Abstract
Background
High neuroticism is a personality risk factor that captures much of the genetic vulnerability to Major Depressive Disorder (MDD), and low extraversion may increase risk as well. Both have been linked to the serotonin system.
Objectives
To test whether MDD patients in selective serotonin reuptake inhibitors (SSRIs) treatment report greater changes in neuroticism and extraversion than patients receiving inert-placebo; and to examine the state-effect hypothesis, that self-reported personality change during SSRI treatment is merely a change of depression-related measurement bias.
Design/Setting
Personality was measured during a placebo-controlled trial in research clinics.
Patients
Adult moderate-to-severe MDD patients randomized to receive paroxetine (n=120), placebo (n=60), or cognitive therapy (CT) (n=60).
Outcome Measures
NEO Five-Factor Inventory; Hamilton Rating Scale for Depression.
Results
Paroxetine patients reported greater personality change than did placebo patients, even after controlling for depression improvement (p≤.002). The advantage of paroxetine over placebo in antidepressant efficacy was no longer significant after controlling for change in personality (p≥.14). Paroxetine patients reported 6.8 times as much change on neuroticism and 3.5 times as much change on extraversion as placebo patients matched for depression improvement. Although placebo patients exhibited substantial depression improvement (−1.2 SD, p<.001), they reported little change on neuroticism (−0.18 SD, p=.08) or extraversion (0.08 SD, p=.50). CT produced greater personality change than placebo (p≤.01); but its advantage on neuroticism was no longer significant after controlling for depression (p=.14). Neuroticism reduction during treatment predicted lower relapse rates among paroxetine responders (p=.003), but not among CT responders (p=.86).
Conclusion
Paroxetine appears to have a specific pharmacological effect on personality that is distinct from its effect on depression. If replicated, this pattern would disconfirm the state-effect hypothesis and instead support the notion that SSRIs’ effects on personality go beyond and perhaps contribute to their antidepressant effects.
Neuroticism and extraversion are two of the five primary personality dimensions in the Five-Factor Model of Personality1-3. Neuroticism refers to a tendency to experience negative emotions and emotional instability; extraversion encompasses social extraversion, dominance, and a tendency to experience positive emotions1-3. Neuroticism and extraversion are largely independent constructs, as evidenced by correlations between them of −.28 and −.38 in two normative samples4.
Results from longitudinal studies have consistently shown that neuroticism predicts both the onset and the chronicity of MDD5-11. For example, in a study of more than 800 children, precursors of neuroticism measured at age 3 predicted whether these children would develop MDD at age 2111. In some longitudinal studies, lower levels of extraversion have also predicted the onset of MDD9, 12, but in other studies this pattern has not been replicated8, 10.
Neuroticism and extraversion are both moderately heritable, with genetic factors determining 50-60% of their variance2, 13. Twin studies have found substantial overlap in the genetic factors that are associated with high neuroticism and those that predispose persons to MDD10, 14-17. Neuroticism, therefore, appears to reflect much of the genetic vulnerability to MDD10, 16, 17.
Research on medical disorders with known causes often focuses on the impact of treatments on these causal factors. Despite the accumulating evidence associating neuroticism and extraversion with a causal path to MDD, little research exists on how SSRI treatment affects these personality risk factors. Patients have reported that SSRIs make them less reactive to stress, less sensitive to rejection, and more outgoing and vivacious18-23. These descriptions are consistent with decreases in neuroticism and increases in extraversion, but these reports have not come from patients in double-blind, placebo-controlled studies18-22. In the one published placebo-controlled study of neuroticism change during the SSRI treatment of MDD, Agosti and McGrath did not find that fluoxetine patients reported significantly greater neuroticism reduction than placebo patients24. However, fewer than half of the patients in that study (42%) completed both treatment and assessment, making these findings difficult to interpret24. Thus, it remains unclear whether neuroticism and extraversion change in response to SSRI treatment of MDD, above and beyond their natural history and the placebo effect.
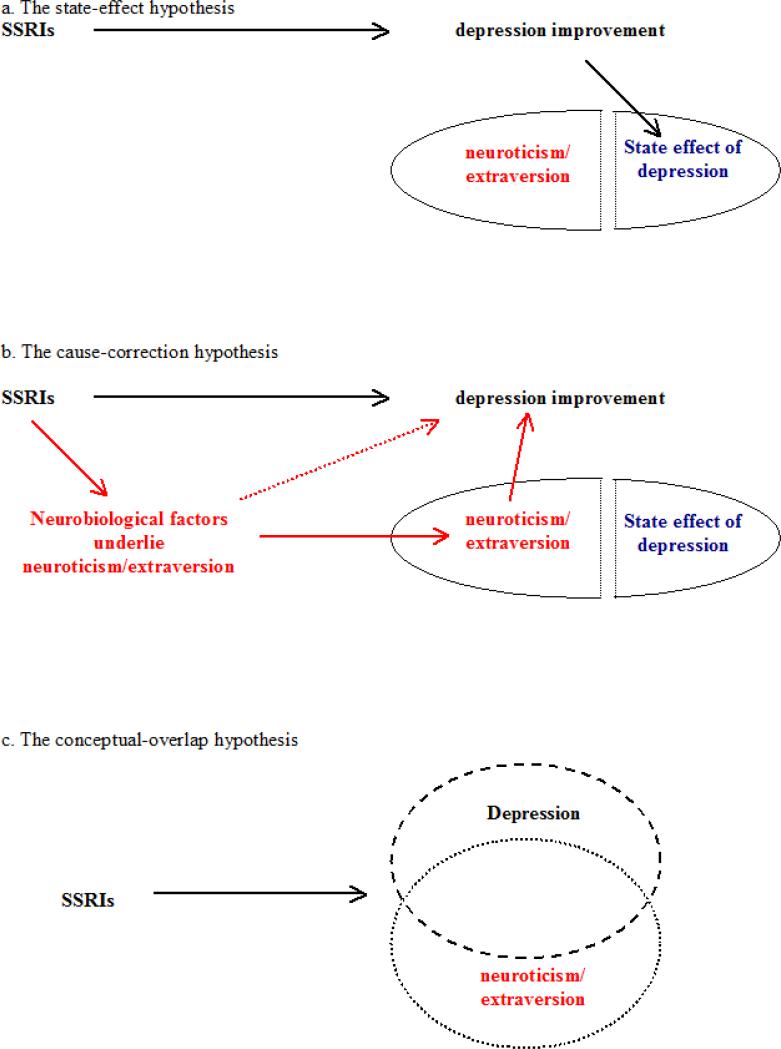
When change in personality is reported during SSRI treatment, it is usually assumed to reflect nothing more than the state effect of depression on personality measurement21, 25, 26. When in depressive episodes, patients often describe themselves as more neurotic and less extroverted than they are in their inter-morbid or pre-morbid states. This is consistent with the idea that there is a state effect of depression on personality measurement5, 6, 8. The state-effect hypothesis holds that reported personality change during SSRI treatment is just change in state effect: as depression improves, the state effect of depression will decline, and personality measurement will change as a consequence21, 25, 26. This hypothesis thus regards reported personality change as an inconsequential byproduct of depression improvement. The main evidence for this hypothesis is the finding that personality change correlates with depression improvement during SSRI treatment18, 21.
