Abstract
BACKGROUND:
Hospitalized infants undergo multiple, repeated painful procedures. Despite continued efforts to prevent procedural pain and improve pain management, clinical guidelines and standards frequently do not reflect the highest quality evidence from systematic reviews.
OBJECTIVE:
To critically appraise all systematic reviews on the effectiveness of procedural pain interventions in hospitalized infants.
METHODS:
A structured review was conducted on published systematic reviews and meta-analyses of pharmacological and nonpharmacological interventions of acute procedural pain in hospitalized infants. Searches were completed in the Cochrane Database of Systematic Reviews, MEDLINE, EMBASE, CINAHL and PsycINFO. Two reviewers independently selected articles for review and rated the methodological quality of the included reviews using a validated seven-point quality assessment measure. Any discrepancies were resolved by a third reviewer.
RESULTS:
Of 1469 potential systematic reviews on interventions for painful procedures in hospitalized infants, 11 high-quality reviews were included in the analysis. Pharmacological interventions supported by research evidence included premedication for intubation, dorsal penile nerve block and EMLA (AstraZeneca Canada, Inc) for circumcision, and sucrose for single painful procedures. Non-nutritive sucking, swaddling, holding, touching, positioning, facilitative tucking, breast feeding and supplemental breast milk were nonpharmacological interventions supported for procedural pain.
CONCLUSION:
There is a growing number of high-quality reviews supporting procedural pain management in infants. Ongoing research of single, repeated and combined pharmacological and nonpharmacological interventions is required to provide the highest quality evidence to clinicians for decision-making on optimal pain management.
Keywords: Acute pain, Infants, Pain management, Systematic review
Abstract
HISTORIQUE :
Les nourrissons hospitalisés subissent de nombreuses interventions douloureuses à répétition. Malgré les efforts constants pour prévenir la douleur associée à ces interventions et améliorer le traitement de la douleur, souvent, les directives cliniques et les normes ne reflètent pas les preuves de la plus haute qualité provenant des analyses systématiques.
OBJECTIF :
Évaluer de façon critique toutes les revues systématiques ayant porté sur l’efficacité des interventions pour soulager les douleurs associées aux traitements chez les nourrissons hospitalisés.
MÉTHODE :
Les auteurs ont procédé à une analyse structurée des revues systématiques et des méta-analyses publiées sur les interventions pharmacologiques et non pharmacologiques pour soulager la douleur aiguë associée aux traitements chez les nourrissons hospitalisés. La base de données Cochrane des revues systématiques, et les bases de données MEDLINE, EMBASE, CINAHL et PsycINFO ont été interogées. Deux examinateurs ont sélectionné les articles séparément en vue de l’analyse et ont coté la qualité méthodologique des revues recensées à l’aide d’un barème validé d’évaluation de la qualité en sept points. Le cas échéant, les discordances étaient tranchées par un troisième examinateur.
RÉSULTATS :
Parmi les 1 469 revues systématiques potentielles sur des interventions analgésiques lors de traitements douloureux chez des nourrissons hospitalisés, 11 revues de grande qualité ont été incluses dans l’analyse. Parmi les interventions pharmacologiques appuyées par des résultats de recherches, mentionnons la prémédication avant l’intubation, le bloc nerveux pénien dorsal et l’application d’EMLA (AstraZeneca Canada, Inc.) pour la circoncision et le sucrose pour les interventions douloureuses simples. Parmi les interventions non pharmacologiques utilisées pour contribuer à soulager les douleurs liées aux traitements, mentionnons : leur donner une suce, les bercer, les tenir, les toucher, les positionner, les envelopper, les allaiter ou leur administrer un supplément de lait maternel.
CONCLUSION :
Un nombre croissant d’analyses de grande qualité appuie le traitement de la douleur en cours d’intervention chez les nourrissons. Il faut poursuivre la recherche sur des interventions pharmacologiques et non pharmacologiques simples et répétées ou concomitantes pour fournir des preuves de la meilleure qualité possible aux médecins qui ont à prendre des décisions pour la prise en charge optimale de la douleur.
