Abstract
Type II topoisomerases (TOP2) introduce transient double-stranded DNA breaks through a covalent TOP2-DNA intermediate. Anticancer agents like etoposide kill cells by trapping covalent TOP2-DNA cleavable complexes. Pathways influencing the repair of cleavable complexes are expected to be major determinants of therapeutic response to etoposide. Rb1 is required to enforce cell cycle checkpoints in response to DNA damage, but evidence for a direct role in the processing and repair of DNA lesions is lacking. We observe that degradation of trapped TOP2 cleavable complexes, liberation of DNA strand breaks, and repair of those breaks occurs more efficiently in cells expressing Rb1 protein (pRb). Cells lacking pRb are more sensitive to etoposide induced cytotoxicity. Rb1 mediated processing and repair of TOP2 cleavable complexes is genetically separable from its ability to bind E2F and enforce DNA damage induced cell cycle checkpoints. Rb1 protein binds both TOP2 and BRCA1 in intact cells, and pRb is required for association between TOP2 and BRCA1. These results suggest that pRb facilitates processing and repair of TOP2 cleavable complexes by recruiting proteins like BRCA1 to the damaged site. The functional status of pRb, therefore, may influence sensitivity to etoposide by facilitating the repair of trapped TOP2-DNA complexes as well as by enforcing cell cycle checkpoints.
Keywords: Retinoblastoma tumor suppressor gene, Topoisomerase II, Etoposide, DNA damage, DNA repair
Introduction
DNA topoisomerases are important enzymes involved in maintaining proper DNA topology during transcription, replication, and chromosome segregation (Wang, 1996). Type II topoisomerases (TOP2) make transient double-stranded cuts in the DNA through a covalent TOP2-DNA intermediate called the cleavable complex. The mode of action of many commonly used anticancer drugs like etoposide (VP16), teniposide, adriamycin, and mitoxantrone target TOP2 by poisoning their activity (Chen and Liu, 1994 ; Liu, 1989). The mechanism of tumor cell killing by many TOP2 poisons involves the trapping of TOP2-DNA cleavable complexes (Li and Liu, 2001). Since the double-stranded DNA break (DSB) within the cleavable complex is protected by covalent linkage to TOP2, the DNA break cannot be repaired. DNA repair first requires proteasome mediated degradation of TOP2 and subsequent exposure of the DNA DSB (Mao et al., 2001; Xiao et al., 2003). The selectivity of TOP2 poisons for cancer cells is expected to be determined, in large part, by the cellular pathways that influence the processing and repair of trapped cleavable complexes.
TOP2 is present as two major isoforms with TOP2α associated predominantly with DNA replication (Niimi et al., 2001; Woessner et al., 1991) and TOP2β primarily associated with transcription (Govoni et al., 1995 ; Tsutsui et al., 2001 ; Yang et al., 2000). Trapped TOP2β cleavable complexes are efficiently degraded in a transcription and proteasome dependent manner throughout the cell cycle (Mao et al., 2001 ; Xiao et al., 2003). These findings have suggested that collision of the RNA transcription machinery with the TOP2-DNA cleavable complex triggers repair of the lesion by initiating proteasome mediated degradation of TOP2β. While the molecular pathways regulating the processing, and repair of TOP2-DNA cleavable complexes are expected to influence chemosensitivity to TOP2 poisons, these pathways are incompletely characterized.
The Rb1 tumor suppressor protein (pRb) is required for efficient activation of cell cycle checkpoints in response to a variety of DNA lesions, including those induced by VP16 (Bosco et al., 2004 ; Brugarolas et al., 1999 ; Harrington et al., 1998 ; Knudsen et al., 2000 ; Naderi et al., 2002). Rb1 protein can also affect the DNA damage response by regulating the expression of genes involved in DNA repair (Cam and Dynlacht, 2003). Typically pRb is targeted to particular genes by interaction with sequence specific DNA binding transcription factors like the E2F family. Rb1 protein serves as an adaptor to recruit chromatin modifying factors to these genes to regulate their expression. Thus Rb can indirectly influence the response to TOP2 poisons by altering gene expression and enforcing cell cycle checkpoints through interaction with E2F transcription factors. Evidence implicating pRb directly in the actual processing and repair of DNA lesions, however, is lacking.
Rb1 protein physically interacts with human TOP2α (Bhat et al., 1999). Since TOP2α and TOP2β share 72% identity in primary amino acid sequence, it is possible that pRb also interacts with TOP2β. Moreover, Rb interacts with other cellular proteins like BRCA1 that participate in the repair of VP16 induced DNA damage (Aprelikova et al., 1999 ; Fan et al., 2001 ; Yarden and Brody, 1999). The BRCA1/BARD1 heterodimer has E3 ubiquitin ligase activity that can target substrates for proteasome dependent degradation (Hashizume et al., 2001 ; Ruffner et al., 2001 ; Xia et al., 2003). BRCA1 may also function in nonhomologous end joining (NHEJ)(Zhong et al., 2002b ; Zhong et al., 2002a), an important pathway for repair of the DSB generated by trapped TOP2 cleavable complexes(Adachi et al., 2003 ; Adachi et al., 2004). Thus pRb may serve as an adaptor protein to recruit processing and repair factors like BRCA1 to TOP2 cleavable complexes, facilitating the repair of VP16 induced DNA damage. To test this hypothesis, we have examined VP16 induced TOP2β degradation, DSB repair, and cytotoxicity in cells with wild type or mutant pRb.
