Abstract
The field of neonatal neurology, and specifically its focus on the premature infant, had its inception in neuropathological studies. Since then, the development of advanced imaging techniques has guided our developing understanding of the etiology and nature of neonatal brain injury. This review promotes the concept that neonatal brain injury has serious and diverse effects on subsequent brain development, and that these effects likely are more important than simple tissue loss in determining neurological outcome. Brain injury in the premature infant is best illustrative of this concept. This “encephalopathy of prematurity” is reviewed in the context of the remarkable array of developmental events actively proceeding during the last 16-20 weeks of human gestation. Recent insights into the brain abnormalities in survivors of preterm birth obtained by both advanced MRI and neuropathological techniques suggest that this encephalopathy is a complex amalgam of destructive and developmental disturbances. The interrelations between destructive and developmental mechanisms in the genesis of the encephalopathy are emphasized. In the future, advances in neonatal neurology will likely reiterate the field's dependence upon neuropathological studies, including new cellular and molecular approaches in developmental neurobiology.
Historical Overview
Personal Perspective
This presentation will focus on the brain of the premature infant. My interest in premature infants began in the early 1970s when neonatal intensive care began its ascendancy to the highly accomplished medical discipline of today. I was inspired by the seminal neuropathological studies of Betty Banker, Jeanne-Claudie Larroche, E. Pierson Richardson, Gilles Lyon, Dawna Armstrong, Lucy Rorke, Floyd Gilles, Jonathan Wigglesworth, and my colleague of the past 20 years, Hannah Kinney. My review of the neurology of prematurity and, indeed, of the entire field of neonatal neurology was heavily biased by my neurology training with Raymond Adams, Miller Fisher and Philip Dodge. This training placed great emphasis on neuropathology as the starting point for understanding the nature of disease and the subsequent neurological deficits. Dodge, my lifelong mentor, recognizing my particular interest in brain development, encouraged me in the late 1960s and early 1970s to embark on neonatal neurology as a career emphasis. Despite my reluctance to focus my young career in an uncertain area of pediatric neurology, I accepted his wise counsel and have been greatly stimulated and challenged by the field over the many years since.
Major Earlier Challenges and Advances
The major earlier challenges in neonatal neurology, especially the neurology of prematurity, related in considerable part to the problems of imaging the brain. Indeed, in the past 30 years, the particular emphasis of the neurology of prematurity has varied largely according to the capabilities of available imaging methods. In the late 1970s cranial ultrasound scanning, and slightly later, CT scanning, with their particular strengths for detection of hemorrhage, led to a great emphasis on intraventricular hemorrhage and its complications as the major sources of disability in survivors of premature birth. In the late 1980s and early 1990s, with the advent of MRI scanning, it became clear that cerebral white matter injury is the dominant pathology of prematurity. Near the turn of the century and to the present, more advanced MRI methodologies showed that cerebral white matter abnormality is accompanied by disturbances of gray matter structures in cerebrum, diencephalon, brain stem and cerebellum. Only very recently has it become apparent that these abnormalities of the neuronal-axonal unit are in large part disturbances of development, likely initiated by the initial injury. This constellation of white and gray matter abnormality, i.e., the “encephalopathy of prematurity”, is the principal determinant of neurologic outcome.
The Future
My view of the optimal future course of discovery in the field of the neurology of prematurity and, likely, all of neonatal neurology is that we must return to study of the human neuropathology, but now with approaches that include a very broad spectrum of modern-day, state-of-the-art cellular and molecular probes. The goal must be to define more clearly the fundamental nature of the brain injury, i.e., the extents to which primary injury and secondary developmental disturbances are operative. To fully delineate the developmental disturbances, deeper insights into the progression of brain development in the third trimester of human gestation are needed. The observations in recent years of Kostovic, Rakic, Kinney and coworkers, among others, are revealing a remarkable and complex world of developmental events occurring in human brain during the premature period. This renewed focus on human neuropathology and brain development is perhaps a classic example of “back to the future”.
Emerging Interface of Neonatal Neurology and Developmental Neurobiology
This review thus will focus on interrelations between brain injury in the premature infant and brain development. Over the past three to four decades, as neonatal neurology has evolved to arguably the most active discipline in neonatal medicine, the principal emphasis, as just noted, has been the definition of the regional and cellular characteristics of brain lesions, and the effects of this primary injury and the related tissue loss on subsequent neurological function. During the same time period, developmental neurobiology, at the molecular, cellular and “systems” levels, has evolved to one of the most extraordinarily complex and diverse areas of basic science. Only in recent years have neonatal neurology and developmental neurobiology significantly interfaced.
The principal results of the nascent interactions of neonatal neurology and developmental neurobiology, thus far, have been the delineation of the pathophysiology of brain injury and the role of maturation-dependent factors in rendering specific regions and cell types vulnerable to injury. Insights into pathophysiology have led to formulation of neuroprotective interventions, a few of which have reached the clinical arena. Insights into the roles of maturation-dependent factors in vulnerability include, among others, the discovery of the overexpression of excitatory amino acid receptors by developing neurons and oligodendroglia. These overly abundant receptors are important for normal development but become the source of deadly excitotoxicity under conditions of hypoxiaischemia and related insults. Notwithstanding these advances, it now appears clear that developmental neurobiology can provide critical insights into the structural and functional consequences of neonatal brain injury. Specifically, as understanding of human brain development mounts, it is becoming apparent that brain injury has serious and diverse effects on subsequent developmental events, and that these effects likely are more important than simple tissue loss in determining neurological outcome.
Introduction
The purpose of this review is to further the concept that neonatal brain injury and its subsequent clinical and anatomic consequences must be viewed as an amalgam of destructive and developmental disturbances.1 (This concept was introduced in a recent report,1 and this review will draw heavily on that discussion.) As noted above, traditionally the clinical and anatomic consequences of neonatal brain injury have been considered a result of “lesions” and tissue loss. With continuing insights into the development of human brain in the premature, neonatal and early infantile periods, it has become clear that this traditional subtractive approach is incomplete and often misleading. Perhaps the best example of the importance of viewing clinical and anatomic consequences in the context of relevant brain development is brain injury of the premature infant.1
The extraordinary importance of brain injury in the premature infant relates in part to the fact that 1.5% of the more than 4,000,000 live births in the United States, approximately 63,000 infants, are born yearly with a very low birth weight (VLBW; ≤1500 g).2 Of the approximately 90% of VLBW infants who survive, the resulting brain abnormalities account for the subsequent occurrence of cognitive, behavioral, attentional, and socialization defects in 25-50%, and of major motor deficits (e.g., cerebral palsy) in 5-10%.3-13 Notably, therefore, cognitive deficits without major motor deficits are now the dominant neurodevelopmental sequelae in the survivors of early preterm birth. Worthy of emphasis is the increasingly important contribution to this burden of disability by the most premature infants. In infants of <1000 gm birth weight, survival rates approach 70%, but more than 50% exhibit subsequent disability.14-16 Thus, overall the initial injury in the very premature population occurs at a time period equivalent to the interface of the second and third trimesters of human gestation and very shortly thereafter.
The neuropathology of brain injury in premature infants consists of multiple lesions: periventricular leukomalacia (PVL) and accompanying neuronal/axonal abnormalities; severe germinal matrix-intraventricular hemorrhage, especially with periventricular hemorrhagic infarction; and posthemorrhagic hydrocephalus, among others. Neuroimaging studies indicate that PVL in its various forms is by far the most common, occurring in 50% or more of VLBW infants.10 Because the neuronal/axonal abnormalities principally accompany PVL, I have used the term “encephalopathy of prematurity” for this constellation.1, 17 Because quantitatively this encephalopathy appears to account for most of the subsequent neurological sequelae, the remainder of this review will focus on this entity.
In the following, I will review first the neuropathology of the encephalopathy of prematurity, next describe the relevant brain developmental events that occur in the late second and third trimesters of gestation, and then discuss the interrelations between destructive and developmental mechanisms in the genesis of the encephalopathy. As noted earlier, the thesis of this review will be that this encephalopathy is a complex amalgam of primary destructive disease and secondary developmental disturbances.1
Neuropathology – Encephalopathy of Prematurity
The neuropathology of encephalopathy of prematurity consists of PVL and the often-associated neuronal/axonal disease. These two aspects are discussed briefly next.
PVL
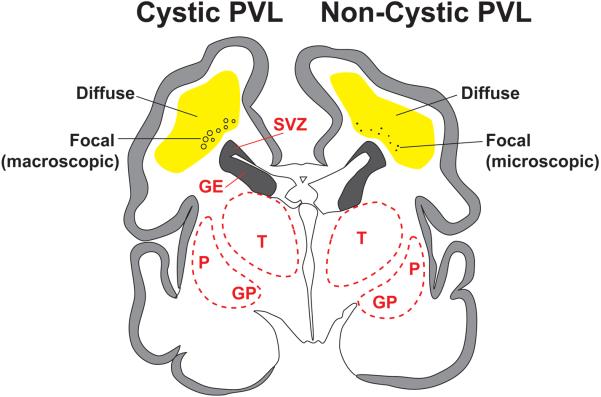
PVL refers to injury to cerebral white matter, generally more severe in deep than superficial white matter, and consists of two major components, focal necrosis deep in the white matter with loss of all cellular elements, and a more diffuse component in central cerebral white matter, with injury to premyelinating oligodendrocytes (pre-OLs) and a marked astrocytosis and microgliosis (Fig. 1).10 The focal necrotic lesions may be macroscopic in size (several millimeters or more) and evolve to cysts, readily visualized on cranial ultrasound or MRI scans. This severe lesion occurs in less than 5% of VLBW infants in modern neonatal intensive care units.18-22 Much more commonly, the focal necrotic lesions are microscopic in size and evolve to small glial scars, not easily seen on neuroimaging.