Findings inconsistent with the state effect hypothesis were reported from two studies among healthy subjects without current MDD. In both studies, SSRIs were found to affect behaviors and affects that are closely associated with neuroticism and extraversion27, 28. Recent findings from neuroscience investigations also support associations between these personality dimensions and the serotonin system. For example, molecular genetic and positron emission tomography (PET) studies have associated neuroticism with serotonin receptor polymorphisms and binding29-31. Individual studies of neuroticism and the functional polymorphic variants of the serotonin transporter gene have yielded mixed results, but two meta-analyses of these studies have converged on the conclusion that the link is statistically significant32, 33. Moreover, the serotonin system has been implicated as a substrate for behaviors related to extraversion in numerous animal and human studies27, 28, 34-36. SSRI treatment, which targets the serotonin system, might thus affect neuroticism and extraversion directly, in a manner that does not depend on its antidepressant effects.
Cognitive therapy (CT), a leading psychosocial treatment of MDD, has been shown to reduce depression symptom severity to a degree similar to that produced by SSRIs37-39. While some studies of CT have examined pre-treatment personality as predictors of outcome40, 41, very little theoretical or empirical work exists on how CT might impact neuroticism and extraversion. Although the state-effect hypothesis might also apply to personality changes reported in CT, it is also plausible that CT changes personality through cognitive or behavioral pathways. For example, CT therapists routinely encourage socially withdrawn patients to seek out social interactions, and to test whether these interactions are as unpleasant as they predict. CT also teaches patients to challenge internal and stable negative attributions, such as “I am an inferior person.” Such interventions, if successful, might result in changes in neuroticism and extraversion.
This project examined self-reported personality changes in a randomized, placebo-controlled clinical trial of MDD. In the trial, 240 patients were randomized such that 60 patients received placebo for 8 weeks, 120 patients received paroxetine for 16 weeks, and 60 patients received CT for 16 weeks37. Our first goal was to test whether paroxetine patients or CT patients reported greater changes in neuroticism and extraversion at week 8, relative to placebo patients.
If paroxetine or CT were observed to produce significantly greater personality change than placebo, our second goal was to test whether such effects could be attributed to between-group differences in depression improvement. We examined this in two ways: a) a linear regression that accounted for depression improvement statistically; and b) a matching analysis in which placebo patients were compared against paroxetine patients matched on amount of depression improvement. The state-effect hypothesis would predict that the between-group differences in personality change would be largely eliminated in both of these analyses.
Our third goal was to examine the state-effect hypothesis by analyzing data obtained from the patients assigned to eight weeks of treatment with placebo. In studies of MDD treatments, placebo patients tend to experience substantial improvement in depression42-46. Thus, their personality data provide a direct test of the state-effect hypothesis, which predicts that these placebo patients should also report substantial change in personality. Because this test is not confounded by the effects of active treatments, it might be the cleanest method to test this hypothesis. Moreover, during the eight weeks that followed the placebo phase, 31 placebo patients accepted the offer of free subsequent treatment with an SSRI. This set up a within-subject comparison between their placebo phase and their SSRI phase. The state-effect hypothesis predicts that the magnitude of reported personality change during each phase should be roughly proportional to the magnitude of depression improvement during that same phase.
Our final goal was to examine whether reported neuroticism reduction during SSRI (or CT) treatment predicted subsequent relapse, since neuroticism is a key risk factor that reflects much of the genetic cause of MDD. If reported neuroticism reduction during active treatment was merely a change in the state effect of depression, then it should have no long-term clinical consequences.
Methods
Participants
Institutional review boards approved the clinical trial protocol, and written informed consent was obtained from all participants. Participants were 240 moderate-to-severely depressed adult outpatients; patient characteristics, as well as the procedures of the trial, have been detailed elsewhere37, 47. All patients met criteria for MDD and scored ≥ 20 at both screening and intake evaluations on the HRSD42. Inclusion criteria were: (1) DSM-IV MDD diagnosis48; (2) aged 18 to 70; (3) English speaking; and (4) willingness and ability to give informed consent. Exclusion criteria were: (1) history of bipolar I disorder; (2) substance abuse or dependence judged to require treatment; (3) current or past psychosis; (4) another DSM-IV Axis I disorder judged to require priority treatment; (5) antisocial, borderline, and/or schizotypal disorders (all other Axis II disorders were permitted); (6) suicide risk requiring immediate hospitalization; (7) a medical condition that contraindicated study medications; or (8) non-response to an adequate trial of paroxetine in the preceding year.