Results
pRb is required for efficient processing of trapped TOP2β-DNA cleavable complexes
In response to VP16, TOP2β is rapidly degraded in a transcription and proteasome dependent manner to liberate trapped DSBs for repair. We have tested whether pRb is required for efficient TOP2β degradation in response to VP16. Mouse embryonic fibroblasts (MEFs) containing wild type or null alleles of Rb1 are treated with VP16, the type I topoisomerase inhibitor camptothecin (CPT), or vehicle control. Nuclease is added to cell extracts to liberate any TOP2 covalently linked to DNA. The relative levels of TOP2β are then compared by western blotting. VP16 induces a rapid decrease in TOP2β levels relative to the vehicle control (Figure 1A). In contrast, the TOP2β levels in pRb null MEFs are not significantly affected by VP16 treatment. Quantitation of the relative signals from multiple western blots suggests that TOP2β levels in VP16 treated wild type cells are 50–60% of the levels in VP16 treated pRb null cells. Loss of TOP2β is specific since the TOP1 poison camptothecin (CPT) does not affect TOP2β levels. Consistent with previously published reports, the VP16 induced decline in TOP2β levels is not observed in the presence of the proteasome inhibitor MG132 (data not shown), indicating that TOP2β levels decline due to protein degradation. To confirm that the defect in TOP2β degradation was specifically due to loss of pRb, we have tested whether exogenous expression of pRb could rescue VP16 induced TOP2β degradation in Rb null MEFs. Expression of a wild type GFP-Rb fusion protein in Rb1 null MEFs is able to restore the VP16 induced decline in TOP2β levels (Figure 1B). Expression of GFP has no discernable effect on TOP2β levels. Together, the above results suggest that efficient degradation of TOP2β trapped in cleavable complexes is specifically dependent on pRb. Previous studies in tumor cells have demonstrated that degradation of TOP2 cleavable complexes depends on transcription, but not protein synthesis (Mao et al., 2001 ; Xiao et al., 2003). We have confirmed this in the MEF model system. Inhibition of transcription by 5, 6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB) blocks VP16 induced TOP2β degradation (Figure 1C). The protein-synthesis inhibitor cycloheximide, however, has no effect on VP16-induced TOP2β degradation. Hence TOP2β degradation is not dependent on new protein synthesis and thus is not dependent on potential pRb mediated changes in protein expression. The effect of DRB is likely due to blocking elongation of the transcriptional machinery itself rather than altering the levels of gene expression.
Figure 1. VP16 induced TOP2β degradation is impaired in pRb deficient cells.
(A) Rb1 null and wild type MEFs were treated with DMSO (V), 250 µM VP16 or 25 µM CPT. After 4 hrs, cells were extracted by alkaline lysis and treated with staphylococcal S7 nuclease. Proteins were resolved by SDS-PAGE and TOP2β levels determined by western blotting. Blots were stained with an HSP70 antibody to serve as a loading control. (B) Rb1 null MEFs expressing GFP or GFPRb were treated with DMSO (V), VP16 or CPT and analyzed by western blotting as in A. (C) Wild type MEFs were treated with 250 µM VP16 in the presence and absence of 150 µM DRB or 50 µM CHX. TOP2β levels were analyzed as in A. (D) Rb1 null and wild type MEFs were treated with DMSO (V), 250 µM VP16 or 25 µM CPT. Four hours later, cells were extracted with SDS-PAGE sample buffer. TOP2β levels were ascertained by western blotting as in A.
We used a band depletion assay to control for possible differences in the extent of VP16 induced TOP2β trapping in wild type and Rb null MEF. Cells were briefly treated with VP16 but nuclease treatment of the resulting extracts was omitted so that TOP2β trapped in covalent linkage with DNA would not enter the gel. Hence the proportion of TOP2β trapped in cleavable complexes was proportional to the reduction in TOP2β as assessed by western blotting. Both wild type and pRb null cells showed similar levels of TOP2β band depletion upon VP16 treatment (Figure 1D). As expected, CPT treatment did not cause TOP2β band depletion. Hence a substantial fraction of TOP2β was trapped in cleavable complexes in pRb null MEF, but this trapped TOP2β was not degraded as efficiently as in wild type MEFs.
Rb1 is required for efficient repair of VP16 induced DNA damage
Since pRb is required for efficient degradation of trapped TOP2β cleavable complexes, we tested whether lack of pRb also compromises the repair of VP16 induced DSB. The levels of phosphorylated ATM or H2AX are proportional to the levels of free DSBs (Motoyama and Naka, 2004). Hence, we have measured the levels of phospho-ATM and phospho-H2AX as markers to monitor the generation and repair of VP16 induced DSB. Treatment of wild type MEFs with VP16 induced significant phosphorylation of ATM and H2AX as detected by immunofluorescent staining (Figures 2A,B). However VP16 induced phosphorylation of ATM or H2AX was not detected in pRb null MEFs under the conditions used. Hence, liberation of DSBs from VP16 trapped TOP2 cleavable complexes and subsequent induction of ATM and H2AX phosphorylation was inefficient in the absence of pRb.
Figure 2. Processing and repair of VP16 induced DNA lesions is impaired in pRb deficient cells.
(A) Rb1 null and wild type MEFs were treated with 250 µM VP16. After 1.5 hrs, cells were fixed and stained with anti-phosphorylated ATM antibody. Cells were counterstained with DAPI and samples visualized under fluorescence microscopy using a 10X objective. (B) Cells were treated, fixed, stained with anti-phosphorylated H2AX antibody, and analyzed as in A. (C) Rb1 null and wild type MEFs were treated with DMSO (V) or 250 µM VP16. After treatment and recovery as indicated, cells were extracted and protein analyzed by immunoblotting with anti-phosphorylated H2AX antibody. MG132 represents a 0 hour recovery sample treated with VP16 in the presence of the proteasome inhibitor MG132 at 10 µM. Hsp70 protein levels serve as a loading control. (D) The data from two independent experiments as in C were quantitated by densitometry using ImageQuant software. The data was expressed relative to the 0 recovery timepoint and represents the mean and standard deviation from the mean.