Fig. 1.
Schematic diagram of cystic and noncystic periventricular leukomalacia (PVL). Coronal sections of cerebrum from a 28-week premature infant show focal necrotic and diffuse components of PVL. In cystic PVL (left) the focal necrotic lesions (circles) are macroscopic in size and evolve primarily to cysts, and in noncystic PVL, the focal necrotic lesions (dots) are microscopic in size and evolve primarily to glial scars. The diffuse component (yellow) is characterized by pre-OL injury, astrogliosis and microgliosis. Abbreviations: SVZ, subventricular zone; GE, ganglionic eminence; T, Thalamus; P, putamen; GP, globus pallidus. Reproduced with permission. (1)
The injury to pre-OLs in the diffuse component of PVL may consist of cell death or loss of cell processes on viable cells or both.23-26 The not-unexpected consequence of both types of PVL is a deficiency of fully differentiated OLs and hypomyelination with ventriculomegaly, easily identified by neuroimaging.27-34 The recent demonstration by advanced diffusion tensor MRI of lower axial diffusivity and higher radial diffusivity in the abnormal cerebral white matter supports the notion that a failure of ensheathment of axons by pre-OLs is a critical cause of the hypomyelination.35
Notably, attempts at replenishment of the oligodendroglial lineage appear to occur in human premature brain. Thus, the initial decrease in pre-OLs is counteracted by an increase in oligodendroglial progenitors.26 This reparative response of progenitors has been shown in several developing animal models of PVL.36, 37 However, these cells seem not to have the capacity for full differentiation to myelin-producing cells. Notably in animal models these progenitors are exquisitely vulnerable to a subsequent hypoxicischemic insult,24 a common clinical feature in premature infants and perhaps important in explaining why the incidence of PVL increases in premature infants as a function of postnatal age.10 Although precise correlations of neuroimaging and neuropathology are lacking, the correlates of the diffuse component of PVL on MRI in the neonatal period seem to include diffuse signal abnormalities and disturbances in diffusion parameters.3, 19, 22, 35, 38-46
Neuronal/Axonal Disease
Neuronal/axonal disease, a previously under-recognized accompaniment of PVL, is the other major element of the encephalopathy of prematurity. The following reviews the major regions of involvement, i.e., cerebral white matter (axons and subplate neurons), thalamus, basal ganglia, and cerebral cortex. (Cerebellum and brain stem also are affected, as reviewed elsewhere,1, 47 but not included here because of space limitations.)
Cerebral white matter – axons
During the peak period of vulnerability for PVL in the human premature infant, cerebral white matter axons (projection, commissural and association fibers) are in a phase of rapid growth (see later). The occurrence of axonal injury in the necrotic foci of severe PVL has been known for many years.25, 48-52 Unexpectedly, however, Haynes and coworkers,53 utilizing the apoptotic marker fractin, showed widespread axonal degeneration in the diffuse component of PVL, separate from the focal necroses. Consistent with these observations, diffusion tensor MRI studies of cerebral white matter in noncystic PVL show blunting of the normal maturational increase in fractional anisotropy in various axonal tracts.34, 42, 46, 54-62 However, the possibility remains that some or all of these diffusion tensor results relate to a failure of pre-OL ensheathment (see later).35
Cerebral white matter - subplate neurons / late migrating neurons
The two principal neuronal types in cerebral white matter during the premature period are subplate neurons and late migrating GABAergic neurons. Subplate neurons are located in the subcortical white matter, and the late migrating neurons, primarily in central white matter. Both of these neuronal types are critical for cerebral cortical and thalamic development, and both are transient populations (see later). Subplate neurons contain excitatory amino acid receptors and have been shown in a developing animal model to be selectively vulnerable to hypoxia-ischemia.63 Because hypoxia-ischemia and excitotoxicity appear to be important in the pathogenesis of PVL10 and because PVL is associated with volumetric deficits of the cerebral cortex and thalamus, it is reasonable to hypothesize that with PVL there is concomitant injury to subplate neurons. Late migrating neurons also may be intrinsically vulnerable. Scant human data are available. However, in a brief autopsy study, increased apoptosis (activated caspase-3 expression) was observed in the subplate of premature infants with PVL versus those without PVL.25 A decrease in GABAergic neurons (GAD (glutamic acid decarboxylase)-67) in central white matter also was documented.25 A more detailed study has recently shown a prominent decrease in subcortical and central white matter neurons in infants with PVL.64
Thalamus
Involvement of thalamus in premature infants initially became most clearly apparent from volumetric MRI studies of infants at term equivalent age and later in childhood and adolescence.20, 22, 28-30, 65-68 Studies that specifically assessed the presence of PVL noted that the thalamic volumetric deficit occurred especially in association with imaging features of PVL.20, 22 Consistent with the in vivo observations, a detailed neuropathological analysis of 41 premature infants from a modern neonatal intensive care unit identified in thalamus frequent neuronal loss (40%) and gliosis (60%) in the presence of PVL; neuronal loss was absent in infants without PVL.69 A subsequent more detailed neuropathological study of thalamus in 22 cases of PVL showed neuronal loss, gliosis and axonal degeneration (fractin expression) in fully 60%.70 Whether the findings related to a primary injury or a secondary trophic disturbance could not be determined (see later). Notably the mediodorsal nucleus was particularly affected, and this crucial nucleus, which has reciprocal connections to prefrontal cortex and limbic cortex, is involved in integration of cognitive and affective functions, deficits of which are common in survivors of premature birth.
Basal ganglia
As with thalamus, MRI volumetric deficits of basal ganglia were found to be frequent in VLBW infants at term equivalent age or older.20, 27, 29, 30, 65, 67, 68, 71 In a study that specifically addressed the presence of noncystic PVL, a clear relation of the basal ganglia deficit with the white matter abnormality was apparent.20 A large neuropathological study showed gliosis in basal ganglia in 50-60% and overt neuronal loss in 15-30% in infants with PVL, whereas these findings were observed much less commonly or not at all in the absence of PVL.69 As for thalamus, the neuropathological findings do not allow distinction of primary injury from secondary trophic effects (see later).1
Cerebral cortex
Cerebral cortical involvement in premature infants with PVL appears likely but remains to be carefully elucidated. Thus, in living infants volumetric MRI studies over the past 10 years have repeatedly demonstrated deficits in multiple cortical regions as early as term equivalent age.20, 72, 73 Parieto-occipital cortex, which overlies the white matter region most susceptible to PVL, is most commonly involved. Infants studied later in childhood, adolescence and adulthood have shown persisting volumetric deficits, with the most pronounced deficits generally in parieto-occipital, sensorimotor, premotor, temporal and hippocampal cortices.28-30, 33, 67, 68, 71, 74, 75 As expected, these cortical deficits correlate with a wide spectrum of cognitive deficits on follow-up.
In contrast to the MRI studies, neuropathological observations thus far have not demonstrated pronounced cortical deficits. Although earlier work showed cortical neuronal abnormalities in infants with particularly severe cystic PVL,48, 76-78 later neuropathological studies of the now common, less severe, noncystic PVL show neuronal loss or gliosis or both in no more than 13-30% of cases.69 What is the reason for the apparent disconnect between the MRI and neuropathological data? The answer likely lies in the relative deficiencies of conventional neuropathological analysis. Consistent with this notion, a recent study that utilized advanced computer-based techniques to quantitate neuronal density showed markedly diminished neuronal density in layer V of sensory-related cortical areas.79 These neurons, of course, represent the outflow to thalamus, and the neuronal deficit could relate to primary injury, or perhaps more likely, a secondary disturbance caused by injury of the efferent axons in cerebral white matter, loss of their targets in thalamus, or impairment of their reciprocal afferent input from thalamus (see later). Similar quantitative studies of neuronal populations and axonal and dendritic ramifications in cerebral cortex are needed.