Clinical Trial
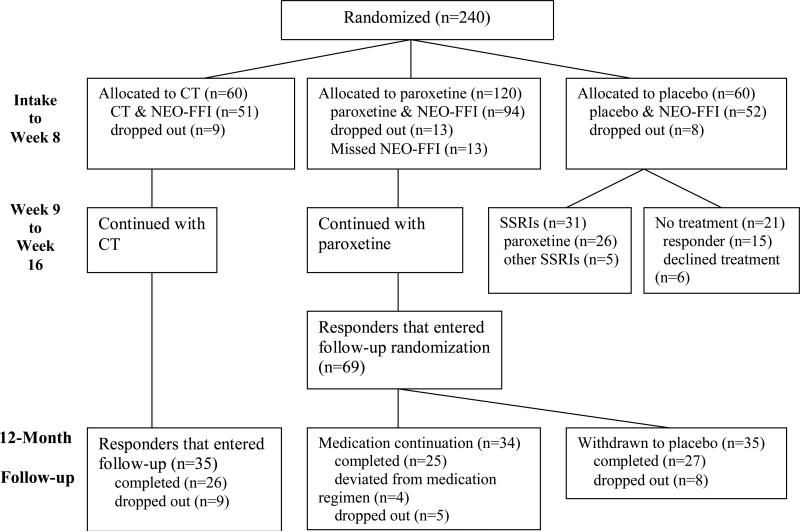
The trial randomized 60 patients into the CT group, 120 patients into the paroxetine group, and 60 patients into the pill-placebo group (Figure 1). The patients who dropped out or who did not complete personality assessments did not differ significantly from the other patients at intake on depression severity, neuroticism, or extraversion.
Figure 1.
A flow chart of the clinical trial patients utilized in our analyses.
For patients in the paroxetine or CT conditions, response was defined in terms of HRSD scores in the period surrounding the end of 16 weeks of treatment, using the following criteria : (1) week-16 HRSD ≤ 12, and either week-14 HRSD ≥ 14, or week-10 and week-12 HRSD ≤ 12; or (2) week-12, week-14, and week-18 HRSD ≤ 1237. These criteria prevented a transient exacerbation of depressive symptoms at week 14 or 16 from precluding a patient as being recognized as a responder.
After acute treatment, responders to paroxetine or CT entered the 12-month continuation phase (Figure 1). Paroxetine responders were randomized into two subgroups: 34 remained on medication at the same dosage, and 35 were withdrawn onto inert pill-placebos. The 35 CT responders were allowed 3 booster sessions, scheduled at least one month apart. All responders were asked not to pursue depression treatment outside the research protocol during this continuation phase.
The patients who had initially been assigned to placebo completed participation in the study proper at week 8. Thirty-one of these placebo patients then opted for subsequent SSRI treatment (Figure 1).
Measurements
Personality variables were assessed with the NEO Five-Factor Inventory (NEO-FFI), a widely used self-report measure based on the Five-Factor Model of Personality (FFM)3, the dominant paradigm in current personality research1-3. The NEO-FFI has been validated by spouse and peer ratings3, and 3-month test-retest reliability coefficients have been reported as .79 for both neuroticism and extraversion3. NEO-FFI scales have well-established normative sample means, neuroticism: mean=19.1, SD=7.7; extraversion: mean=27.7, SD=5.83. In this article, SD for neuroticism and extraversion refers to the standard deviation estimates obtained in the normative sample.
Depression was measured with the 17-item version of the HRSD, modified to assess atypical symptoms42. The Longitudinal Interval Follow-Up Evaluation II (LIFE-II) was the primary means of evaluation during follow-up49. The LIFE-II tracks whether patients have reentered treatment and whether they had depressive episodes between evaluations49. Patients met criteria for relapse if: (1) they were given an HRSD ≥ 14 for two consecutive weeks (three weeks during the period of medication withdrawal); or (2) LIFE-II interviews yielded a diagnosis of MDD.
Statistical Analysis
The main effects were tested by regressing week-8 scores against treatment assignment in a standard linear regression, with intake scores as covariates. All main effect analyses used last available observations as week-8 scores for patients who dropped out or missed assessments. We also conducted analyses restricted to only those patients with both intake and week-8 scores, and no notable differences emerged. Effect sizes for main effects were estimated by dividing the difference in the respective least square mean at week 8 by the pooled standard deviation of the respective mean. They are basically Cohen's d for least square means, and effect sizes above .8 can be considered “large” in magnitude, between .5 and .8 “medium”, and between .2 and .5 “small” 50. Unless otherwise specified, multiple regressions results reported reflect the incremental changes attributed to the predictor variable in question, and not to the full model. Within-subject comparisons between two time points were examined with paired t-tests. Repeated-measure ANOVA was used to evaluate whether the placebo patients showed greater changes during their placebo phase than their SSRI phase. Survival curves and relapse rates were estimated using the Cox Proportional Hazards Model51, and the observations that reflected those patients who dropped out, deviated from medication regimen, or sought outside treatment prior to relapse were censored.
Matching Analysis
In an exploratory analysis, we identified placebo patients for whom we could find a paroxetine patient with matching amounts of depression improvement from intake to week 8. When such a match could not be found for a placebo patient, that patient was dropped. When a placebo patient could be matched with more than one paroxetine patient, the paroxetine patient whose intake HRSD score was closest to the placebo patient's was chosen. If more than one match remained, the paroxetine patient closest to the placebo patient on study identification number was chosen. This matching algorithm eliminated subjective choices, and it was unaffected by personality scores. It successfully matched 44 of the 52 placebo patients (85%) with intake and week-8 personality data with 44 paroxetine patients. Rather than conducting paired comparisons of these matched groups, we analyzed for the differences in group means. (While we attempted the same analysis with placebo and CT, we will not detail the results in this article because matching CT patients were found for only 28 of the 52 placebo patients (54%), and results from these 28 pairs were perfectly consistent with the results of regression analyses in Table 3.)
Table 3.
Between-group comparisons of personality change from intake to week 8 after accounting for depression improvement.
| Paroxetine vs. placebo | CT vs. paroxetine | CT vs. placebo | |
|---|---|---|---|
| Neuroticism | p<.001 | p=.07 | p=.14 |
| Extraversion | p=.004 | p=.50 | p=.05 |
Results
Main Effects
At intake, the mean neuroticism score was 34 (1.9 SD above normative sample mean) and the mean extraversion score was 20 (1.3 SD below normative sample mean). The treatment groups did not differ significantly on neuroticism (p=.27) or extraversion (p=.84) at intake. At week 8, treatment assignment (placebo, paroxetine, and CT) significantly predicted depression severity, neuroticism, and extraversion. The main effect statistics are presented in Table 1; although no significant differences emerged between the two active treatment groups, paroxetine and CT each outperformed placebo in changing depression, neuroticism, and extraversion. Interestingly, in the comparisons with placebo, the effect sizes on depression improvement were smaller than those on neuroticism and extraversion for both paroxetine and CT (Table 1).