Hospitalized neonates undergo an average of 10 to 14 painful procedures per day (1,2), with as many as 53 procedures being reported during the first two weeks of life (1). Early exposure to repeated painful events can alter pain processing and perception at the spinal and supraspinal levels (3). Most recently, response to pain at the cortical level in the neonate’s developing pain system has been described (4,5). Stress during this critical period in development has immediate and long-term consequences that can influence physiological, social and cognitive outcomes (6). Furthermore, infants exposed to repeated heel lances early in life may become conditioned to pain, experience higher pain intensities during future painful events (7), and be predisposed to persistent or chronic pain states (8,9).
Pediatric pain guidelines, accreditation standards and policy statements have been developed for assessing and managing acute pain in infants (10–12). The Joint Commission on Accreditation of Healthcare Organizations (13) and Accreditation Canada (14) developed organizational standards for infant pain assessment and management. Consensus statements by the American Academy of Pediatrics and the Canadian Paediatric Society on prevention and management of infant pain (15,16) highlight the importance of assessing pain and providing the appropriate pharmacological, physical, behavioural and environmental interventions to manage pain in infants (17). Most recently, recommendations from the Neonatal Pain Control Group, led by Anand et al (18), have called for improvements in the education of health care professionals to enable the use of the latest evidence on pain management interventions to improve clinical and health outcomes. Pain guidelines and position statements continue to be developed nationally and internationally, and across health disciplines; some examples are the National Association of Neonatal Nurses position statement on pain management in infants (19) and the Royal Australasian College of Physicians guideline statement (20) on managing procedural pain in neonates. Despite these efforts, there has been no significant global improvement in pain management in infants (1,9,12,21); it remains suboptimal.
Results from systematic reviews have been incorporated into infant pain guidelines and policies (eg, sucrose for procedural pain [22]). However, the frequency of effective use of this practice remains unknown. Although comprehensive literature reviews and summaries on infant pain management strategies (23), and systematic reviews of the literature (22) exist, there are no rigorous evaluations of systematic reviews using validated quality assessment measures. Therefore, the purpose of the present review is to critically appraise high-quality systematic reviews on acute pain management in hospitalized infants, using a validated quality assessment evaluation measure. This appraisal will provide a structured and comprehensive synthesis of the nature and scope of published scientific evidence. The ultimate goal is to provide practitioners with ready access to high-quality evidence for clinical decision-making in the prevention or minimization of acute pain in infants.
METHODS
Data sources
Electronic searches were conducted by Librarian Information Specialists (EU, TAW) in The Cochrane Database of Systematic Reviews, MEDLINE (1966 to May 2006), EMBASE (1980 to May 2006), CINAHL (1982 to May 2006) and PsycINFO (1985 to 2006). Subject headings and MeSH terms included ‘pain’, ‘pain measurement’ and ‘pain assessment’. Key words and abbreviations used included ‘infant:’, ‘bab’, ‘baby’, ‘babies’, ‘neonat:’, ‘newborn:’, ‘premature:’, ‘preemie:’, ‘pediatric’, ‘paediatric’ and ‘child:’. Other keywords, such as ‘meta analysis’, ‘systematic review:’ and ‘system review’, were also used to search for the ideal publication type. Reference lists from retrieved reviews were screened for additional systematic reviews. All search titles and abstracts were independently rated for relevance by two trained research assistants (JL, AD). To establish inter-rater reliability of accurate eligibility selection, each reviewer pilot-tested 10 review articles using the selection criteria. There was 97% agreement on the selected review articles.
Study selection
Multiple systematic reviews exist on pain in hospitalized children from birth to 18 years of age. For the present review, the selection criteria were narrowed to infants from birth to 12 months of age who were undergoing acute procedural pain. Only published systematic reviews in English were included. Study designs within the relevant systematic reviews included randomized controlled trials (RCTs) and quasi-RCTs.