Major Developmental Events in Human Brain During the Premature Period
The encephalopathy of prematurity, i.e., both PVL and the associated neuronal/axonal disease, occurs during a period of extraordinarily rapid and complex events in human brain development.1, 10 The developmental events between 24-40 weeks involve, particularly: (1) in cerebral white matter – pre-OLs, axons, microglia, and neurons (subplate and late migrating GABAergic neurons); (2) two proliferative zones – the dorsal cerebral subventricular zone (SVZ) and the ventral germinative epithelium of the ganglionic eminence (GE); and (3) key neuronal structures – thalamus, cerebral cortex and basal ganglia. Because of the rapidity and complexity of these developmental events, they are postulated to be vulnerable to exogenous and endogenous insults, such as ischemia, inflammation, excitotoxicity and free-radical attack. 1 This concept of enhanced vulnerability of rapidly developing events in brain was championed and corroborated decades ago by classic studies of the effects of infantile undernutrition and other exogenous insults by Dobbing and colleagues.80, 81
Pre-OLs
Pre-OLs are early differentiating premyelinating oligodendrocytes. The nomenclature and details of these differentiating phases of the oligodendrocyte lineage are reviewed elsewhere.10 Suffice it to say here that pre-OLs are a key cellular target in PVL, are the predominant forms of the lineage present in human cerebral white matter from 24-40 weeks of gestation, and are in a phase of rapid differentiation, including ensheathment of developing axons in preparation for full differentiation to myelin-producing oligodendrocytes (Fig. 2A).82-85 The latter do not become abundant in cerebral white matter until after term. Pre-OLs exhibit maturation-dependent characteristics that render them especially vulnerable to such insults as ischemia and inflammation, which lead to excitoxicity and generation of free radicals.86 These multiple maturation-dependent characteristics are discussed elsewhere.10, 23, 24, 86-96 It should only be emphasized here that a remarkable confluence of maturation-dependent characteristics at this time conspire to make the pre-OL a very vulnerable cell to injurious insults.
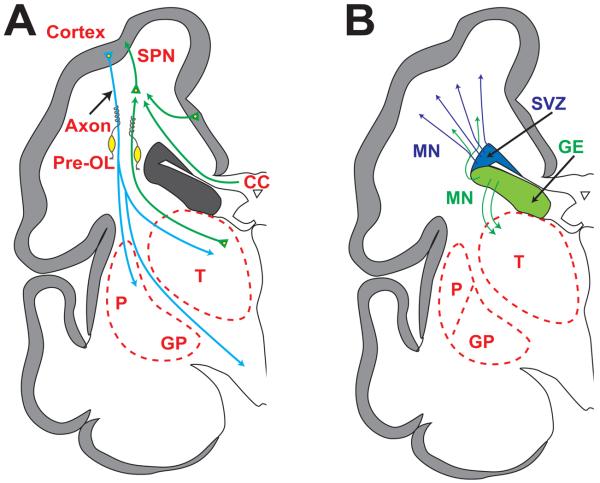
Fig. 2.
Schematic diagrams of cerebrum (coronal sections) at 28 weeks' gestation depicting critical events in cerebral development. (A) Axons (green) emanate from thalamus (T) (projection fibers), corpus callosum (CC) (commissural fibers) and other cortical sites (association fibers) and synapse initially on subplate neurons (SPN). SPNs send axons to cortex to promote cortical development before the thalamo-cortical, commissural-cortical and cortico-cortical fibers enter the cortex. From cortex, axons (blue) descend to thalamus, basal ganglia and corticospinal (and corticopontine) tracts. Premyelinating oligodendrocytes (preOLs) (yellow) ensheath afferent and efferent axons before full differentiation to mature myelin-producing oligodendrocytes, especially after term. (B) The proliferation and migration of GABAergic interneurons from the dorsal telencephalic subventricular zone (SVZ) (blue) and the ventral germinative epithelium of the ganglionic eminence (GE) (green) are shown. Neurons from the SVZ (blue) migrate radially to the cortex, and those from the GE (green) tangentially and then radially to the cortex. The migrating stream of GABAergic interneurons (green) to the dorsal thalamus (T) also is shown. Other abbreviations: GP, globus pallidus; P, putamen. Reproduced with permission. (1)
Microglia
Microglia are a prominent cellular presence in the diffuse component of PVL (see earlier). Interestingly, in normal human brain these cells become prominent in the forebrain from 16-22 weeks of gestation97-100 and reach a peak abundance in cerebral white matter in the third trimester, with a deep to superficial gradient.100 These normal features are consistent with recognized key roles for microglia in brain development, involving apoptosis, vascularization, axonal development and myelination.1 However, microglia, when activated, as in PVL, are key effectors of cellular injury initiated by ischemia or inflammation or both. These cells generate free radicals, secrete injurious cytokines and enhance excitotoxicity.91, 92, 101-105 Because microglia are particularly abundant in normal cerebral white matter in the third trimester, they are in the right place at the right time in large numbers to lead to injury to white matter constituents (pre-OLs, axons, subplate neurons, late migrating neurons).
Axons
Axonal development, in concert with growth of the subplate, is markedly exuberant over the last 20 weeks of gestation (Fig. 2A). The three major categories of cerebral axons destined for the cortex, i.e., projection, association and commissural fibers, are involved (Table 1).106-110 These fibers synapse initially on subplate neurons, and “wait” as cortical neurons differentiate sufficiently to provide sites of later contact. Projection axons from thalamus arrive at the subplate initially during this period and then enter cortex between 24-32 weeks. Commissural and association (cortico-cortical) fibers enter the subplate at 24-32 weeks and then the cortex at 33-35 weeks (Fig. 2A).
Table 1.
Development of Cerebral White Matter Axons and Subplate Neurons During the Premature Period
| 20-24 Weeks |
|
|
| 24-32 Weeks |
|
|
|
|
| 32-36 Weeks |
|
|
Adapted from Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009;8(4):110-24; and data primarily from Kostovic and coworkers: Kostovic I, Judas M, Rados M, Hrabac P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex 2002;12(5):536-44; Kostovic I, Jovanov-Milosevic N. The development of cerebral connections during the first 20-45 weeks' gestation. Semin Fetal Neonatal Med 2006;11:415-22; and Kostovic I, Judas M. Transient patterns of cortical lamination during prenatal life: do they have implications for treatment? Neurosci Biobehav Rev 2007;31(8):1157-68.
Subplate Neurons
This remarkable transient population of neurons located beneath the cortical plate reaches peak size and maximal developmental impact at 24-32 weeks of gestation, the peak period for the occurrence of PVL (Fig. 2A) (Table 1).1, 106, 107, 109-111 These neurons originate prior to the premature period, primarily from the dorsal telencephalic ventricular zone (glutamatergic), and to a lesser extent from the ventral telencephalic GE (GABAergic). Development of the subplate is closely linked with development of cerebral cortex, deep nuclear structures (especially thalamus) and axons (projection, commissural and association) (Table 2).1, 106, 112-118
Table 2.
Major Roles of Subplate Neurons
|
|
|
|
|
See text for references
Subventricular Zone and Late Migrating GABAergic Neurons
The dorsal telencephalic SVZ, which gives rise to projection neurons early in gestation, previously has been considered to be principally gliogenic after 20 weeks of gestation.111 This notion has been revised in recent years by studies showing that after the 20th gestational week and extending into at least weeks 25-27, the SVZ actively generates neurons, mainly GABAergic neurons for the upper cortical layers, the hallmark of the human cortex.1, 111, 119, 120 These later arriving neurons are generated largely (65%) from the dorsal telencephalic SVZ and migrate radially, although approximately 35% are generated from the ventral GE, from which they migrate first tangentially, parallel to the cortical plate, to the region of the dorsal SVZ from which they migrate radially to the cortex (Fig. 2B).111, 119, 120 The late proliferation and migration of cortical GABAergic neurons have been documented recently in developing animals.121, 122
A critical unanswered question is when does the late migration of GABAergic neurons cease in human brain? Extrapolating from the experimental data, it seems likely that this migration continues well into the third trimester. Indeed, the SVZ is a prominent structure during the entire premature period.123 The particular importance of cortical GABAergic neurons relates to several facts: 1) they constitute 20-30% of all cortical neurons; 2) they are concentrated in upper cortical layers, which are disproportionally thickened in human cortex; 3) they increase in number and complexity with evolution; and 4) they are critical for the coordination and integration of cortical function, thereby playing a key role in cognitive phenomena and modulation of excitation.