To assess the rate of DSB repair, we measured relative phospho-H2AX levels at varying times after recovery from VP16 treatment in drug-free media. Consistent with Figures 2A and B, wild type MEF initially had higher phospho-H2AX levels than pRb null MEF upon VP16 treatment (Figure 2C). Treatment with the proteasome inhibitor MG132 during VP16 treatment decreased the initial level of phospho-H2AX in both wild type and pRb null MEF. This data was consistent with the notion that proteasome-mediated degradation of trapped TOP2 liberates the DNA DSB and that the lower initial phospho-H2AX level in VP16 treated pRb null MEFs was due to the lower rate of TOP2β degradation. Despite the initially higher levels of phospho-H2AX in wild type MEF, the relative rate of phospho-H2AX decline and hence of DSB repair was significantly greater in wild type MEFs than in pRb null MEFs (Figure 2D). This data suggested that Rb1 status also affected the repair rate of the DSBs liberated from trapped TOP2 cleavable complexes.
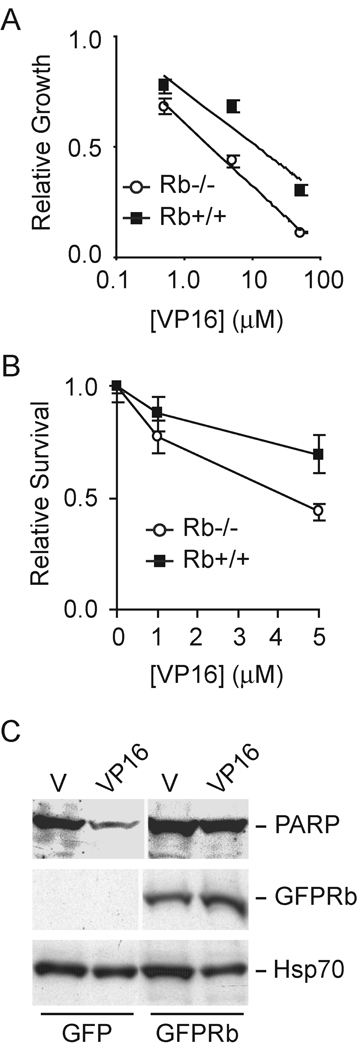
Given the inefficient processing and repair of trapped TOP2 cleavable complexes in the absence of pRb, we asked whether Rb1 status affected the chemosensitivity of MEFs to VP16. VP16 cytotoxicity was assayed by relative cell proliferation and clonogenic survival. In the presence of pRb, MEFs were more resistant to VP16 as measured by proliferation of viable cells (Figure 3A). The concentration of VP16 required to inhibit cell growth to 50% of vehicle control was 10.7µM in wild type MEFs and 2.4µM in Rb1 null MEFs, a greater than four-fold difference in chemosensitivity. Clonogenic survival of cells after treatment with VP16 was also higher in wild type MEF versus pRb null MEF (Figure 3B). The increased sensitivity of pRb null MEF to VP16 was due, at least in part, to greater levels of apoptotic cell death as indicated by increased degradation of the caspase substrate poly(ADP-ribose) polymerase in the absence of pRb (Figure 3C).
Figure 3. VP16 cytotoxicity is greater in pRb deficient cells.
(A) Rb1 null and wild type MEFs were treated with different concentrations of VP16. Four days later, viable cells were measured by MTT assay. The optical densities were normalized to the vehicle treated control. The data represent the mean and standard deviation of two independent experiments each done in triplicate. The logarithmic trendline shown was calculated by least squares. (B) Rb1 null and wild type MEFs were treated with different concentration of VP16 for 1 hr and replenished with fresh medium. After 2 weeks, the number of surviving colonies was counted. The data are expressed relative to the vehicle treated control and represent the mean and standard deviation of two experiments. (C) 293 cells expressing GFP or GFPRb were treated with DMSO (V) or VP16 for 4 hrs. Cells were extracted and analyzed by western blotting with antibodies directed against PARP, GFP, and HSP70 as indicated.
pRb mediated repair of VP16 induced DNA damage is genetically separable from its cell cycle regulatory activity
A number of indirect mechanisms may account for the effects of pRb on the processing and repair of trapped TOP2 cleavable complexes, including cell cycle checkpoint activation or regulation of gene expression. To test for the involvement of these indirect Rb mechanisms, we have examined the activity of a partially deficient pRb mutant in facilitating the repair of VP16 induced DNA damage. We have generated mice containing a mutation in the native Rb1 gene that results in a tryptophan for arginine substitution at codon 654 (Chang and Goodrich, unpublished). These mice have been used to generate MEFs homozygous for the mutation (R654W). This mouse mutation is the same as the tryptophan for arginine substitution mutation at human pRb codon 661 identified in carriers of partially penetrant hereditary retinoblastoma (Harbour, 2001). The protein encoded by this mutant allele fails to bind or regulate E2F transcription factors (Otterson et al., 1997 ; Otterson et al., 1999 ; Sellers et al., 1998 ; Whitaker et al., 1998). As a result the mutant is deficient in enforcing cell cycle checkpoints, yet it retains other known pRb functions such as the ability to promote cellular differentiation.
Treatment of wild type MEFs with VP16 or CPT causes activation of a G1/S phase cell cycle checkpoint that is reflected in a decrease in the percentage of cells incorporating the nucleotide analogue BrdU, relative to a vehicle treated control (Figure 4A). In contrast, BrdU incorporation in pRb null or R654W MEF continues unimpeded upon treatment with VP16 or CPT. This data confirms that the R654W pRb mutant is deficient in enforcing DNA damage induced cell cycle checkpoints. Like pRb null MEF, R654W MEF are also defective in normal cell cycle control when cultured in vitro at high or low cell density (H. Sung and D.W. Goodrich, unpublished observation). These cell cycle defects are likely due to the inability of the R654W pRb mutant to bind and regulate E2F transcription factors.
Figure 4. pRb mediated processing and repair of VP-16 induced DNA lesions is genetically separable from its cell cycle regulatory activity.