Table 1.
Clinical trial main effects from intake to week 8.
| Treatment Assignment | Paroxetine vs. CT | CT vs. placebo | Paroxetine vs. placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | effect size | F | p | effect size | F | p | effect size | |
| HRSD | 3.2 | .04 | .0044 | .95 | .01 | 5.6 | .02 | .37 | 5.3 | .02 | .38 |
| Neuroticism | 6.4 | .002 | .41 | .52 | .10 | 6.5 | .01 | .46 | 13 | <.001 | .57 |
| Extraversion | 7.7 | <.001 | .13 | .71 | .07 | 3.0 | <.001 | .56 | 14 | <.001 | .63 |
Personality Change from Intake to Week 8
Table 2 presents the magnitude of change (in SD) among patients who completed both intake and week 8 personality measurements, and Table 3 compares reported personality change across the three groups after accounting for change in depression. Changes reported in all groups are in the direction of normalization: HRSD and neuroticism decreased, and extraversion increased. Consistent with prior studies43-46, placebo patients reported substantial depression improvement (1.2 SD), equal to 75% of the depression improvement shown by CT or ADM patients (1.6 SD each). Contrary to the prediction of the state-effect hypothesis, placebo patients reported little change in neuroticism (.18 SD, p=.08) or extraversion (.08 SD, p=.50) despite such considerable depression improvement.
Table 2.
Change from intake to week 8 among patients who completed NEO-FFI at intake and week 8.
| Placebo (n=52) | CT (n=51) | Paroxetine (n=94) | |
|---|---|---|---|
| HRSD, SD | −1.2, p<.001 | −1.6, p<.001 | −1.6, p<.001 |
| Neuroticism, SD | −.18, p=.08 | −.54, p<.001 | −.80, p<.001 |
| Extraversion, SD | .08, p=.50 | .49, p<.001 | .64, p<.001 |
From intake to week 8, CT patients reported substantial changes on neuroticism and extraversion (Table 2). After accounting for depression improvement, CT patients still reported significantly greater improvement than placebo on extraversion, but the two groups’ difference on neuroticism was no longer significant (Table 3). Furthermore, after accounting for depression improvement, CT patients reported less change on neuroticism than paroxetine patients, at the level of a non-significant trend (Table 3).
Paroxetine patients reported changes in neuroticism and extraversion that were 4 to 8 times as large as the changes reported by placebo patients (Table 2). Regression analyses suggested that such large differences were unlikely to be explained by the modest between-group difference on depression improvement (1.2 SD vs. 1.6 SD): after accounting for depression improvement, paroxetine still significantly outperformed placebo in changing both neuroticism and extraversion (Table 3). Furthermore, the difference between paroxetine and placebo in depression reduction was no longer significant after controlling for neuroticism reduction F(1, 145)=.74, p=.46, effect size=.19, or change in extraversion F(1,145)=1.5, p=.14, effect size=.23.
Matching Analysis
As linear regression approaches might model the non-linear relationships between depression and personality poorly, we reexamined these variables in the paroxetine and placebo patients in an exploratory matching analysis. For each placebo patient, we attempted to identify a paroxetine patient with the same amount of depression improvement. We found matching paroxetine patients for 44 of the 52 placebo patients with intake and week-8 personality data.
Personality change of these patients from intake to week-8 are presented in Table 4. Although matched on depression improvement (and thus the state effect of depression), these paroxetine patients still reported far greater personality change than these placebo patients. On neuroticism, the matched paroxetine patients showed 3.5 times as much reduction as the placebo patients; on extraversion, the matched paroxetine patients showed 6.8 times as much improvement as the placebo patients.
Table 4.
Personality change from intake to week 8 of the placebo patients and paroxetine patients matched on depression improvement.
| Placebo (n=44) | Paroxetine (n=44) | Magnitude Ratio: Paroxetine vs. placebo | P value: placebo vs. paroxetine | |
|---|---|---|---|---|
| HRSD, SD | −1.4 | −1.4 | 100% | matched by design |
| Neuroticism, SD | −.23 | −.80 | 347% | p<.001 |
| Extraversion, SD | .08 | .54 | 675% | p=.006 |
Placebo Patients Within-Subject Comparison
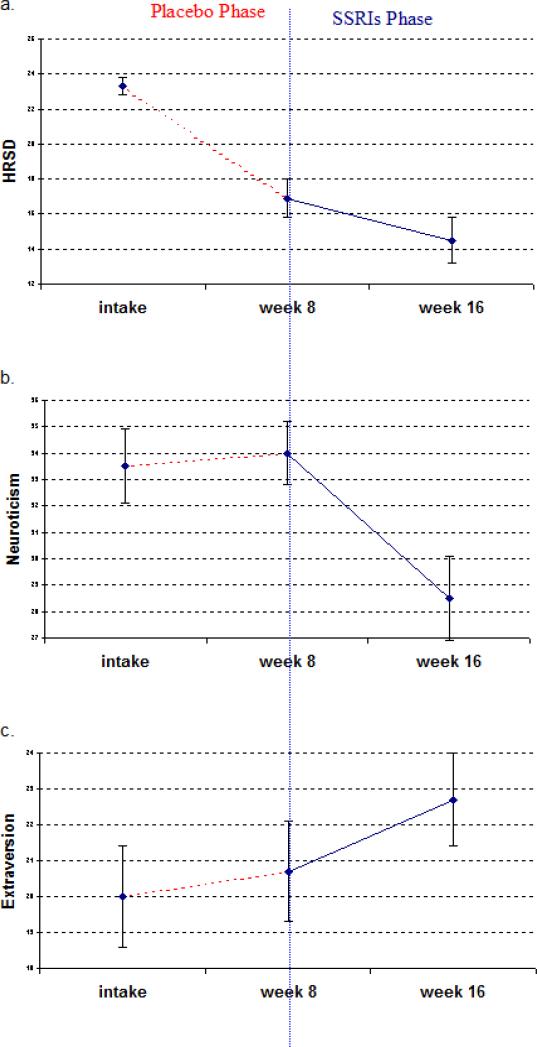
Figure 2 shows the pattern of change for the 31 placebo patients who opted for subsequent SSRI treatment after week 8. Their HRSD score decreased by an average of 6.4 points during the placebo phase (from intake to week 8), compared to 2.4 points during the subsequent SSRIs phase (from week 8 to week 16; see Figure 2a). A repeated-measure ANOVA confirmed that HRSD changed significantly more during the placebo phase than during the subsequent SSRI phase: F(2,28)=9.0, p=.005.