Thalamus
Development of the thalamus, particularly thalamic GABAergic neurons, exhibits important similarities to cerebral cortical development. Thus, as with cortex, the majority of initial neuronal acquisition occurs prior to 20 weeks' gestation, in this case primarily from the diencephalic ventricular zone.111 However, as with cortex, recent data show a second, later wave of neurons that are generated in the ventral telencephalic GE and migrate to dorsal thalamus (Fig. 2B).111, 120, 124 Continuing the analogy with cortex, these neurons are mainly GABAergic. Similar to cortex, approximately 30% of the neurons in every thalamic nucleus are GABAergic.125, 126 This telencephalon-derived wave of migration to dorsal thalamus appears unique to human brain and likely leads to an increase in the population of GABAergic neurons in the large association nuclei (the mediodorsal and pulvinar nuclei).111 Notably the first demonstration of a higher number of human neurons in the brain of newborns compared with the adult involved the mediodorsal nucleus.127 The number of neurons in the newborn is 75% higher than in the adult. Recall that the mediodorsal nucleus of thalamus in particular shows neuronal loss and gliosis in premature infants with PVL. The unique telencephalon-derived neurons in human brain are probably linked to the expansion of the thalamic association nuclei, which in turn are anatomically linked to the enlargement of association cortices involved in multiple higher cognitive function.1, 124 As with late migrating GABAergic neurons to cortex, the timing of this critical later development of thalamus is not entirely known but probably occurs during a long period “from 15 to approximately 34 weeks” of gestation.124
Cerebral Cortex
Advanced MRI techniques during the premature period have shown two striking characteristics of cerebral cortical development: 1) a four-fold increase in cerebral cortical volume from 28-40 weeks, and 2) in parallel, dramatic increases in both cortical surface area and gyral development. The anatomic substrates for these findings are becoming clear. Thus, most, but not all, of the cortical neurons have migrated from the early proliferative ventricular/subventricular zones, especially to deeper cortical layers, before 24 weeks of gestation.1, 10, 111 From 24-32 weeks of gestation, synapses appear in this deep cortical plate as thalamocortical axons exit the subplate and enter the cortex (Table 1).109 Parallel acceleration in cortex of dendritic differentiation and extensive elaboration of afferent axon terminals from thalamic, commissural and association fibers that enter the cortex after synapsing on subplate neurons (see earlier) together lead to the striking four-fold increase in cerebral cortical volume documented by MRI between 28 and 40 weeks post-conception.128, 129
Particularly important in the increase in cortical surface area and gyral formation is the disproportionate increase in thickness of upper cortical layers.111 As described earlier, this thickening results at least in part because of the late-arriving GABAergic interneurons from the dorsal SVZ and ventral GE (Fig. 2B). (The importance of a disproportionate increase in superficial versus deeper cortical areas in gyral formation has been reviewed.)10 As noted earlier, the time of termination of this later GABAergic neuronal migration to cortex is unknown, but probably extends well into the third trimester.1, 111, 119
Encephalopathy of Prematurity -- Combination of Destructive and Developmental Disturbances
As noted in the Introduction, the thesis of this review is that the encephalopathy of prematurity, i.e., the combination of PVL and neuronal/axonal disease described under Neuropathology, is a complex amalgam of primary destructive and secondary developmental disturbances.1 The developmental disturbances include impaired cell-cell interactions, involving intercellular trophic support, retrograde effects (“dying back”), and anterograde effects (e.g., Wallerian degeneration, trans-synaptic degeneration), among others.1 The relative importance of primary destructive versus secondary developmental disturbances and the nature and extent of the interactions between these two mechanisms remain to be fully elucidated. However, it appears quite possible that the developmental disturbances may propagate and build upon each other, thereby causing apparently self-limited primary injury to result in a multifaceted maturational impairment.
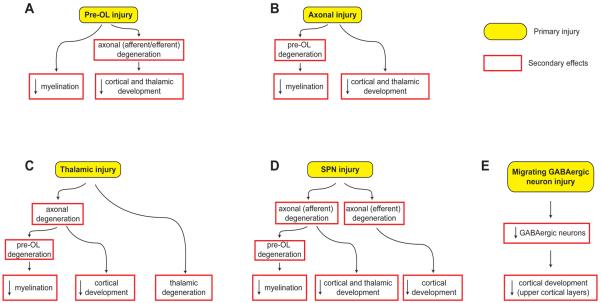
Five potential scenarios of primary injurious and secondary trophic/maturational disturbances appear most likely (Fig. 3). These are discussed separately next. The principal outcomes, shown by neuroimaging and by neuropathology, are impairments of myelination and of cortical and thalamic development.
Fig. 3.
Potential sequences of events leading to the major brain sequelae observed in premature infants with periventricular leukomalacia. The major sequelae include hypomyelination, and impaired cortical and thalamic development. For each sequence, the initiating primary injury is shown in yellow, and the subsequent secondary effects, shown in red boxes, are postulated to occur because of maturational/trophic/trans-synaptic disturbances, as described in the text. Abbreviations: Pre-OL, premyelinating oligodendrocyte; SPN, subplate neuron. Reproduced with permission. (1)
Pre-OL Injury
The pre-OL in the premature period is a highly vulnerable cell, and pre-OL injury appears to be a key initial finding in the diffuse component of PVL (Figs. 3A and 4A). The propensity for pre-OL injury has been documented both in human PVL23-26 and in several well-established animal models.1, 10, 130-133 This injury includes cell death or process loss (with intact cell soma) or both. Excitotoxic mechanisms appear to be involved in both, i.e., activation of calcium-permeable AMPA receptors on cell somata results in cell death,93, 134-141 and activation of NMDA receptors on cell processes, process loss with intact soma.1, 10, 95, 142-145 The ultimate result of either event would be a deficit in mature myelin-producing oligodendroglia, and thereby hypomyelination, the hallmark of PVL (Fig. 3A).
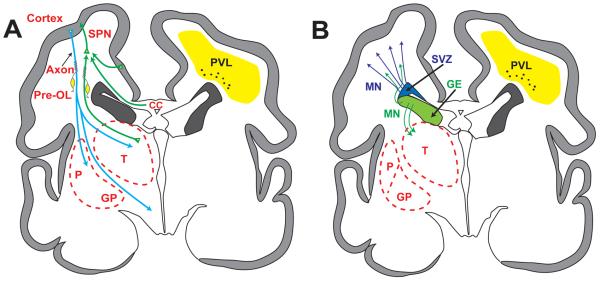
Fig. 4.
Schematic diagrams of coronal sections of cerebrum from a 28-week gestation premature infant depicting the anatomical relationships between the major developmental events and the topography of noncystic periventricular leukomalacia. For purposes of clarity the developmental events are separated (A and B). Abbreviations as in Figs. 1 and 2. Reproduced with permission. (1)
Pre-OL injury also could lead to the neuronal/axonal deficits observed with PVL (Fig. 3A). The principal mechanisms would involve failure of axonal development and ultimately axonal degeneration.1 The important trophic role of oligodendrocytes for axonal development, survival and function has been established in experimental models.146-157 As noted earlier, accompanying the ensheathment of axons by pre-OLs, axonal growth in the cerebral white matter is markedly exuberant during the premature period, and thus a particular need for trophic support is apparent. Diffusion-based MRI studies of cerebral white matter in premature infants show abnormalities consistent with axonal deficiency, which could reflect impaired axonal development or axonal degeneration or both.34, 35, 42, 54-58, 60, 158 The consequences of the axonal deficiency would be diminished cerebral cortical and thalamus/basal ganglia volumes secondary to retrograde and anterograde (trans-synaptic) effects (i.e., projection fibers to and from cortex, thalamus and basal ganglia, and commissural and association fibers to and from cortex) (Figs. 3A and 4A).1
Axonal Injury
Axonal injury could be a primary event with PVL (Fig. 3B). As noted earlier, axonal disruption occurs in the areas of focal necrosis. Perhaps more importantly, axonal degeneration has been discovered in the wider spread, diffuse component of PVL, detected by the apoptotic marker, fractin,.53 This observation is consistent with the finding of axonal injury in experimental models of hypoxic-ischemic injury analogous to PVL.159-162 The active axonal development in cerebral white matter in premature infants (see earlier) could make these fibers especially vulnerable. Although it is unclear whether the axonal degeneration in diffuse PVL is a primary injury or secondary effect, if the former did occur, the expected secondary developmental effects would be hypomyelination (due to failure of axonal ensheathment and thereby axonal/oligodendroglial interactions) and decreased cortical and thalamus/basal ganglia volumes (via anterograde and retrograde effects) (Figs. 3B and 4A).
Thalamic Injury
The possibility of thalamic injury as a primary event is suggested by experimental observations in a developing animal model of hypoxic-ischemic injury.163 Neuropathological study of human infants with PVL shows thalamic neuronal/axonal abnormalities in approximately 60%.69, 70 However, the principal findings, i.e., neuronal loss, gliosis, axonal degeneration, do not allow distinction of primary injury from secondary trophic/developmental effects. If primary thalamic neuronal injury did occur, expected secondary effects would involve white matter axons with subsequent hypomyelination, and impaired development of both cerebral cortex and thalamus (Figs. 3C and 4A).1 More data are needed.