(A) Asynchronously growing pRb null, R654W, and wild-type MEFs were treated with DMSO (V), 5 µM VP16, or 2.5 µM CPT for 16 hrs. Following treatment, cells were pulsed with 100 µM BrdU for 1 hr and subsequently fixed and stained to determine the percentage of cells incorporating BrdU. The data is normalized to the vehicle treated control for each genotype and represents the mean and standard deviation of two experiments done in duplicate. (B) Rb1 null, R654W, and wild-type MEFs were treated with DMSO (V), 250 µM VP16 or 25 µM CPT. After 4 hrs, cells were extracted and analyzed by western blotting as in Figure 1A. (C) Rb1 null, R654W, and wild-type MEFs were treated with DMSO (V) or 250 µM VP16 for 4 hrs. After treatment and 0 or 4 hrs recovery, cells were extracted and analyzed by immunoblotting with anti-phosphorylated H2AX antibodies. Hsp70 protein level serves as a loading control. (D) Data from two independent experiments as in C were quantitated by densitometry using ImageQuant software. The data were normalized to the vehicle control and represent the mean and standard deviation. (E) Rb1 null, R654W, and wild-type MEFs were treat with different concentrations of VP16. After 4 days, viable cells were measured by MTT assay as in Figure 3A. The data are the mean and standard deviation of two experiments done in triplicate. The logarithmic trendline shown was calculated by least squares.
We analyzed TOP2β levels in R654W MEF after treatment with VP16 to determine if the mutant pRb was able to mediate efficient TOP2β degradation. As above, TOP2β levels in wild type MEF, but not in pRb null MEFs, declined rapidly and specifically upon treatment with VP16 (Figure 4B). Despite the cell cycle defects associated with the codon 654 pRb mutant, TOP2β levels declined at least as rapidly upon VP16 treatment in R654W MEF as in wild type MEF. Further, the relative rate of decline in VP16 induced phospho-H2AX levels was greater in R654W MEF than in wild type MEF (Figures 4C, D) suggesting that the R654W pRb was competent for efficient processing and repair of VP16 induced DSB. The ability of the R654W MEF to efficiently process and repair trapped cleavable complexes correlated with their relative resistance to the cytotoxic effects of VP16. The relative proliferation of R654W MEFs in the presence of increasing doses of VP16 was significantly greater than wild type or pRb null MEFs (Figure 4E). Hence resistance of MEF to VP16 induced cytotoxicity correlates with pRb mediated processing and repair of trapped TOP2 cleavable complexes, not pRb mediated cell cycle checkpoint activation.
pRb associates with both TOP2 and BRCA1 and is required for TOP2/BRCA1 interaction
The ability of pRb to facilitate rapid degradation and repair of trapped cleavable complexes was independent of its ability to bind E2F transcription factors and to regulate the cell cycle. This suggested the possibility that a more direct molecular mechanism was involved. A direct mechanism would be consistent with the observation that pRb-mediated TOP2β processing and DSB repair were detectable within two hours of VP16 treatment. A direct mechanism would also be consistent with the ability of pRb to bind TOP2α(Bhat et al., 1999). To explore this possibility, we tested whether pRb could also interact with TOP2β. Exogenously expressed GFPRb fusion protein was immunoprecipitated from 293 cell extracts and the immunoprecipitates analyzed for the presence of TOP2β, TOP2α, and BRCA1. All three proteins coimmunoprecipitated with GFPRb (Figure 5A). None of the proteins coimmunoprecipitated with GFP or a mutant GFPRbN fusion protein lacking the carboxy-terminal pRb protein binding pocket. Our data were consistent with previous results demonstrating that the carboxy-terminal protein binding pocket of pRb was required for interaction with TOP2α and BRCA1(Aprelikova et al., 1999 ; Bhat et al., 1999), and suggested that TOP2β interacts with the same region of pRb. Interestingly, the human R661W mutant pRb (GFP661) analogous to the mouse R654W mutant was also able to efficiently coimmunoprecipitate TOP2α, TOP2β, and BRCA1. Hence the pRb activity that facilitates processing and repair of trapped TOP2 cleavable complexes correlates with the ability to bind TOP2 and BRCA1, but not with the ability to bind E2F.
Figure 5. pRb associates with TOP2 and BRCA1 in cells.
(A) Protein extracts from 293 cells expressing GFP, GFPRb, GFPRbN, or GFP661 respectively were immunoprecipitated with polyclonal anti-GFP antibody, immunoprecipitates resolved by SDS-PAGE, and immunoblots stained with the indicated antibodies. GFP661 is the human pRb mutant analogous to the mouse R654W mutant analyzed in MEF. (B) Protein extracts from pRb null, R654W, or wild type MEFs were immunoprecipitated with polyclonal anti-Rb antibody or polyclonal anti-TOP2β antibody and immunoprecipitates resolved by SDS-PAGE. Immunoblots were stained with anti-TOP2β or anti-pRb antibodies as indicated. (C) Protein extracts from pRb null or wild type MEFs were immunoprecipitated with polyclonal anti-BRCA1 antibody, immunoprecipitates resolved by SDS-PAGE, and immunoblots stained with anti-TOP2β or anti-BRCA1 antibody as indicated. (D) C33A cells expressing GFPRb or GFPRbN were treated with DMSO or 250µM VP16. Four hours later, cells were extracted, and the proteins analyzed by immunoblotting with the indicated antibodies. HSP70 protein levels serve as a loading control.
To confirm the pRb/TOP2β interaction occurs between endogenous proteins in normal cells, we tested whether pRb and TOP2β interacted with one another in MEF. TOP2β was detected in pRb immunoprecipitates from wild type and R654W MEF, but not from pRb null MEF (Figure 5B). Likewise, pRb was detected in TOP2β immunoprecipitates from wild type, but not pRb null MEF. Rb1 status did not affect TOP2β levels in these cells. Reciprocal coimmunoprecipitation of pRb and TOP2β suggests that this complex exists in intact cells.