Data extraction
Although measures have been developed to enhance the quality of reporting meta-analyses for both observational studies (24) and RCTs (25), these measures are not designed or validated for rating the methodological quality of systematic reviews. A validated rating tool developed by Oxman and Guyatt (26) was selected to evaluate the methodological and scientific quality of the systematic reviews included in the present overview. The tool rates systematic reviews on a seven-point scale, where a score of 1 (lowest) signifies extensive methodological flaws and a score of 7 (highest) is indicative of minimal flaws (26,27). The overall scientific quality of the systematic reviews is based on the scoring of 10 items – two items related to the quality of the search methods, one item on inclusion criteria of the studies included in the review, one item assessing the avoidance of bias, two items on the methodological validity of the included studies, two items addressing the methods used to combine studies, one item on the conclusions stated in the reviews and an overall item evaluating the rating of the scientific quality of the reviews (26–28). Before rating the reviews, the quality assessment measure was pilot tested on 10 systematic reviews independently rated by two authors (JY, JS) using the quality assessment measure. There was a 92% agreement between the two reviewers. Any disagreements in ratings were resolved by a third reviewer for both relevance and quality testing (BS). Two raters (JY and JL) independently extracted information from the papers on year of publication, study design, participants, study focus (ie, type of pain intervention) and main results of the reviews, which included results from a meta-analysis when possible, or qualitative reports of results.
Data synthesis
When available, effects were reported in terms of mean effect size, weighted effect size, mean difference (MD), standardized MD and weighted MD. If a meta-analysis had been performed, the effect’s significance or nonsignificance was recorded. If quantitative summary measures of effectiveness were not used, the range of effects across studies was reported. If this information was not available, the author’s main conclusions were reported.
RESULTS
Description of studies
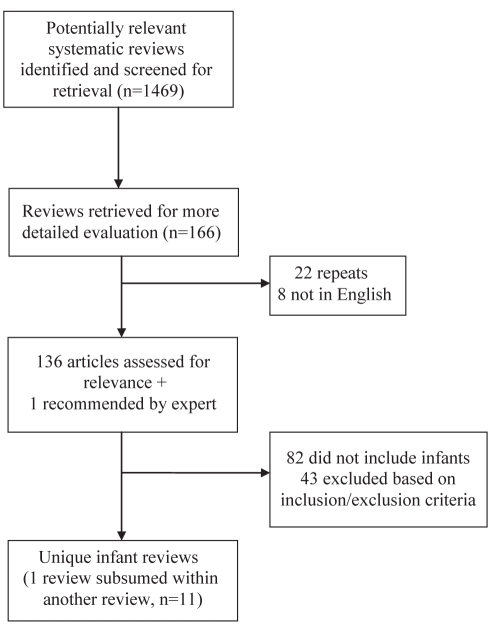
A total of 1469 articles were retrieved from the electronic searches. Of these, 166 articles were selected for further consideration. Thirty articles were removed after accounting for duplicates and languages other than English. After retrieving and reviewing the full text of the remaining 136 articles, 82 articles involving children older than one year of age were excluded from the present review. One additional article that was accepted for publication was recommended by a key informant. From the 55 remaining articles, a further 41 reviews were not systematic and were therefore excluded, leaving 14 articles for evaluation. Results of one review (29) were subsumed within a larger review (30) of the same topic; both articles were counted as one review. A review that compared venipuncture with heel lance, and another review that assessed the use of midazolam for sedation were excluded because their focus was not on pain-relieving strategies. Therefore, a total of 11 unique systematic reviews were included in the present overview of reviews (Figure 1) (22,29–39). Five of these articles, plus the article subsumed into the larger study (29), were Cochrane Systematic Reviews (22,31–33,37), while the remaining six (30,34–36,38,39) were published in a variety of peer-reviewed journals. A summary of the 11 reviews describing the number of studies included in each review, the total quality scores using the validated quality assessment tool, the interventions and the main results is provided in Tables 1 and 2. Quantitative meta-analyses were used in seven of the reviews (22,29–32,36,37,39). The remaining four reviews (33–35,38) were qualitative systematic reviews, in which the results of primary studies were not statistically pooled. Where possible, effect sizes and weighted MDs are reported.
Figure 1).
Study selection process
TABLE 1.