Subplate Neuronal Injury
Injury to subplate neurons could have major secondary trophic/maturational disturbances affecting both cerebral cortex and thalamus, in view of the key role of this transient neuronal population in the development of thalamo-cortical and cortico-cortical circuits (see earlier). Considerable experimental data support this contention.1, 10, 106, 112-118, 164 A brief earlier report showed increased apoptosis in the subplate of infants with PVL.25 A more detailed recent report has shown a prominent decrease in subcortical white matter neurons, presumably subplate neurons, in infants with PVL.64 If these observations reflect a primary neuronal injury, secondary anterograde effects would involve cerebral cortical targets, and retrograde effects would impact afferent white matter axons and their originating neurons in thalamus and distant cortical regions (Figs. 3D and 4A). Axonal degeneration would lead to subsequent hypomyelination, as discussed earlier, for pre-OL and axonal injury. Notably, selective subplate neuronal death was identified in a neonatal rat model of hypoxic-ischemic injury akin to PVL.63
Late Migrating Neuronal Injury
Because the diffuse component of PVL includes the migrating path of the late-generated GABAergic neurons from the SVZ and GE, the possibility of direct injury to these critical cells is real. An earlier report showed a deficit in GABAergic neurons in central white matter in infants with PVL.25 A larger recent study also showed a pronounced deficit in central white matter neurons in such infants.64 Whether these findings reflect direct injury or decreased generation from an impaired SVZ is unclear. Notably, however, in several experimental models of selective white matter injury similar to PVL, the SVZ responded by generating oligodendroglial progenitors after the insult.36, 37 Nevertheless, an earlier report suggests that precursor cells in the dorsal telencephalic SVZ, the source of 65% of the GABAergic neurons destined for human cortex, are vulnerable to hypoxia-ischemia.165 On balance, it appears that there is a deficit in late-migrating GABAergic neurons in human PVL, but the mechanism is unclear. Because these GABAergic neurons contribute importantly to the thickness of upper cortical layers, a blunting or diminution of this migration could have important structural and functional consequences (Figs. 3E and 4B).1
Conclusions
Which of the five potential scenarios depicted in Fig. 3 is most important in the encephalopathy of prematurity is unclear. Pre-OL injury may be the most common starting point. However, it seems likely that more than one and perhaps all of the scenarios shown in Fig. 3 operate to a varying extent.1 Determinants of the relative importance of each scenario could relate to such factors as the gestational age of the infant, the timing and nature of the insult(s), such critical associated factors as disturbed nutritional state, exposure to glucocorticoids or other drugs, and many still-to-be-defined parameters. The most important lesson is that the encephalopathy of prematurity is a complex amalgam of destructive and developmental disturbances, and its full understanding will require deep insight into the extraordinary spectrum of developmental events occurring in the human premature period.
Acknowledgement
This work was supported by the NINDS Grant #P01-NS038475.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(4):110–24. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin JA, Kung HC, Mathews TJ, Hoyert DL, Strobino DM, Guyer B, et al. Annual summary of vital statistics: 2006. Pediatrics. 2008;121(4):788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- 3.Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128:2578–87. doi: 10.1093/brain/awh618. [DOI] [PubMed] [Google Scholar]
- 4.Bayless S, Stevenson J. Executive functions in school-age children born very prematurely. Early Hum Dev. 2007;83(4):247–54. doi: 10.1016/j.earlhumdev.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Platt MJ, Cans C, Johnson A, Surman G, Topp M, Torrioli MG, et al. Trends in cerebral palsy among infants of very low birthweight (<1500 g) or born prematurely (<32 weeks) in 16 European centres: a database study. Lancet. 2007;369(9555):43–50. doi: 10.1016/S0140-6736(07)60030-0. [DOI] [PubMed] [Google Scholar]
- 6.Larroque B, Ancel PY, Marret S, Marchand L, Andre M, Arnaud C, et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet. 2008;371(9615):813–20. doi: 10.1016/S0140-6736(08)60380-3. [DOI] [PubMed] [Google Scholar]
- 7.Kobaly K, Schluchter M, Minich N, Friedman H, Taylor HG, Wilson-Costello D, et al. Outcomes of extremely low birth weight (<1 kg) and extremely low gestational age (<28 weeks) infants with bronchopulmonary dysplasia: effects of practice changes in 2000 to 2003. Pediatrics. 2008;121(1):73–81. doi: 10.1542/peds.2007-1444. [DOI] [PubMed] [Google Scholar]
- 8.Allin M, Walshe M, Fern A, Nosarti C, Cuddy M, Rifkin L, et al. Cognitive maturation in preterm and term born adolescents. J Neurol Neurosurg Psychiatr. 2008;79(4):381–6. doi: 10.1136/jnnp.2006.110858. [DOI] [PubMed] [Google Scholar]
- 9.Limperopoulos C, Bassan H, Sullivan NR, Soul JS, Robertson RL, Moore M, et al. Positive screening for autism in ex-preterm infants: Prevalence and risk factors. Pediatrics. 2008;121(4):758–65. doi: 10.1542/peds.2007-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpe JJ. Neurology of the Newborn. 5th ed. Elsevier; Philadelphia: 2008. [Google Scholar]
- 11.Lowe J, MacLean PC, Shaffer ML, Watterberg K. Early working memory in children born with extremely low birth weight: assessed by object permanence. J Child Neurol. 2009;24(4):410–5. doi: 10.1177/0883073808324533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuban KC, O'Shea TM, Allred EN, Tager-Flusberg H, Goldstein DJ, Leviton A. Positive screening on the Modified Checklist for Autism in Toddlers (M-CHAT) in extremely low gestational age newborns. J Pediatr. 2009;154(4):535–40. doi: 10.1016/j.jpeds.2008.10.011. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson S, Marlow N. Positive screening results on the modified checklist for autism in toddlers: implications for very preterm populations. J Pediatr. 2009;154(4):478–80. doi: 10.1016/j.jpeds.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 14.Marlow N, Hennessy EM, Bracewell MA, Wolke D. Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 2007;120(4):793–804. doi: 10.1542/peds.2007-0440. [DOI] [PubMed] [Google Scholar]
- 15.Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child. 2005;90:F134–F40. doi: 10.1136/adc.2004.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolke D, Samara M, Bracewell M, Marlow N. Specific language difficulties and school achievement in children born at 25 weeks of gestation or less. J Pediatr. 2008;152(2):256–62. doi: 10.1016/j.jpeds.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Volpe JJ. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics. 2005;116:221–25. doi: 10.1542/peds.2005-0191. [DOI] [PubMed] [Google Scholar]
- 18.Larroque B, Marret S, Ancel PY, Arnaud C, Marpeau L, Supernant K, et al. White matter damage and intraventricular hemorrhage in very preterm infants: the EPIPAGE study. J Pediatr. 2003;143:477–83. doi: 10.1067/S0022-3476(03)00417-7. [DOI] [PubMed] [Google Scholar]
- 19.Miller SP, Cozzio CC, Goldstein RB, Ferriero DM, Partridge JC, Vigneron DB, et al. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR Am J Neuroradiol. 2003;24(8):1661–69. [PMC free article] [PubMed] [Google Scholar]
- 20.Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–94. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 21.Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147:609–16. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 22.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–94. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 23.Haynes RL, Folkerth RD, Keefe R, Sung I, Swzeda LI, Rosenberg PA, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes is accompanied by microglial activation in periventricular leukomalacia in the human premature infant. J Neuropath Exp Neurol. 2003;62:441–50. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 24.Back SA, Luo NL, Mallinson RA, O'Malley JP, Wallen LD, Frei B, et al. Selective vulnerability of preterm white matter to oxidative damage defined by F(2)-isoprostanes. Ann Neurol. 2005;58(1):108–20. doi: 10.1002/ana.20530. [DOI] [PubMed] [Google Scholar]
- 25.Robinson S, Li Q, Dechant A, Cohen ML. Neonatal loss of gamma-aminobutyric acid pathway expression after human perinatal brain injury. J Neurosurg. 2006;104(6 Suppl):396–408. doi: 10.3171/ped.2006.104.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Billiards SS, Haynes RL, Folkerth RD, Borenstein NS, Trachtenberg FL, Rowitch DH, et al. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 2008;18(2):153–63. doi: 10.1111/j.1750-3639.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Scheinder KC, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–47. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 28.Nosarti C, Al-Asady MHS, Frangou S, Stewart AL, Rifkin L, Murray RM. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125:1616–23. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 29.Reiss AL, Kesler SR, Vohr B, Duncan CC, Katz KH, Pajot S, et al. Sex differences in cerebral volumes of 8-year-olds born preterm. J Pediatr. 2004;145:242–9. doi: 10.1016/j.jpeds.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 30.Kesler SR, Ment LR, Vohr B, Pajot SK, Schneider KC, Katz KH, et al. Volumetric analysis of regional cerebral development in preterm children. Pediatr Neurol. 2004;31:318–25. doi: 10.1016/j.pediatrneurol.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allin M, Henderson M, Suckling J, Nosarti C, Rushe T, Fearon P, et al. Effects of very low birthweight on brain structure in adulthood. Dev Med Child Neurol. 2004;46(1):46–53. doi: 10.1017/s0012162204000088. [DOI] [PubMed] [Google Scholar]