Since pRb can interact with both TOP2β and BRCA1, pRb may facilitate formation of a protein complex containing both proteins. To test this possibility, we have determined whether TOP2β coimmunoprecipitates with BRCA1 in both wild type and pRb null MEF. TOP2β can be detected within BRCA1 immunoprecipitates from wild type extracts, but not from pRb null extracts (Figure 5C). Loss of pRb does not significantly affect the levels of either BRCA1 or TOP2β in MEFs (Figures 5B, C). TOP2β and BRCA1, therefore, associate with each other in intact normal cells, but this interaction requires the presence of pRb. Interestingly, BRCA1 levels also decline upon exposure of cells to VP16, and this decline is dependent on pRb. C33A cells expressing wild type GFPRb show a relative decline in BRCA1 levels upon VP16 treatment (Figure 5D). Cells expressing the pocket domain mutant GFPRbN do not show a decline in BRCA1 levels subsequent to VP16 treatment, but the electrophoretic mobility of BRCA1 isolated from these cells does shift. This shift is reminiscent of that caused by BRCA1 phosphorylation in response to DNA damage (Cortez et al., 1999 ; Tibbetts et al., 2000). In the absence of pRb, VP16 induced DNA damage is presumably recognized resulting in BRCA1 phosphorylation, but BRCA1 and TOP2β are inefficiently processed.
Discussion
Cells respond to DNA damage by activating a complex response pathway that regulates cell-cycle checkpoint activation, cell survival, and DNA repair networks (Zhou and Elledge, 2000). Accumulating evidence suggests that these responses are coupled such that proteins like BRCA1 are important for both cell cycle checkpoint activation and DNA repair (Venkitaraman, 2002). VP16 trapped TOP2 cleavable complexes are a unique form of DNA damage because the DSB are sealed by covalent linkage to TOP2. Hence repair of this lesion requires degradation of TOP2 to reveal the DNA break. BRCA1 may be important for the repair of trapped TOP2 cleavable complexes at multiple levels. BRCA1/BARD1 heterodimers have ubiquitin E3 ligase activity that may facilitate proteasome dependent degradation of TOP2β (Baer and Ludwig, 2002) and BRCA1 is important for DSB repair, in part by recruiting other DNA repair proteins (Jasin, 2002; Motoyama and Naka, 2004). The increasingly appreciated connections between cell cycle checkpoints and DNA repair prompted us to re-evaluate the role of pRb, an important cell cycle regulator, in DNA repair. Since pRb is known to interact with both BRCA1 and TOP2, we have tested the hypothesis that pRb is required for efficient processing and repair of trapped TOP2 cleavable complexes.
We report several observations that support this hypothesis. One, VP16 induced TOP2β degradation is more efficient in MEFs expressing wild type pRb. The observed TO2β degradation is specific for pRb since rapid degradation can be restored in Rb1 null MEFs by exogenous expression of a wild type GFPRb fusion protein. Increased processing of trapped cleavable complexes in the presence of pRb facilitates liberation of the DSBs as recognized by subsequent phosphorylation of H2AX and ATM. Two, once DSB are liberated from the trapped cleavable complexes, MEFs expressing pRb are more proficient in the repair of the DSB. Although initially higher, phosphorylation of H2AX declines more rapidly in the presence of pRb. While this observation does not directly implicate pRb in the repair of DSB, it does suggest that the presence of pRb facilitates this process. Three, decreased processing and repair of trapped TOP2 cleavable complexes in pRb null MEF is associated with increased sensitivity to VP16 cytotoxicity.
Several possible mechanisms may underlie the effects of pRb on processing and repair of VP16 induced DNA lesions. It is well established that pRb is required to enforce cell cycle checkpoints in response to various forms of DNA damage, including VP16 (Bosco et al., 2004 ; Brugarolas et al., 1999 ; Harrington et al., 1998 ; Knudsen et al., 2000 ; Naderi et al., 2002). The principal pRb target in orchestrating cell cycle checkpoint activation is the E2F family of transcription factors (Bracken et al., 2004 ; Stevens and La Thangue, 2004). Our data support this conclusion since the E2F binding deficient R654W pRb mutant fails to enforce cell cycle checkpoints in response to VP16. E2F transcription factors also regulate genes involved in DNA repair. Hence pRb may influence the VP16 induced DNA damage response by altering the expression of E2F regulated DNA repair genes. Our data, however, are inconsistent with this interpretation. The R654W pRb mutant (human R661W) is capable of mediating both rapid TOP2β degradation and DSB repair in response to VP16. This pRb mutant is also able to establish relative resistance to VP16 cytotoxicity. Yet this well-characterized mutant fails to bind E2F transcription factors or enforce cell cycle checkpoints (Harbour, 2001). Hence the pRb mediated mechanism involved in facilitating rapid processing and repair of TOP2 cleavable complexes is independent of E2F or cell cycle checkpoint activation.
We suggest that the mechanism underlying the pRb-mediated effects on processing of TOP2 cleavable complexes may be direct (Figure 6). This model is based on the fact that TOP2-DNA covalent complexes need to be processed before DNA repair factors can access the DSBs. We propose that pRb functions as an adaptor protein to facilitate recruitment of processing and repair factors like BRCA1 to trapped TOP2 cleavable complexes. Once recruited, BRCA1/BARD1 or other similar factors mark TOP2 for proteasome dependent degradation. Once the DSB are liberated, pRb may than facilitate repair, again through its interaction with BRCA1. NHEJ is the repair pathway responsible for repair of VP16 DSB. While BRCA1 is best known for its role in repair of DSB by homologous recombination, it is also known associate with additional repair factors (e.g. Rad50, Mre11 and NBS1) that may play a more direct role in NHEJ. Recent studies have implicated BRCA1 in facilitating efficient NHEJ-mediated DSB repair, consistent with this possibility (Zhong et al., 2002b ; Zhong et al., 2002a)(Adachi et al., 2003 ; Adachi et al., 2004). The model predicts that the functional status of pRb influences the ultimate fate of the damaged cell and, hence, is a determinant of VP16 sensitivity.
Figure 6. A model for the role of pRb in repair of VP16 induced DNA lesions.