Systematic reviews of pharmacological interventions
| Reference, number of studies | Quality score | Focus | Main results |
|---|---|---|---|
| Bellù et al (31), 2005, n=13 | 7 | Opioids for mechanical ventilation | Opioids resulted in reduced PIPP scores compared with the control group (WMD = −1.71, 95% CI −3.18 to −0.24) but the reduction was not deemed to be clinically significant; morphine in very preterm infants delayed time to reach full enteral feedings compared with control group (WMD = 2.10 days, 95% CI 0.35 to 3.85); opioid group showed no statistically significant differences in mortality, duration of mechanical ventilation, and long-and short-term neurodevelopmental outcomes compared with control groups |
| Brady-Fryer et al (32), 2004, n=35 | 7 | Dorsal penile nerve block (DPNB), EMLA* and sucrose for reducing pain in neonatal circumcision | DPNB resulted in significantly lower HR (WMD = 35 beats/min; 95% CI −41 to −30), decreased crying time (WMD = 54%; 95% CI −64 to −44) and increased SpO2 (WMD = 3.7%; 95% CI 2.7 to 3.7) compared with placebo; DPNB resulted in significantly lower HR (WMD = −17 beats/min, 95% CI −23 to −11) and lower pain scores compared with EMLA*; DPNB resulted in a significantly decreased crying time (MD = −166 s, 95% CI −211 to −121) and lower HR (WMD = −27 beats/min, 95% CI −33 to −20) compared with sucrose; EMLA* resulted in lower facial action scores (WMD = −46.5, 95% CI −80.4 to −12.6), decreased time crying (WMD = −15.2%, 95% CI −21 to −9.3) and a lower HR (WMD = −15 beats/min; 95% CI −19 to −10) compared with placebo; minor bleeding, swelling and hematoma with DPNB; erythema and mild skin pallor with EMLA*; reported methemoglobin levels were within normal limits for the EMLA* group |
| Shah and Ohlsson (38), 2002, n=9 | 6 | Premedication for endotracheal intubation | 4/9 studies had evidence that premedication reduced physiological indicators of pain/distress; 4/9 studies reported significant adverse effects associated with premedication; 2/9 studies reported that premedication reduced the duration of the intubation; adverse event of chest wall rigidity was associated with fentanyl |
| Stevens et al (22), 2004, n=21 | 7 | Sucrose for pain from heel lance | Sucrose significantly reduced PIPP scores compared with control group at 30 s (WMD = −1.64, 95% CI −2.47 to −0.81) and 60 s (WMD = −2.05, CI −3.08 to −1.02) after heel lance; 6 studies reported adverse events. One of these studies reported that both the placebo and sucrose; groups had decreased SpO2 compared with the control group; saturation levels recovered spontaneously |
| Taddio et al (30), 1998, n=11 (procedural pain, including 3 circumcision papers below) | 6 | Lidocaine-prilocaine cream (EMLA*) for acute procedural pain | EMLA* group had reduced crying times, reduced facial grimacing and a lower HR (WMD = −12 to −27 beats/min) for circumcision compared with placebo; inconclusive evidence for EMLA* for venipuncture, arterial puncture and percutaneous venous catheter placement |
| Taddio et al (29), 2000, n=3 (circumcision) | EMLA* (single dose) ineffective for heel lance pain and lumbar puncture (no significant difference between control and EMLA* groups); methemoglobin levels not different between EMLA* and placebo-treated infants (WMD = −0.11%; 95% CI −0.31 to 0.10) |
AstraZeneca Canada, Inc. HR Heart rate; MD Mean difference; PIPP Premature Infant pain Profile; SpO2 Oxygen saturation; WMD Weighted mean difference
TABLE 2.