- 32.Fearon P, O'Connell P, Frangou S, Aquino P, Nosarti C, Allin M, et al. Brain volumes in adult survivors of very low birth weight: a sibling-controlled study. Pediatrics. 2004;114:367–71. doi: 10.1542/peds.114.2.367. [DOI] [PubMed] [Google Scholar]
- 33.Lodygensky GA, Rademaker KJ, Zimine S, Gex-Fabry M, Lieftink A, Lazeyras F, et al. Structural and functional brain developmental after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics. 2005;116:1–7. doi: 10.1542/peds.2004-1275. [DOI] [PubMed] [Google Scholar]
- 34.Vangberg TR, Skranes J, Dale AM, Martinussen M, Brubakk AM, Haraldseth O. Changes in white matter diffusion anisotropy in adolescents born prematurely. NeuroImage. 2006;32(4):1538–48. doi: 10.1016/j.neuroimage.2006.04.230. [DOI] [PubMed] [Google Scholar]
- 35.Cheong JL, Thompson DK, Wang HX, Hunt RW, Anderson PJ, Inder TE, et al. Abnormal white matter signal on MR imaging is related to abnormal tissue microstructure. AJNR Am J Neuroradiol. 2009;30(3):623–8. doi: 10.3174/ajnr.A1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Z, Covey MV, Bitel CL, Ni L, Jonakait GM, Levison SW. Sustained neocortical neurogenesis after neonatal hypoxic/ischemic injury. Ann Neurol. 2007;61(3):199–208. doi: 10.1002/ana.21068. [DOI] [PubMed] [Google Scholar]
- 37.Sizonenko SV, Camm EJ, Dayer A, Kiss JZ. Glial responses to neonatal hypoxicischemic injury in the rat cerebral cortex. Int J Dev Neurosci. 2008;26:37–45. doi: 10.1016/j.ijdevneu.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Maalouf EF, Duggan PJ, Rutherford MA, Counsell SJ, Fletcher AM, Battin M, et al. Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. J Pediatr. 1999;135:351–57. doi: 10.1016/s0022-3476(99)70133-2. [DOI] [PubMed] [Google Scholar]
- 39.Inder TE, Anderson NJ, Spencer C, Wells SJ, Volpe J. White matter injury in the premature infant: a comparison between serial cranial ultrasound and MRI at term. AJNR Am J Neuroradiol. 2003;24:805–09. [PMC free article] [PubMed] [Google Scholar]
- 40.Debillon T, Guyen SN, Muet A, Quere MP, Moussaly F, Roze JC. Limitations of ultrasonography for diagnosing white matter damage in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2003;88:F275–F79. doi: 10.1136/fn.88.4.F275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inder TE, Wells SJ, Mogridge N, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant - a qualitative magnetic resonance imaging study. J Pediatr. 2003;143:171–79. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 42.Counsell SJ, Allsop JM, Harrison MC, Larkman DJ, Kennea NL, Kapellou O, et al. Diffusion weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003;112:1–7. doi: 10.1542/peds.112.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Volpe JJ. Cerebral white matter injury of the premature infant - more common than you think. Pediatrics. 2003;112:176–79. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 44.Dyet LE, Kennea NL, Counsell SJ, Mallouf EF, Ajayi-Obe M, Duggan PJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118:536–48. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 45.Krishnan ML, Dyet LE, Boardman JP, Kapellou O, Allsop JM, Cowan F, et al. Relationship between white matter apparent diffusion coefficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics. 2007;120(3):e604–9. doi: 10.1542/peds.2006-3054. [DOI] [PubMed] [Google Scholar]
- 46.Counsell SJ, Edwards AD, Chew AT, Anjari M, Dyet LE, Srinivasan L, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–08. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- 47.Volpe JJ. Cerebellum of the premature infant -- rapidly developing, vulnerable, clinically important. J Child Neurol. doi: 10.1177/0883073809338067. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banker BQ, Larroche JC. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7:386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- 49.Arai Y, Deguchi K, Mizuguchi M, Takashima S. Expression of β-amyloid precursor protein in axons of periventricular leukomalacia brains. Pediatr Neurol. 1995;13:161–63. doi: 10.1016/0887-8994(95)00149-a. [DOI] [PubMed] [Google Scholar]
- 50.Deguchi K, Oguchi K, Takashima S. Characteristic neuropathology of leukomalacia in extremely low birth weight infants. Pediatr Neurol. 1997;16:296–300. doi: 10.1016/s0887-8994(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 51.Meng SZ, Arai Y, Deguchi K, Takashima S. Early detection of axonal and neuronal lesions in prenatal-onset periventricular leukomalacia. Brain Dev. 1997;19:480–84. doi: 10.1016/s0387-7604(97)00068-5. [DOI] [PubMed] [Google Scholar]
- 52.Deguchi K, Oguchi K, Matsuura N, Armstrong DD, Takashima S. Periventricular leukomalacia: Relation to gestational age and axonal injury. Pediatr Neurol. 1999;20:370–74. doi: 10.1016/s0887-8994(99)00010-7. [DOI] [PubMed] [Google Scholar]
- 53.Haynes RL, Billiards SS, Borenstein NS, Volpe JJ, Kinney HC. Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr Res. 2008;63:656–61. doi: 10.1203/PDR.0b013e31816c825c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, et al. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44:584–90. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 55.Miller SP, Vigneron DB, Henry RG, Bohland MA, Ceppi-Cozzio C, Hoffman C, et al. Serial quantitative diffusion tensor MRI of the premature brain: Development in newborns with and without injury. J Magn Reson Imaging. 2002;16:621–32. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- 56.Huppi PS, Murphy B, Maier SE, Zientara GP, Inder TE, Barnes PD, et al. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107:455–60. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- 57.Martinussen M, Fischl B, Larsson HB, Skranes J, Kulseng S, Vangberg TR, et al. Cerebral cortex thickness in 15-year-old adolescents with low birth weight measured by an automated MRI-based method. Brain. 2005;128:2588–96. doi: 10.1093/brain/awh610. [DOI] [PubMed] [Google Scholar]
- 58.Anjari M, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD, et al. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. NeuroImage. 2007;35:1021–27. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 59.Counsell S, Shen Y, Boardman JP, Larkman D, Kapellou O, Ward P, et al. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on MRI at term equivalent age. Pediatrics. 2006;117:376–86. doi: 10.1542/peds.2005-0820. [DOI] [PubMed] [Google Scholar]
- 60.Counsell SJ, Dyet LE, Larkman DJ, Nunes RG, Boardman JP, Allsop JM, et al. Thalamo-cortical connectivity in children born preterm mapped using probabilistic magnetic resonance tractography. NeuroImage. 2007;34:896–904. doi: 10.1016/j.neuroimage.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 61.Bassi L, Ricci D, Volzone A, Allsop JM, Srinivasan L, Pai A, et al. Probabilistic diffusion tractography of the optic radiations and visual function in preterm infants at term equivalent age. Brain. 2008;131(Pt 2):573–82. doi: 10.1093/brain/awm327. [DOI] [PubMed] [Google Scholar]
- 62.Drobyshevsky A, Bregman J, Storey P, Meyer J, Prasad PV, Derrick M, et al. Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Dev Neurosci. 2007;29(45):289–301. doi: 10.1159/000105470. [DOI] [PubMed] [Google Scholar]
- 63.McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci. 2003;23(8):3308–15. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andiman SE, Haynes RL, Folkerth RD, Sleeper LA, Volpe JJ, Kinney HC. Loss of white matter neurons in periventricular leukomalacia: Implications for cognitive deficits in survivors of prematurity. Soc Neurosci. 2009 Abstract. [Google Scholar]
- 65.Lin Y, Okumura A, Hayakawa F, Kato T, Kuno K, Watanabe K. Quantitative evaluation of thalami and basal ganglia in infants with periventricular leukomalacia. Dev Med Child Neurol. 2001;43:481–85. doi: 10.1017/s0012162201000883. [DOI] [PubMed] [Google Scholar]
- 66.Boardman JP, Counsell SJ, Rueckert D, Kapellou O, Bhatia KK, Aljabar P, et al. Abnormal deep grey matter development following preterm birth detected using deformation based morphometry. NeuroImage. 2006;32:70–78. doi: 10.1016/j.neuroimage.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 67.Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 2007;131:205–17. doi: 10.1093/brain/awm282. [DOI] [PubMed] [Google Scholar]
- 68.Kesler SR, Reiss AL, Vohr B, Watson C, Schneider KC, Katz KH, et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008;152(4):513–20. doi: 10.1016/j.jpeds.2007.08.009. 20 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pierson CR, Folkerth RD, Billards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, et al. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114:619–31. doi: 10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ligam P, Haynes RL, Folkerth RD, Liu L, Yang M, Volpe JJ, et al. Thalamic damage in periventricular leukomalacia: Novel pathologic observations relevant to cognitive deficits in survivors of prematurity. Pediatr Res. 2008 doi: 10.1203/PDR.0b013e3181998baf. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abernethy LJ, Cooke RW, Foulder-Hughes L. Caudate and hippocampal volumes, Intelligence, and motor impairment in 7-year-old children who were born preterm. Pediatr Res. 2004;55(5):884–93. doi: 10.1203/01.PDR.0000117843.21534.49. [DOI] [PubMed] [Google Scholar]