We suggest pRb serves as an adaptor protein to recruit processing and repair factors to VP16 trapped TOP2 cleavable complexes. BRCA1/BARD1 E3 ubiquitin ligase activity facilitates degradation of TOP2 to reveal free DSBs that are then repaired by BRCA1 associated DSB repair factors. The model is consistent with the observed requirement for an E2F independent pRb activity in the efficient processing and repair of VP16 induced DNA lesions.
Several observations reported here are consistent with this model. Rb1 protein can associate with both TOP2 and BRCA1 in intact, normal cells as indicated by reciprocal coimmunoprecipitation. Importantly, pRb is also required for association between TOP2β and BRCA1. The simplest interpretation of this result is consistent with the model; pRb serves as a physical link between TOP2 and processing and repair factors like BRCA1. However, since the pRb/TOP2/BRCA1 complex has not been sufficiently characterized, indirect mechanisms cannot be excluded. For example, pRb could affect the posttranslational modification of TOP2 and/or BRCA1 thereby influencing their interaction. The recently discovered effects of pRb on chromatin structure (Narita et al., 2003) could also influence the recognition and subsequent repair of VP16 trapped cleavable complexes. It should be noted that processing and repair of VP16 trapped cleavable complexes occurs in the absence of pRb, albeit at a much slower rate. Hence pRb is not required, but it does improve the efficiency of processing and repair. Additional experiments are required to definitively assess whether pRb plays a direct role in the effects observed here.
Interestingly, the R654W pRb mutant is apparently more proficient at processing and repair of TOP2 cleavable complexes than wild type pRb. Several possible explanations for this observation come to mind. The lack of cell cycle checkpoint activation in the R654W MEF may contribute to the increased processing and repair rate. For example, cycling cells may have a distinct gene expression profile or chromatin structure that facilitates repair. However, given that processing and repair of trapped cleavable complexes occurs within 2 hours in asynchronously proliferating cells and that loss of pRb does not affect VP16 induced trapping of TOP2β, these explanations seem unlikely. Alternatively, the mutant protein may have greater specific activity for the relevant pRb dependent mechanism. Rb1 protein binds a large number of different cellular proteins (Morris and Dyson, 2001), with the E2F family being a major target. Since E2F is unable to efficiently bind R654W pRb, there may be a greater pool of free pRb available for binding other protein partners. This reshuffling of pRb protein complexes may facilitate repair of VP16 induced DNA damage. Precedent for a mechanism involving reshuffling of pRb protein complexes has been documented(Lee et al., 2002).
Irrespective of the precise molecular mechanism involved, the findings reported here have significant implications for cancer chemotherapy based on TOP2 poisons like etoposide. Our data predict that cancer cells completely lacking pRb will be more sensitive to the cytotoxic effects of TOP2 poisons. Resistance to TOP2 poisons are predicted to correlate with the ability of pRb to facilitate processing and repair of cleavable complexes, rather than to the ability to regulate E2F dependent gene expression and enforce cell cycle checkpoints. Most cancers express pRb protein that is presumed inactive due to hyperphosphorylation. Rb1 protein hyperphosphorylation is known to inhibit pRb/E2F binding and pRb mediated cell cycle checkpoint activation (Harbour et al., 1999), defects that are observed in cancer cells. Yet the functional status E2F-independent mechanisms of pRb action in cancer cells are often unknown. Like the R654W pRb mutant, residual pRb protein activity in cancer cells may contribute to adverse clinical outcome by mediating resistance to VP16. While the effects of phosphorylation on pRb mediated repair of VP16 induced DNA lesions are unknown, correlations between pRb hyperphosphorylation and VP16 resistance have been noted in human cancer cells (Yamamoto et al., 1998).
VP16 reveals a function of pRb biologically relevant to the repair of trapped TOP2 cleavable complexes. Since cells are unlikely to encounter such DNA lesions under normal physiological conditions, the effects of pRb on TOP2 cleavable complexes noted here may reflect an activity that is important for the normal function of TOP2. Loss of pRb and TOP2 activity are both increasingly recognized to cause genomic instability (Cortes et al., 2003 ; Hernando et al., 2004). The activity identified here may provide a functional link between these observations and suggests a novel mechanism potentially involved in pRb tumor suppression.
Materials and Methods
Reagents and cell lines
VP16, CPT and MG132 were purchased from Sigma. Stock solutions were prepared in DMSO. Antibodies against TOP2β were the generous gift of Dr. Leroy Liu (UMDNJ, Piscataway, NJ). All other antibodies were purchased from commercial suppliers: BRCA1 (Santa Cruz), phosphorylated H2AX (Travigen), GFP (BD Biosciences), Rb (Pharmingen and NeoMarker), TOP2α (TopoGEN), phosphorylated ATM (Rockland), and PARP (Santa Cruz). GFPRb fusion protein expression vectors were constructed using the BD creator DNA cloning kit (BD Biosciences). Transfection was carried out with Lipofectamin2000 following manufacture’s instructions (Invitrogen). Heterozygous Rb null mice were provided by Dr. Eldad Zacksenhaus and were originally engineered by Dr. Tyler Jacks(Jacks et al., 1992). The Rb codon 654 mutant mice were constructed by targeted homologous recombination in ES cells using a mutated knockin targeting vector. Details of the construction will be described in a forthcoming publication. MEFs were derived from 13.5 day post coitum embryos resulting from intermating of heterozygous mice using standard procedures. C33A and HEK293 cell lines were obtained from ATCC. All cells were cultured in DMEM supplemented with 10% FCS.
Cytotoxicity and BrdU incorporation assays
Cell proliferation was assayed by MTT assay using standard methods. Five thousand cells per well were plated in 96-well plates and subsequently treated. Four days later, cell proliferation relative to vehicle treated control (DMSO) was assayed. For the clonogenic survival assay, 250 cells per well were plated in six-well plates and cultured overnight. Cells were treated with for 1 hour, washed, and replenished with fresh medium. After 2 weeks, cells were stained with 10 mg/ml methylene blue (Sigma) in 50% methanol, and the colony number counted.