Figure 2. Time courses of depression, neuroticism, and extraversion for the placebo patients who opted for subsequent SSRI treatment after week 8.
The figure is based on the 31 placebo patients who took inert-placebo from intake to week 8 and then opted for SSRI treatment from week 8 to week 16. Error bars reflect standard errors.
Based on this pattern of depression improvement, the state-effect hypothesis predicts that personality measurements should change considerably more during the placebo phase than during the SSRI phase. However, the mean Neuroticism score of these patients did not decrease at all during the placebo phase (it increased slightly instead), but it decreased .66 SD during the subsequent SSRI phase (Figure 2b). Similarly, their mean Extraversion score increased only .12 SD during the placebo phase, but it increased .34 SD during the subsequent SSRI phase (Figure 2c). Repeated-measure ANOVA confirmed that Neuroticism and Extraversion changed less during the placebo phase than during the subsequent SSRI phase: Neuroticism, F(2,28)=11.5, p=.002; Extraversion, F(2,28)=4.2, p=.03.
Long-term Clinical Consequence
Among the 69 paroxetine responders, extraversion improvement during acute treatment did not predict relapse rates, X2(1)=.27, p=.60. However, greater neuroticism reduction predicted significantly lower likelihood of relapse, X2(1)=8.6, p=.003. This relationship remained statistically significant even after accounting for pre-treatment and end-of-treatment HRSD, pre-treatment neuroticism, and whether the responder was assigned to active medication or placebo during the continuation phase, X2(1)=12, p=.0006. (While medication continuation vs. withdrawn to placebo predicted lower relapse rates47, it did not interact with neuroticism improvement in predicting relapse, X2(1)=.025, p=.87.) To better understand the strength of this relationship between neuroticism improvement and relapse, we divided the paroxetine responders into three subgroups based on the magnitude of their neuroticism reduction: top third, neuroticism reduction ≥ 15 points; middle third, 9 points < neuroticism reduction < 15 points; and bottom third, neuroticism reduction ≤ 9 points. The relapse rate was 35% for the top third (n=23), 54% for the middle third (n=23), and 84% for the bottom third (n=23).
Among the 35 CT responders, personality change during treatment did not predict relapse rates: neuroticism, X2(1)=.028, p=.86; extraversion, X2(1)=.12, p=.73.
Comment
To the best of our knowledge, these are the first findings from a randomized placebo-controlled trial to suggest that an SSRI treatment of MDD produces greater changes in neuroticism and extraversion than an inert-placebo. In other words, paroxetine demonstrated a “true” drug effect on neuroticism and extraversion scores, reflecting pharmacological specificity.
The state-effect hypothesis predicts that drug-placebo differences in reported personality change should largely disappear after controlling for depression improvement. However, after accounting for depression improvement in regression analyses and in a procedure that matched paroxetine and placebo patients on depression improvement, paroxetine patients still reported far greater personality change than did the placebo patients. This pattern of findings should not obtain if personality change conformed to the state-effect hypothesis.
The observed course of change in patients who, in sequence, were given placebo for eight weeks, followed by eight weeks of SSRIs would, likewise, not be predicted by the state-effect hypothesis. Depression reduction was substantial during the placebo phase, and was much less so during the paroxetine phase. Personality change evidenced the opposite pattern. Perhaps the most surprising pattern we observed was the relation between neuroticism reduction during acute SSRI treatment and subsequent resistance to relapse. This finding, too, is not in line with the state-effect hypothesis.
The Cause-correction Model
If the state-effect hypothesis (Figure 3a) proves incorrect in future research, then what alternatives should be considered? One possibility is that the biochemical properties of SSRIs directly produce real personality change. Furthermore, because neuroticism is an important risk factor that captures much of the genetic vulnerability for MDD, change in neuroticism (and in neurobiological factors underlying neuroticism) might have contributed to depression improvement. Indeed, our regression analyses suggest that personality change can explain the advantage of paroxetine over placebo in antidepressant efficacy, rather than vice versa.
Figure 3. The state-effect hypothesis and two alternative hypotheses.
Depression etiology research has not determined whether neuroticism and extraversion are causes of depression or risk factors reflecting other underlying causes of depression. The cause-correction hypothesis retains this ambiguity in that changes in neuroticism/extraversion and changes in factors underlying neuroticism/extraversion might both contribute to depression improvement.
These possibilities can be integrated into the cause-correction model (Figure 3b): SSRI treatment produces changes in neuroticism/extraversion and the neurobiological factors underlying them; these changes then contribute to depression improvement. This model could be directly tested by tracking a large sample of pre-morbid subjects, waiting for them to develop MDD, and then treating them in a SSRIs clinical trial. How much SSRI treatment changes personality could then be measured by comparing the pre-morbid and post-treatment personality scores of responders in full remission. It would be challenging to obtain an adequate sample for such a study: a recent study found that only 262 subjects developed their first episode of MDD after tracking 4263 subjects for 2 years6.
Among responders to paroxetine, those for whom neuroticism changed the most during treatment were also those least likely to relapse. The predictive power of change in neuroticism was not accounted for by pre-treatment levels of neuroticism, or by post-treatment levels of depressive symptoms. It would appear, then, that change in neuroticism – or in a phenomenon related to self-reported neuroticism – served to reduce vulnerability to relapse. This explanation of the relapse prediction finding is consistent with the cause-correction model. In this model, greater neuroticism reduction reflects more correction of the personality risk factors of depression, which then reduces the risk for relapse. From this perspective, the paroxetine responders who recovered without experiencing much neuroticism reduction resembled the placebo patients in that they might not have benefited much from the biochemical properties of SSRIs. The alternative explanation of the relapse prediction finding is that confounding variables might have influenced both neuroticism and relapse rates; as a result, patients with lower relapse risk also happened to show greater neuroticism improvement during treatment.