- 72.Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara P, Barnes PD, et al. Periventricular white matter injury in the premature infant is associated with a reduction in cerebral cortical gray matter volume at term. Ann Neurol. 1999;46:755–60. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 73.Peterson BS, Anderson AW, Ehrenkranz RA, Staib LH, Tageldin M, Colson E, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111:939–48. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- 74.Isaacs E, Lucas A, Chong WK, wood SJ, Johnson CL, Marshall C, et al. Hippocampal volume and everyday memory in children of very low birth weight. Pediatr Res. 2000;47:713–20. doi: 10.1203/00006450-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 75.Thompson DK, Wood SJ, Doyle LW, Warfield SK, Lodygensky GA, Anderson PJ, et al. Neonate hippocampal volumes: prematurity, perinatal predictors, and 2-year outcome. Ann Neurol. 2008;63(5):642–51. doi: 10.1002/ana.21367. [DOI] [PubMed] [Google Scholar]
- 76.Armstrong DL, Sauls CD, Goddard-Finegold J. Neuropathologic findings in short-term survivors of intraventricular hemorrhage. Am J Dis Child. 1987;141:617–21. doi: 10.1001/archpedi.1987.04460060035027. [DOI] [PubMed] [Google Scholar]
- 77.Marin-Padilla M. Developmental neuropathology and impact of perinatal brain damage. 2. White matter lesions of the neocortex. J Neuropathol Exp Neurol. 1997;56(3):219–35. doi: 10.1097/00005072-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 78.Kinney HC, Armstrong DL. Perinatal neuropathology. In: Graham DI, Lantos PE, editors. Greenfield's Neuropathology. 7 ed. Arnold Publishers; London: 2002. pp. 519–606. [Google Scholar]
- 79.Andiman SE, Haynes RL, Trachtenberg FL, Billiards SS, Folkerth RD, Volpe JJ, et al. The cerebral cortex overlying periventricular leukomalacia: Analysis of pyramidal neurons. Soc Neurosci. 2009 doi: 10.1111/j.1750-3639.2010.00380.x. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dobbing J, Hopewell JW, Lynch A, Sands J. Vulnerability of developing brain. I. Some lasting effects of x-irradiation. Exp Neurol. 1970;28(3):442–9. doi: 10.1016/0014-4886(70)90181-0. [DOI] [PubMed] [Google Scholar]
- 81.Dobbing J. The later growth of the brain and its vulnerability. Pediatrics. 1974;53(1):2–6. [PubMed] [Google Scholar]
- 82.Back SA, Volpe JJ. Cellular and molecular pathogenesis of periventricular white matter injury. Ment Retard Dev Disabil Res Rev. 1997;3:96–107. [Google Scholar]
- 83.Back SA, Khan R, Gan X, Rosenberg PA, Volpe JJ. A new alamar blue viability assay to rapidly quantify oligodendrocyte death. J Neurosci Meth. 1999;91:47–54. doi: 10.1016/s0165-0270(99)00062-x. [DOI] [PubMed] [Google Scholar]
- 84.Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21(4):1302–12. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kinney HC, Back SA. Human oligodendroglial development: Relationship to periventricular leukomalacia. Semin Pediatr Neurol. 1998;5:180–89. doi: 10.1016/s1071-9091(98)80033-8. [DOI] [PubMed] [Google Scholar]
- 86.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–F61. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Back SA, Gan X, Li Y, Rosenberg PR, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18(16):6241–53. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Folkerth RD, Keefe RJ, Haynes RL, Trachtenberg FL, Volpe JJ, Kinney HC. Interferon-gamma expression in periventricular leukomalacia in the human brain. Brain Pathol. 2004;14(3):265–74. doi: 10.1111/j.1750-3639.2004.tb00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Folkerth RD, Haynes RL, Borenstein NS, Belliveau RA, Trachtenberg F, Rosenberg PA, et al. Developmental lag in superoxide dismutases relative to other antioxidant enzymes in premyelinated human telencephalic white matter. J Neuropathol Exp Neurol. 2004;63:990–9. doi: 10.1093/jnen/63.9.990. [DOI] [PubMed] [Google Scholar]
- 90.Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci USA. 2005;102:9936–41. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buntinx M, Moreels M, Vandenabeele F, Lambrichts N, Raus J, Steels P, et al. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J Neurosci Res. 2004;76:834–45. doi: 10.1002/jnr.20118. [DOI] [PubMed] [Google Scholar]
- 92.Pang Y, Cai ZW, Rhodes PG. Effect of tumor necrosis factor-alpha on developing optic nerve oligodendrocytes in culture. J Neurosci Res. 2005;80(2):226–34. doi: 10.1002/jnr.20450. [DOI] [PubMed] [Google Scholar]
- 93.Talos DM, Follett PL, Folkerth RD, Fishman RE, Trachtenberg FL, Volpe JJ, et al. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J Comp Neurol. 2006;497:61–77. doi: 10.1002/cne.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DeSilva TM, Kinney HC, Borenstein NS, Trachtenberg F, Irwin N, Volpe JJ, et al. The glutamate transporter is transiently expressed in developing human cerebral white matter. J Comp Neurol. 2007;501:879–90. doi: 10.1002/cne.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karadottir R, Attwell D. Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience. 2007;145(4):1426–38. doi: 10.1016/j.neuroscience.2006.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Manning SM, Talos DM, Zhou C, Selip DR, Park H-K, Park C-J, et al. NMDA receptor blockade with memantine attenuates white matter deficits in a rat model of periventricular leukomalacia. J Neurosci. 2008;28:6670–78. doi: 10.1523/JNEUROSCI.1702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17:13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 98.Rezaie P, Dean A, Male D, Ulfig N. Microglia in the cerebral wall of the human telencephalon at second trimester. Cereb Cortex. 2005;15:938–49. doi: 10.1093/cercor/bhh194. [DOI] [PubMed] [Google Scholar]
- 99.Monier A, Evrard P, Gressens P, Verney C. Distribution and differentiation of microglia in the human encephalon during the first two trimesters of gestation. J Comp Neurol. 2006;499(4):565–82. doi: 10.1002/cne.21123. [DOI] [PubMed] [Google Scholar]
- 100.Billiards SS, Haynes RL, Folkerth RD, Trachtenberg FL, Liu LG, Volpe JJ, et al. Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2006;497:199–208. doi: 10.1002/cne.20991. [DOI] [PubMed] [Google Scholar]
- 101.Agresti C, D'Urso D, Levi G. Reversible inhibitory effects of interferon-γ- and tumour necrosis factor-α on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur J Neurosci. 1996;8:1106–16. doi: 10.1111/j.1460-9568.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 102.Andrews T, Zhang P, Bhat NR. TNF-α potentiates IFNγ-induced cell death in oligodendrocyte progenitors. J Neurosci Res. 1998;54:574–83. doi: 10.1002/(SICI)1097-4547(19981201)54:5<574::AID-JNR2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 103.Xie Z, Wei M, Morgan TE, Fabrizio P, Han D, Finch CE, et al. Peroxynitrite mediates neurotoxicity of amyloid β-peptide 1-42- and lipopolysaccharide-activated microglia. J Neurosci. 2002;22:3484–92. doi: 10.1523/JNEUROSCI.22-09-03484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lehnardt S, Massillon L, Follet P, Jensen FE, Ratan RR, Rosenberg PA, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–19. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lechpammer M, Manning SM, Samonte F, Nelligan J, Sabo E, Talos DM, et al. Minocycline treatment following hypoxic-ischemic injury attenuates white matter injury in a rodent model of periventricular leukomalacia. Neuropathol Appl Neurobiol. 2008;34:379–93. doi: 10.1111/j.1365-2990.2007.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kostovic I, Judas M. Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec. 2002;267:1–6. doi: 10.1002/ar.10069. [DOI] [PubMed] [Google Scholar]
- 107.Kostovic I, Judas M, Rados M, Hrabac P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12(5):536–44. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- 108.Haynes RL, Borenstein NS, DeSilva TM, Folkerth RD, Liu LG, Volpe JJ, et al. Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2005;484:156–67. doi: 10.1002/cne.20453. [DOI] [PubMed] [Google Scholar]
- 109.Kostovic I, Jovanov-Milosevic N. The development of cerebral connections during the first 20-45 weeks' gestation. Semin Fetal Neonatal Med. 2006;11:415–22. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 110.Kostovic I, Judas M. Transient patterns of cortical lamination during prenatal life: do they have implications for treatment? Neurosci Biobehav Rev. 2007;31(8):1157–68. doi: 10.1016/j.neubiorev.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 111.Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9(2):110–22. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- 112.Volpe JJ. Subplate neurons - missing link in brain injury of the premature infant? Pediatrics. 1996;97:112–13. [PubMed] [Google Scholar]
- 113.McConnell SK, Ghosh A, Shatz CJ. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989;245(4921):978–82. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- 114.Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347(6289):179–81. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- 115.Ghosh A, Shatz CJ. Involvement of subplate neurons in the formation of ocular dominance columns. Science. 1992;255(5050):1441–3. doi: 10.1126/science.1542795. [DOI] [PubMed] [Google Scholar]
- 116.Kanold PO, Kara P, Reid RC, Shatz CJ. Role of subplate neurons in functional maturation of visual cortical columns. Science. 2003;301:521–25. doi: 10.1126/science.1084152. [DOI] [PubMed] [Google Scholar]