For the BrdU incorporation assay, asynchronously growing Rb null, Rb 654/654, and wild-type MEFs were treated with DMSO, 5 µM VP16, or 2.5 µM CPT for 16 hrs. Following treatment, cells were pulsed with 100 µM BrdU for 1 hr and subsequently fixed and stained to determine the percentage of cells incorporating BrdU. Immunofluorescent images were captured on a Zeiss Axioplan micoscope and Axiovision software.
Co-Immunoprecipitation and immunoblotting
Cells were lysed in IPP150 buffer (10 mM Tris-HCl pH8.0, 150 mM NaCl, 0.1% NP40) with protease inhibitors (50 µg/ml leupeptin, 50 µg/ml aprotinin, 50 µg/ml pepstatin A, 1 mM PMSF). The cleared lysates were mixed with polyclonal anti-GFP antibodies or polyclonal anti-Rb antibodies and inverted for 4 hours at 4°C. Protein G/protein A agarose beads (CalBiochem) were added and incubated overnight at 4°C. Beads were washed with IPP150 buffer and immunoprecipitates resolved by SDS-PAGE. Western blotting was performed as described previously(Doostzadeh-Cizeron et al., 1999).
Immunoblotting analysis of TOP2-DNA covalent complexes was performed essentially as described(Xiao et al., 2003). Treated cells were lysed either directly with SDS sample buffer (band depletion assay) or by alkaline lysis (200 mM NaOH, 2 mM EDTA). The alkaline lysis extract was neutralized by 1.2 M Tris (pH 8.0) and 2 M HCl. The neutralized lysate was mixed with 10x S7 nuclease reaction buffer (50 mM MgCl2, 50 mM CaCl2, 5 mM DTT, 1 mM EDTA, 50 µg/ml leupeptin, 50 µg/ml aprotinin, 50 µg/ml pepstatin A, 1 mM PMSF) and 60 units of staphylococcal S7 nuclease. Staphylococcal S7 nuclease treatment was omitted for the band-depletion assay. The samples were resolved by SDS-PAGE and analyzed by western blotting.
Immunofluorescence microscopy
MEFs were seeded on coverslips and treated with VP16 for 1.5 hrs. Cells were washed with phosphate buffered saline (PBS) and fixed in 4% formaldehyde. Fixed cells were incubated with primary antibody according to manufacturers’ recommendations. Rhodamine conjugated goat anti-rabbit IgG secondary antibody (Molecular Probe) was used at 1:200 dilution. Cells were counterstained with DAPI and mounted in Vectashield (Vector Laboratories). Immunofluorescent images were captured on a Zeiss Axioplan micoscope and Axiovision software.
Acknowledgements
We thank Dr. Leroy Liu for providing the anti-TOP2β antibodies. We acknowledge Yanjie Chang for preparing the MEFs and other members of the Goodrich lab for insightful discussions. This work was supported by NIH grant CA70292 (D.W.G.).
References
- Adachi N, Iiizumi S, So S, Koyama H. Biochem.Biophys.Res.Commun. 2004;318:856–861. doi: 10.1016/j.bbrc.2004.04.099. [DOI] [PubMed] [Google Scholar]
- Adachi N, Suzuki H, Iiizumi S, Koyama H. J.Biol.Chem. 2003;278:35897–35902. doi: 10.1074/jbc.M306500200. [DOI] [PubMed] [Google Scholar]
- Aprelikova ON, Fang BS, Meissner EG, Cotter S, Campbell M, Kuthiala A, Bessho M, Jensen RA, Liu ET. Proc.Natl.Acad.Sci.U.S.A. 1999;96:11866–11871. doi: 10.1073/pnas.96.21.11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R, Ludwig T. Curr.Opin.Genet.Dev. 2002;12:86–91. doi: 10.1016/s0959-437x(01)00269-6. [DOI] [PubMed] [Google Scholar]
- Bhat UG, Raychaudhuri P, Beck WT. Proc.Natl.Acad.Sci.U.S.A. 1999;96:7859–7864. doi: 10.1073/pnas.96.14.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco EE, Mayhew CN, Hennigan RF, Sage J, Jacks T, Knudsen ES. Nucleic Acids Res. 2004;32:25–34. doi: 10.1093/nar/gkg919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken AP, Ciro M, Cocito A, Helin K. Trends Biochem.Sci. 2004;29:409–417. doi: 10.1016/j.tibs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Brugarolas J, Moberg K, Boyd SD, Taya Y, Jacks T, Lees, JA Proc.Natl.Acad.Sci.USA. 1999;96:1002–1007. doi: 10.1073/pnas.96.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cam H, Dynlacht BD. Cancer Cell. 2003;3:311–316. doi: 10.1016/s1535-6108(03)00080-1. [DOI] [PubMed] [Google Scholar]
- Chen AY, Liu LF. Ann.Rev.Pharmacol.Toxicol. 1994;34:191–218. doi: 10.1146/annurev.pa.34.040194.001203. [DOI] [PubMed] [Google Scholar]
- Cortes F, Pastor N, Mateos S, Dominguez I. Mutation Res. 2003;543:59–66. doi: 10.1016/s1383-5742(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Cortez D, Wang Y, Qin J, Elledge SJ. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- Doostzadeh-Cizeron J, Evans R, Yin S, Goodrich DW. Mol.Biol.Cell. 1999;10:3251–3261. doi: 10.1091/mbc.10.10.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Yuan R, Ma YX, Xiong J, Meng Q, Erdos M, Zhao JN, Goldberg ID, Pestell RG, Rosen EM. Oncogene. 2001;20:4827–4841. doi: 10.1038/sj.onc.1204666. [DOI] [PubMed] [Google Scholar]
- Govoni M, Neri S, Labella T, Sylvester JE, Novello F, Pession A. Biochem.Biophys.Res.Commun. 1995;213:282–288. doi: 10.1006/bbrc.1995.2127. [DOI] [PubMed] [Google Scholar]