Conceptual Overlap
The state-effect hypothesis exemplifies one causal interpretation of the correlation between depression and personality: depression improvement directly causes personality change. The cause-correction model integrates the two other possible causal interpretations, which in the context of our findings would be: (1) reverse causation: personality change caused depression improvement, and (2) third variable causation: changes in underlying neurobiological factors caused both depression improvement and personality change. However, statistical associations that do not reflect causal relations might also exist among these variables. Depression, neuroticism, and extraversion probably overlap conceptually, which could lead to apparent correlations. For example, if SSRIs primarily change depression, but the definitions of depression and neuroticism overlap extensively, then neuroticism also would appear to change (Figure 3c). Nevertheless, such a conceptual-overlap hypothesis cannot explain the dissociations we observed between depression change and neuroticism change in respect to paroxetine and placebo.
CT and Personality
This study is also the first clinical trial to show that CT can produce significantly greater change in neuroticism and extraversion than inert-placebo. However, it remains unclear whether this effect reflects personality change, change in state effect, or measurement artifact related to cognitive therapists’ explicit efforts to address thoughts and behaviors related to high neuroticism and low extraversion.
CT did not produce greater change in neuroticism than pill-placebo after controlling for change in depression, and neuroticism improvement during CT did not predict subsequent relapse, as it did among paroxetine responders. These findings suggest that the nature of reported neuroticism reduction in CT might differ from that in SSRI treatment, and that it might be more closely associated with depression improvement. The fact that rates of subsequent relapse were relatively low in CT regardless of the amount of change in neuroticism suggests that it works through mechanisms other than neuroticism to produce its enduring effect47.
On extraversion, CT outperformed placebo even after controlling for depression improvement. This suggests that extraversion improvement in CT may be independent of depression improvement, which contradicts the state-effect hypothesis (and the conceptual-overlap hypothesis). CT showed no significant differences with paroxetine on extraversion, even after controlling for depression. However, the lack of difference between two active treatments on a process variable is often uninformative in testing mechanism models52. The lack of difference between CT and paroxetine on extraversion can be consistent with the state-effect hypothesis, the conceptual-overlap hypothesis, and even the cause-correction hypothesis. For example, if both CT and paroxetine first change extraversion, which then cause depression to improve (i.e. the cause-correction model), then the two treatments may still appear similar on extraversion improvement. (Presumably, paroxetine and CT would change extraversion via distinct pathways53.)
Limitations
The present findings need to be replicated with paroxetine, with other SSRIs, and with other antidepressant medications, to determine whether the effects we observed are reliable, and to determine whether they are limited to the medication or medication class that we tested18. In previous research on the effects of antidepressant medications, other temperament variables such as harm avoidance, novelty seeking, and reward dependence54, have been used. It remains unclear how these variables relate to the personality dimensions of the FFM. In addition, because this clinical trial measured personality with the more abbreviated NEO-FFI, rather than the Revised NEO Personality Inventory, we could not distinguish the different facets of neuroticism and extraversion3. We also did not investigate which underlying neurobiological system is involved in the cause-correction model. While the serotonin system is a natural candidate, other possibilities should be carefully considered in future research as well. Furthermore, although our main effects analyses were obtained in the context of a placebo-controlled randomized clinical trial, our secondary analyses were non-experimental and may therefore be open to alternative interpretations. Finally, there were a number of dropouts in this trial, and the sample sizes for some analyses were modest.
Implications
In this study, MDD patients reported substantial personality change during SSRIs treatment; such personality change was not dependent upon depression improvement; and it might have contributed to acute and long-term treatment outcomes. Although SSRIs are the most widely used treatment of MDD, our understanding of their mechanisms remains limited55. SSRIs are also effective in treating several anxiety disorders and eating disorders56, 57, and recent studies have suggested that high neuroticism and low extraversion may be general risk factors for these disorders as well5, 58, 59. Investigating how SSRIs impact neuroticism and extraversion may thus lead towards a more parsimonious understanding of the mechanisms of SSRIs.
Acknowledgement
The dataset of this study came from a clinical trial supported by grants MH50129 (R10), MH55875 (R10), and MH01697 (K02) from the National Institute of Mental Health, Bethesda, MD. GlaxoSmithKline of Brentford, Middlesex, United Kingdom, provided medications and pill placebos. The corresponding author, who is independent of any commercial funder, performed the statistical analysis for this study; he had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
Tony Z. Tang, Northwestern University.
Robert J. DeRubeis, University of Pennsylvania.
Steven D. Hollon, Vanderbilt University.
Jay Amsterdam, University of Pennsylvania.
Richard Shelton, Vanderbilt University.
Benjamin Schalet, Northwestern University.
