 10.1002/jcb.10150. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen BT, Dessauer CW. Relaxin stimulates protein kinase C zeta translocation: requirement for cyclic adenosine 3′,5′-monophosphate production. Mol. Endocrinol. 2005 doi: 10.1210/me.2004-0279. In press. [DOI] [PubMed] [Google Scholar]
- 8.Sacchi TB, Bani D, Brandi ML, et al. Relaxin influences growth, differentiation and cell-cell adhesion of human breast-cancer cells in culture. Int. J. Cancer. 1994;57:129–134. doi: 10.1002/ijc.2910570123. [DOI] [PubMed] [Google Scholar]
- 9.Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu. Rev. Pharm. Toxicol. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- 10.Chern Y. Regulation of adenylyl cyclase in the central nervous system. Cell Signal. 2000;12:195–204. doi: 10.1016/s0898-6568(99)00084-4. [DOI] [PubMed] [Google Scholar]
- 11.Bartsch O, Bartlick B, Ivell R. Phosphodiesterase 4 inhibition synergizes with relaxin signaling to promote decidualization of human endometrial stromal cells. J. Clin. Endocrinol. Metab. 2004;89:324–334. doi: 10.1210/jc.2003-030498. [DOI] [PubMed] [Google Scholar]
- 12.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J. Biol. Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- 13.Schwencke C, Yamamoto M, Okumura S, et al. Compartmentation of cyclic adenosine 3′,5′-monophosphate signaling in caveolae. Mol. Endocrinol. 1999;13:1061–1070. doi: 10.1210/mend.13.7.0304. [DOI] [PubMed] [Google Scholar]
- 14.Ostrom RS, Liu X, Head BP, et al. Localization of adenylyl cyclase isoforms and G protein-coupled receptors in vascular smooth muscle cells: expression in caveolin-rich and noncaveolin domains. Mol. Pharmacol. 2002;62:983–992. doi: 10.1124/mol.62.5.983. [DOI] [PubMed] [Google Scholar]