- 117.Kanold PO. Transient microcircuits formed by subplate neurons and their role in functional development of thalamocortical connections. NeuroReport. 2004;15:2149–53. doi: 10.1097/00001756-200410050-00001. [DOI] [PubMed] [Google Scholar]
- 118.Bystron I, Molnar Z, Otellin V, Blakemore C. Tangential networks of precocious neurons and early axonal outgrowth in the embryonic human forebrain. J Neurosci. 2005;25:2781–92. doi: 10.1523/JNEUROSCI.4770-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417(6889):645–9. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- 120.Tan SS. Developmental neurobiology: cortical liars. Nature. 2002;417(6889):605–6. doi: 10.1038/417605a. [DOI] [PubMed] [Google Scholar]
- 121.Inta D, Alfonso J, von Engelhardt J, Kreuzberg MM, Meyer AH, van Hooft JA, et al. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci U S A. 2008;105(52):20994–9. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Petanjek Z, Berger B, Esclapez M. Origins of cortical GABAergic neurons in the cynomolgus monkey. Cerebral Cortex. 2009;19:249–62. doi: 10.1093/cercor/bhn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bayer SA, Altman J. Atlas of Human Central Nervous System Development. CRC Press; London: 2004. The Human Brain During the Third Trimester. [Google Scholar]
- 124.Letinic K, Rakic P. Telencephalic origin of human thalamic GABAergic neurons. Nat Neurosci. 2001;4(9):931–6. doi: 10.1038/nn0901-931. [DOI] [PubMed] [Google Scholar]
- 125.Montero VM. The interneuronal nature of GABAergic neurons in the lateral geniculate nucleus of the rhesus monkey: a combined HRP and GABA-immunocytochemical study. Exp Brain Res. 1986;64(3):615–22. doi: 10.1007/BF00340502. [DOI] [PubMed] [Google Scholar]
- 126.Montero VM, Zempel J. The proportion and size of GABA-immunoreactive neurons in the magnocellular and parvocellular layers of the lateral geniculate nucleus of the rhesus monkey. Exp Brain Res. 1986;62(1):215–23. doi: 10.1007/BF00237420. [DOI] [PubMed] [Google Scholar]
- 127.Abitz M, Nielsen RD, Jones EG, Laursen H, Graem N, Pakkenberg B. Excess of neurons in the human newborn mediodorsal thalamus compared with that of the adult. Cereb Cortex. 2007;17(11):2573–8. doi: 10.1093/cercor/bhl163. [DOI] [PubMed] [Google Scholar]
- 128.Huppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43:224–35. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- 129.Kapellou O, Counsell SJ, Kennea NL, Dyet L, Saeed N, Stark J, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLOS Medicine. 2006;3:e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–41. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22(2):455–63. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bell MJ, Hallenbeck JM. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res. 2002;70(4):570–79. doi: 10.1002/jnr.10423. [DOI] [PubMed] [Google Scholar]
- 133.Duncan JR, Cock ML, Scheerlinck J-PY, Westcott KT, McLean C, Harding R, et al. White matter injury after repeated endotoxin exposure in the preterm ovine fetus. Pediatr Res. 2002;52:941–49. doi: 10.1203/00006450-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 134.Deng W, Rosenberg PA, Volpe JJ, Jensen FE. Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci USA. 2003;100:6801–06. doi: 10.1073/pnas.1136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yoshioka A, Bacskai B, Pleasure D. Pathophysiology of oligodendroglial excitotoxicity. J Neurosci Res. 1996;46:427–38. doi: 10.1002/(SICI)1097-4547(19961115)46:4<427::AID-JNR4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 136.McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–97. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- 137.Matute C, Alberdi E, Domercq M, Perez-Cerda F, Perez-Samartin A, Sanchez-Gomez MV. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001;24:224–30. doi: 10.1016/s0166-2236(00)01746-x. [DOI] [PubMed] [Google Scholar]
- 138.Itoh T, Beesley J, Itoh A, Cohen AS, Kavanaugh B, Coulter DA, et al. AMPA glutamate receptor-mediated calcium signaling is transiently enhanced during development of oligodendrocytes. J Neurochem. 2002;81(2):390–402. doi: 10.1046/j.1471-4159.2002.00866.x. [DOI] [PubMed] [Google Scholar]
- 139.Sanchez-Gomez MV, Alberdi E, Ibarretxe G, Torre I, Matute C. Caspase-dependent and caspase-independent oligodendrocyte death mediated by AMPA and kainate receptors. J Neurosci. 2003;23(29):9519–28. doi: 10.1523/JNEUROSCI.23-29-09519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rosenberg PA, Dai W, Gan XD, Ali S, Fu JM, Back SA, et al. Mature myelin basic protein expressing oligodendrocytes are insensitive to kainate toxicity. J Neurosci Res. 2003;71:237–45. doi: 10.1002/jnr.10472. [DOI] [PubMed] [Google Scholar]
- 141.Deng W, Yue Q, Rosenberg PA, Volpe JJ, Jensen FE. Oligodendrocyte excitotoxicity determined by local glutamate accumulation and mitochondrial function. J Neurochem. 2006;96:213–22. doi: 10.1111/j.1471-4159.2006.03861.x. [DOI] [PubMed] [Google Scholar]
- 142.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–71. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 143.Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–6. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Matute C. Oligodendrocyte NMDA receptors: a novel therapeutic target. Trends Mol Med. 2006;12(7):289–92. doi: 10.1016/j.molmed.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 145.Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–92. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- 146.Bjartmar C, Yin X, Trapp BD. Axonal pathology in myelin disorders. J Neurocytol. 1999;28:383–95. doi: 10.1023/a:1007010205037. [DOI] [PubMed] [Google Scholar]
- 147.Biffiger K, Bartsch S, Montag D, Aguzzi A, Schachner M, Bartsch U. Severe hypomyelination of the murine CNS in the absence of myelin-associated glycoprotein and fyn tyrosine kinase. J Neurosci. 2000;20:7430–37. doi: 10.1523/JNEUROSCI.20-19-07430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gotow T, Leterrier JF, Ohsawa Y, Watanabe T, Isahara K, Shibata R, et al. Abnormal expression of neurofilament proteins in dysmyelinating axons located in the central nervous system of jimpy mutant mice. Eur J Neurosci. 1999;11:3893–903. doi: 10.1046/j.1460-9568.1999.00820.x. [DOI] [PubMed] [Google Scholar]
- 149.Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–74. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 150.Rasband MN, Tayler R, Kaga Y, Yang Y, LappeSiefke C, Nave KA, et al. CNP is required for maintenance of axon-glia interactions at nodes of Ranvier in the CNS. Glia. 2005;50:86–90. doi: 10.1002/glia.20165. [DOI] [PubMed] [Google Scholar]
- 151.Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68(22):S22–S31. doi: 10.1212/01.wnl.0000275229.13012.32. [DOI] [PubMed] [Google Scholar]
- 152.Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci USA. 2007;104(19):8131–6. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, et al. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci. 2006;26(49):12633–41. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Drobyshevsky A, Song SK, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 2005;25(25):5988–97. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: A novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci. 2003;23:4967–74. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dai X, Lercher LD, Clinton PM, Du Y, Livingston DL, Vieira C, et al. The trophic role of oligodendrocytes in the basal forebrain. J Neurosci. 2003;23:5846–53. doi: 10.1523/JNEUROSCI.23-13-05846.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Du YZ, Dreyfus CF. Oligodendrocytes as providers of growth factors. J Neurosci. 2002;68(6):647–54. doi: 10.1002/jnr.10245. [DOI] [PubMed] [Google Scholar]
- 158.Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KA, Martinussen M, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130(Pt 3):654–66. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- 159.Sizonenko SV, Sirimanne E, Mayall Y, Gluckman PD, Inder TE, Williams CE. Selective cortical alteration after hypoxic-ischemic injury in the very immature rat brain. Pediatr Res. 2003;54:263–69. doi: 10.1203/01.PDR.0000072517.01207.87. [DOI] [PubMed] [Google Scholar]
- 160.McCarran WJ, Goldberg MP. White matter axon vulnerability to AMPA/kainate receptor-mediated ischemic injury is developmentally regulated. J Neurosci. 2007;27(15):4220–9. doi: 10.1523/JNEUROSCI.5542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tekkok SB, Goldberg MP. AMPA/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21(12):4237–48. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wakita H, Tomimoto H, Akiguchi I, Matsuo A, Lin -J-X, Ihara M, et al. Axonal damage and demyelination in the white matter after chronic cerebral hypoperfusion in the rat. Brain Res. 2002;924:63–70. doi: 10.1016/s0006-8993(01)03223-1. [DOI] [PubMed] [Google Scholar]
- 163.Northington FJ, Ferriero DM, Flock DL, Martin LJ. Delayed neurodegeneration in neonatal rat thalamus after hypoxia-ischemia is apoptosis. J Neurosci. 2001;21(6):1931–38. doi: 10.1523/JNEUROSCI.21-06-01931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ghosh A, Shatz CJ. A role for subplate neurons in the patterning of connections from thalamus to neocortex. Development. 1993;117:1031–47. doi: 10.1242/dev.117.3.1031. [DOI] [PubMed] [Google Scholar]
- 165.Romanko MJ, Rothstein RP, Levison SW. Neural stem cells in the subventricular zone are resilient to hypoxia/ischemia whereas progenitors are vulnerable. J Cereb Blood Flow Metab. 2004;24:814–25. doi: 10.1097/01.WCB.0000123906.17746.00. [DOI] [PubMed] [Google Scholar]