- Harbour JW. Archives of Ophthalmology. 2001;119:1699–1704. doi: 10.1001/archopht.119.11.1699. [DOI] [PubMed] [Google Scholar]
- Harbour JW, Luo RX, Dei SA, Postigo AA, Dean DC. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- Harrington EA, Bruce JL, Harlow E, Dyson N. Proc.Natl.Acad.Sci.USA. 1998;95:11945–11950. doi: 10.1073/pnas.95.20.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume R, Fukuda M, Maeda I, Nishikawa H, Oyake D, Yabuki Y, Ogata H, Ohta T. J.Biol.Chem. 2001;276:14537–14540. doi: 10.1074/jbc.C000881200. [DOI] [PubMed] [Google Scholar]
- Hernando E, Nahle Z, Juan G, Diaz-Rodriguez E, Alaminos M, Hemann M, Michel L, Mittal V, Gerald W, Benezra R, Lowe SW, Cordon-Cardo C. Nature. 2004;430:797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Jasin M. Oncogene. 2002;21:8981–8993. doi: 10.1038/sj.onc.1206176. [DOI] [PubMed] [Google Scholar]
- Knudsen KE, Booth D, Naderi S, Sever-Chroneos Z, Fribourg AF, Hunton IC, Feramisco JR, Wang JY, Knudsen ES. Molecular & Cellular Biology. 2000;20:7751–7763. doi: 10.1128/mcb.20.20.7751-7763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Cam H, Ziebold U, Rayman JB, Lees JA, Dynlacht BD. Cancer Cell. 2002;2:463–472. doi: 10.1016/s1535-6108(02)00207-6. [DOI] [PubMed] [Google Scholar]
- Li TK, Liu LF. Ann.Rev.Pharmacol.Toxicol. 2001;41:53–77. doi: 10.1146/annurev.pharmtox.41.1.53. [DOI] [PubMed] [Google Scholar]
- Liu LF. Ann.Rev.Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Mao Y, Desai SD, Ting CY, Hwang J, Liu LF. J.Biol.Chem. 2001;276:40652–40658. doi: 10.1074/jbc.M104009200. [DOI] [PubMed] [Google Scholar]
- Morris EJ, Dyson NJ. Advances in Cancer Research. 2001;82:1–54. doi: 10.1016/s0065-230x(01)82001-7. [DOI] [PubMed] [Google Scholar]
- Motoyama N, Naka K. Curr.Opin.Genet.Dev. 2004;14:11–16. doi: 10.1016/j.gde.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Naderi S, Hunton IC, Wang JY. Cell Cycle. 2002;1:193–200. [PubMed] [Google Scholar]
- Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- Niimi A, Suka N, Harata M, Kikuchi A, Mizuno S. Chromosoma. 2001;110:102–114. doi: 10.1007/s004120100140. [DOI] [PubMed] [Google Scholar]
- Otterson GA, Chen W, Coxon AB, Khleif SN, Kaye FJ. Proc.Natl.Acad.Sci.USA. 1997;94:12036–12040. doi: 10.1073/pnas.94.22.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterson GA, Modi S, Nguyen K, Coxon AB, Kaye FJ. American Journal of Human Genetics. 1999;65:1040–1046. doi: 10.1086/302581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffner H, Joazeiro CA, Hemmati D, Hunter T, Verma IM. Proc.Natl.Acad.Sci.U.S.A. 2001;98:5134–5139. doi: 10.1073/pnas.081068398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers WR, Novitch BG, Miyake S, Heith A, Otterson GA, Kaye FJ, Lassar AB, Kaelin WG., Jr Gene.Dev. 1998;12:95–106. doi: 10.1101/gad.12.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C, La Thangue NB. DNA Repair. 2004 Sep;3:1071–1079. doi: 10.1016/j.dnarep.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Tibbetts RS, Cortez D, Brumbaugh KM, Scully R, Livingston D, Elledge SJ, Abraham RT. Genes Dev. 2000;14:2989–3002. doi: 10.1101/gad.851000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Tsutsui K, Sano K, Kikuchi A, Tokunaga A. J.Biol.Chem. 2001;276:5769–5778. doi: 10.1074/jbc.M008517200. [DOI] [PubMed] [Google Scholar]
- Venkitaraman AR. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- Wang JC. Ann.Rev.Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- Whitaker LL, Su H, Baskaran R, Knudsen ES, Wang JYJ. Mol.Cell.Biol. 1998;18:4032–4042. doi: 10.1128/mcb.18.7.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner RD, Mattern MR, Mirabelli CK, Johnson RK, Drake FH. Cell Growth & Differ. 1991;2:209–214. [PubMed] [Google Scholar]
- Xia Y, Pao GM, Chen HW, Verma IM, Hunter T. J.Biol.Chem. 2003;278:5255–5263. doi: 10.1074/jbc.M204591200. [DOI] [PubMed] [Google Scholar]
- Xiao H, Mao Y, Desai SD, Zhou N, Ting CY, Hwang J, Liu LF. Proc.Natl.Acad.Sci.U.S.A. 2003;100:3239–3244. doi: 10.1073/pnas.0736401100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Shimizu E, Masuda N, Takada M, Sone S. Oncology Rep. 1998;5:447–451. doi: 10.3892/or.5.2.447. [DOI] [PubMed] [Google Scholar]
- Yang X, Li W, Prescott ED, Burden SJ, Wang JC. Science. 2000;287:131–134. doi: 10.1126/science.287.5450.131. [DOI] [PubMed] [Google Scholar]
- Yarden RI, Brody LC. Proc.Natl.Acad.Sci.U.S.A. 1999;96:4983–4988. doi: 10.1073/pnas.96.9.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Boyer TG, Chen PL, Lee WH. Cancer Res. 2002a;62:3966–3970. [PubMed] [Google Scholar]
- Zhong Q, Chen CF, Chen PL, Lee WH. J.Biol.Chem. 2002b;277:28641–28647. doi: 10.1074/jbc.M200748200. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]