References
- 1.Digman JM. Personality structure: Emergence of the five-factor model. Annu Rev Psychol. 1990;41:417–440. [Google Scholar]
- 2.McCrae RR, Costa PT. Personality trait structure as a human universal. Am Psychol. 1997;52(5):509–516. doi: 10.1037//0003-066x.52.5.509. [DOI] [PubMed] [Google Scholar]
- 3.Costa PT, McCrae RR. Revised NEO Personality Inventory and NEO Five-Factor Inventory: Professional Manual. Psychological Assessment Resources, Inc.; Lutz, Florida: 1992. [Google Scholar]
- 4.McCrae RR, Costa PT. A contemplated revision of the NEO Five-Factor Inventory. Personality and Individual Differences. 2004;36:587–596. [Google Scholar]
- 5.Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. J Abnorm Psychol. 1994 Feb;103(1):103–116. [PubMed] [Google Scholar]
- 6.Ormel J, Oldehinkel AJ, Vollebergh W. Vulnerability before, during, and after a major depressive episode: a 3-wave population-based study. Arch Gen Psychiatry. 2004 Oct;61(10):990–996. doi: 10.1001/archpsyc.61.10.990. [DOI] [PubMed] [Google Scholar]
- 7.Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry. 2004 Apr;161(4):631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- 8.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of personality and major depression in women. Arch Gen Psychiatry. 1993 Nov;50(11):853–862. doi: 10.1001/archpsyc.1993.01820230023002. [DOI] [PubMed] [Google Scholar]
- 9.Krueger RF, Caspi A, Moffitt TE, Silva PA, McGee R. Personality traits are differentially linked to mental disorders: a multitrait-multidiagnosis study of an adolescent birth cohort. J Abnorm Psychol. 1996 Aug;105(3):299–312. doi: 10.1037//0021-843x.105.3.299. [DOI] [PubMed] [Google Scholar]
- 10.Kendler KS, Gatz M, Gardner CO, Pedersen NL. Personality and major depression: a Swedish longitudinal, population-based twin study. Arch Gen Psychiatry. 2006 Oct;63(10):1113–1120. doi: 10.1001/archpsyc.63.10.1113. [DOI] [PubMed] [Google Scholar]
- 11.Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996 Nov;53(11):1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- 12.Hirschfeld RM, Klerman GL, Lavori P, Keller MB, Griffith P, Coryell W. Premorbid personality assessments of first onset of major depression. Arch Gen Psychiatry. 1989 Apr;46(4):345–350. doi: 10.1001/archpsyc.1989.01810040051008. [DOI] [PubMed] [Google Scholar]
- 13.Tellegen A, Lykken DT, Bouchard TJ, Wilcox KJ, Segal NL, Rich S. Personality similarity in twins reared apart and together. J Pers Soc Psychol. 1988;54:1031–1039. doi: 10.1037//0022-3514.54.6.1031. [DOI] [PubMed] [Google Scholar]
- 14.Jardine R, Martin NG, Henderson AS. Genetic covariation between neuroticism and the symptoms of anxiety and depression. Genet Epidemiol. 1984;1(2):89–107. doi: 10.1002/gepi.1370010202. [DOI] [PubMed] [Google Scholar]
- 15.Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ. The structure of the genetic and environmental risk factors for six major psychiatric disorders in women. Phobia, generalized anxiety disorder, panic disorder, bulimia, major depression, and alcoholism. Arch Gen Psychiatry. 1995 May;52(5):374–383. doi: 10.1001/archpsyc.1995.03950170048007. [DOI] [PubMed] [Google Scholar]
- 16.Kendler KS, Gardner CO, Gatz M, Pedersen NL. The sources of co-morbidity between major depression and generalized anxiety disorder in a Swedish national twin sample. Psychol Med. 2007 Mar;37(3):453–462. doi: 10.1017/S0033291706009135. [DOI] [PubMed] [Google Scholar]
- 17.Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS. A population-based twin study of the relationship between neuroticism and internalizing disorders. Am J Psychiatry. 2006 May;163(5):857–864. doi: 10.1176/ajp.2006.163.5.857. [DOI] [PubMed] [Google Scholar]
- 18.Bagby RM, Levitan RD, Kennedy SH, Levitt AJ, Joffe RT. Selective alteration of personality in response to noradrenergic and serotonergic antidepressant medication in depressed sample: evidence of non-specificity. Psychiatry Res. 1999 Jun 30;86(3):211–216. doi: 10.1016/s0165-1781(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 19.Ekselius L, von Knorring L. Changes in personality traits during treatment with sertraline or citalopram. Br J Psychiatry. 1999;174:444–448. doi: 10.1192/bjp.174.5.444. [DOI] [PubMed] [Google Scholar]
- 20.Brody AL, Saxena S, Fairbanks LA, et al. Personality changes in adult subjects with major depressive disorder or obsessive-compulsive disorder treated with paroxetine. J Clin Psychiatry. 2000;61:349–355. doi: 10.4088/jcp.v61n0505. [DOI] [PubMed] [Google Scholar]
- 21.Du L, Bakish D, Ravindran AV, Hrdina PD. Does fluoxetine influence major depression by modifying five-factor personality traits? J affect disord. 2002 Sep;71(13):235–241. doi: 10.1016/s0165-0327(01)00370-6. [DOI] [PubMed] [Google Scholar]
- 22.Santor DA, Bagby RM, Joffe RT. Evaluating stability and change in personality and depression. J Pers Soc Psychol. 1997 Dec;73(6):1354–1362. doi: 10.1037//0022-3514.73.6.1354. [DOI] [PubMed] [Google Scholar]
- 23.Kramer PD. Listening to Prozac. Viking; New York: 1993. [Google Scholar]
- 24.Agosti V, McGrath PJ. Comparison of the effects of fluoxetine, imipramine and placebo on personality in atypical depression. J Affect Disord. 2002;71:113–120. doi: 10.1016/s0165-0327(01)00393-7. [DOI] [PubMed] [Google Scholar]
- 25.Gracious KS. Do SSRIs affect personality traits? Br J Psychiatry. 1999;175:287. doi: 10.1192/bjp.175.3.287a. [DOI] [PubMed] [Google Scholar]
- 26.Marchevsky D. Selective serotonin reuptake inhibitors and personality change. Br J Psychiatry. 1999;175:589–590. doi: 10.1192/bjp.175.6.589b. [DOI] [PubMed] [Google Scholar]
- 27.Tse WS. Serotonergic involvement in the psychosocial dimension of personality. J Psychopharmacol. 2001;15:195–198. doi: 10.1177/026988110101500313. [DOI] [PubMed] [Google Scholar]
- 28.Knutson B, Wolkowitz OM, Cole SW, et al. Selective alteration of personality and social behavior by serotonergic intervention. Am J Psychiatry. 1998;155:373–379. doi: 10.1176/ajp.155.3.373. [DOI] [PubMed] [Google Scholar]
- 29.Melke J, Westberg L, Nilsson S, et al. A polymorphism in the serotonin receptor 3A (HTR3A) gene and its association with harm avoidance in women. Arch Gen Psychiatry. 2003;60:1017–1023. doi: 10.1001/archpsyc.60.10.1017. [DOI] [PubMed] [Google Scholar]
- 30.Strobel A, Gutknecht L, Rothe C, et al. Allelic variation in 5-HT1A receptor expression is associated with anxiety- and depression-related personality traits. J Neural Transm. 2003 Dec;110(12):1445–1453. doi: 10.1007/s00702-003-0072-0. [DOI] [PubMed] [Google Scholar]
- 31.Tauscher J, Bagby RM, Javanmard M, Christensen BK, Kasper S, Kapur S. Inverse relationship between serotonin 5-HT(1A) receptor binding and anxiety: a [(11)C]WAY-100635 PET investigation in healthy volunteers. Am J Psychiatry. 2001 Aug;158(8):1326–1328. doi: 10.1176/appi.ajp.158.8.1326. [DOI] [PubMed] [Google Scholar]
- 32.Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet B Neuropsychiatr Genet. 2004 May 15;127(1):85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- 33.Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- 34.Young SN, Leyton M. The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacol Biochem Behav. 2002 Apr;71(4):857–865. doi: 10.1016/s0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 35.Huber R, Panksepp JB, Yue Z, Delago A, Moore P. Dynamic interactions of behavior and amine neurochemistry in acquisition and maintenance of social rank in crayfish. Brain Behav Evol. 2001 May;57(5):271–282. doi: 10.1159/000047245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards DH, Kravitz EA. Serotonin, social status and aggression. Curr Opin Neurobiol. 1997 Dec;7(6):812–819. doi: 10.1016/s0959-4388(97)80140-7. [DOI] [PubMed] [Google Scholar]
- 37.DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005 Apr;62(4):409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- 38.Jarrett RB, Schaffer M, McIntire D, Witt-Browder A, Kraft D, Risser RC. Treatment of atypical depression with cognitive therapy or phenelzine: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999 May;56(5):431–437. doi: 10.1001/archpsyc.56.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeRubeis RJ, Gelfand LA, Tang TZ, Simons AD. Medications versus cognitive behavior therapy for severely depressed outpatients: mega-analysis of four randomized comparisons. Am J Psychiatry. 1999 Jul;156(7):1007–1013. doi: 10.1176/ajp.156.7.1007. [DOI] [PubMed] [Google Scholar]
- 40.Quilty LC, De Fruyt F, Rolland JP, Kennedy SH, Rouillon PF, Bagby RM. Dimensional personality traits and treatment outcome in patients with major depressive disorder. J Affect Disord. 2008 Jun;108(3):241–250. doi: 10.1016/j.jad.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 41.Bagby RM, Quilty LC, Segal ZV, McBride CC, Kennedy SH, Costa PT. Personality and differential treatment response in major depression: a randomized controlled trial comparing cognitive-behavioural therapy and pharmacotherapy. Can J Psychiatry. 2008 Jun;53(6):361–370. doi: 10.1177/070674370805300605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988 Aug;45(8):742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 43.Khan A, Warner HA, Brown WA. Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials: An analysis of the Food and Drug Administration database. Arch Gen Psychiatry. 2000;57:311–317. doi: 10.1001/archpsyc.57.4.311. [DOI] [PubMed] [Google Scholar]
- 44.Greenberg RP, Bornstein R, Zborowski MJ, Fisher S, Greenberg ME. A meta-analysis of fluoxetine outcome in the treatment of depression. Journal of Nervous and Mental Disorders. 1994;182:547–551. doi: 10.1097/00005053-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Kirsch I, Moore TJ, Scoboria A, Nicholls SS. The emperor's new drugs: An analysis of antidepressant medication data submitted to the U.S. Food and Drug Administration. Prevention & Treatment. 2002:5. [Google Scholar]
- 46.Kirsch I, Sapirstein G. Listening to Prozac but hearing placebo: A meta-analysis of antidepressant medication. Prevention & Treatment. 1998:1. [Google Scholar]
- 47.Hollon SD, DeRubeis RJ, Shelton RC, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005 Apr;62(4):417–422. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- 48.American Psychological Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition ed. American Psychological Association; Washington, DC: 1994. [Google Scholar]
- 49.Keller MB, Lavori PW, Friedman B, et al. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987 Jun;44(6):540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 50.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- 51.Cox DR, Oakes D. Analysis of Survival Data. Chapman & Hall; London, England: 1984. [Google Scholar]
- 52.Hollon SD, DeRubeis RJ, Evans MD. Causal mediation of change in treatment for depression: discriminating between nonspecificity and noncausality. Psychol Bull. 1987 Jul;102(1):139–149. [PubMed] [Google Scholar]
- 53.Goldapple K, Segal Z, Garson C, et al. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004 Jan;61(1):34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- 54.Hellerstein DJ, Kocsis JH, Chapman D, Stewart JW, Harrison W. Double-Blind Comparison of Sertraline, Imipramine, and Placebo in the Treatment of Dysthymia: Effects on Personality. Am J Psychiatry. 2000;157:1426–1444. doi: 10.1176/appi.ajp.157.9.1436. [DOI] [PubMed] [Google Scholar]
- 55.American Psychiatric Association Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000 Apr;157(4)(Suppl):1–45. [PubMed] [Google Scholar]
- 56.White KS, Barlow DH. Panic disorder and agoraphobia. In: Barlow DH, editor. Anxiety and its disorders. 2nd ed. Guilford; New York: 2002. pp. 328–379. [Google Scholar]
- 57.Walsh BT. Pharmacological treatment of anorexia nervosa and bulimia nervosa. In: Fairburn CG, Brownell KD, editors. Eating disorders and obesity: A comprehensive handbook. 2nd ed. Guilford; New York: 2002. pp. 325–329. [Google Scholar]
- 58.Jorm AF, Christensen H, Henderson AS, Jacomb PA, Korten AE, Rodgers B. Predicting anxiety and depression from personality: is there a synergistic effect of neuroticism and extraversion? J Abnorm Psychol. 2000 Feb;109(1):145–149. doi: 10.1037//0021-843x.109.1.145. [DOI] [PubMed] [Google Scholar]
- 59.Wade TD, Bulik CM, Prescott CA, Kendler KS. Sex influences on shared risk factors for bulimia nervosa and other psychiatric disorders. Arch Gen Psychiatry. 2004 Mar;61(3):251–256. doi: 10.1001/archpsyc.61.3.251. [DOI] [PubMed] [Google Scholar]