Systematic reviews of nonpharmacological interventions
| Reference, number of studies | Quality score | Focus | Main results |
|---|---|---|---|
| Cepeda et al (33), 2006, n=4 | 7 | Music for pain relief for circumcision and heel lance pain | Music reduced pain scores compared with control in 2 studies; music had no effect on pain scores compared with control in 2 studies; none of the papers reported adverse effects of music for infants |
| Cignacco et al (34), 2007, n=15 | 6 | Nonpharmacological interventions for procedural pain | NNS, swaddling and facilitated tucking have positive effects on behavioural and/or physiological indicators of pain; inconclusive evidence of the effects of music, positioning, olfactory and multisensorial stimulation, kangaroo care, and maternal touch on pain; none of the papers reported adverse effects of the nonpharmacological interventions reviewed |
| Pinelli et al (35), 2002, n=4 | 5 | NNS in high-risk infants for procedural pain | Inconclusive evidence of the effectiveness of NNS for procedural pain (heel lance and intravenous insertion) compared with control in 3 studies; pacifiers with sucrose or water significantly reduced PIPP scores for heel lance compared with control in one study; inconclusive evidence on the adverse effects of NNS |
| Prasopkittikun and Tilokskulchai (36), 2003, n=4 | 6 | Nonpharmacological interventions for heel lance | Inconsistent patterns of effect sizes for changes in SpO2 and HR for all interventions (maternal holding and touching, swaddling, and positioning); holding and touching (MES = 0.73, 95% CI 0.41 to 1.04), and swaddling (MES = 0.79, 95% CI 0.53 to 1.05) reduced pain scores compared with control in full-term infants; swaddling (MES = 0.53, 95% CI 0.27 to 0.80) and positioning (MES = 0.64, 95% CI 0.51 to 0.77) reduced pain scores compared with control in preterm infants; the beneficial effect of positioning in preterm newborns persisted after the heel lance, while the beneficial effects of the other interventions decreased after the heel lance; none of the papers reported adverse effects of the nonpharmacological interventions reviewed |
| Shah et al (37), 2006, n=11 | 7 | Breastfeeding or supplemental breast milk to reduce procedural pain | Breastfed group had a lower increase in HR compared with pacifier group and positioned group (for both comparisons, MD = −23 beats/min, 95% CI −34.55 to −11.45); breastfed group had a significantly reduced percentage of time crying compared with pacifier group (MD = −32.6, 95% CI −49.83 to −15.37) and positioned group (MD = −39, 95% CI −55.03 to −22.97); breastfed group had significantly reduced cry duration compared with positioned group (MD = −63.30, 95% CI −74.54 to −52.06) and compared with fasting group (MD = −50.43, 95% CI −78.97 to −21.89); breastfed group had significantly lower PIPP scores compared with placebo group (MD = −5.95, 95% CI −7.42 to −4.48) and positioning in mother’s arms group (MD = −7, 95% CI −8.95 to −6.03), but breastfed group had a significantly higher PIPP score than the glucose group (WMD = 1.30, 95% CI 0.05 to 2.56); breastfed group had significantly lower DAN scores compared with placebo group (MD = −6.24, 95% CI −7.38 to −5.10) and group positioned in mother’s arms (MD = −6.77, 95% CI −7.78 to −5.76); DAN scores between breastfeeding and glucose groups were not significant; supplemental breast milk did not result in a significant change in HR, SpO2 or cry duration when compared with placebo; supplemental breast milk significantly increased duration of crying time (MD = 33.17, 95% CI 12.08 to 54.26) and HR (MD = 13.80, 95% CI 4.23 to 23.37) compared with 25% sucrose; inconclusive evidence for supplemental breast milk in reducing NFCS scores compared with placebo (2 studies had nonsignificant differences and 1 study reported that breast milk significantly reduced NFCS scores); none of the papers reported adverse effects of breastfeeding |
| Shaio et al (39), 1997, n=9 | 6 | NNS for need leinsertions or heel lances | NNS significantly reduced HR during painful stimuli (χ2=69.075, P=0.0001, WES = 1.05, 95% CI 0.60 to 1.50) and increased TcPaO2 (χ2=35.301, P=0.0001, WES = 0.69, 95% CI 0.27 to 1.12); longer duration of NNS resulted in greater effect sizes on reducing HR (2 min WES = 0.46, 95% CI −0.15 to 1.0; 5 min WES = 2.0, 95% CI 1.27 to 2.74) and increasing TcPaO2 (5 min WES =0.44, 95% CI −0.07 to 0.88; 8 min WES = 2.11, 95% CI 1.07 to 3.15); NNS had greater effects on increasing TcPaO2 in preterm infants (WES = 1.45, 95% CI 0.67 to 2.22) compared with term infants (WES = 0.39, 95% CI −0.13 to 0.91); none of the papers reported adverse effects of NNS |
DAN Douleur Aiguë Nouveau-né score; HR Heart rate; MD Mean difference; MES Mean effect size; NFCS Neonatal Facial Coding System; NNS Non-nutritive sucking; PIPP Premature Infant Pain Profile; SpO2 Oxygen saturation; TcPaO2 Transcutaneous arterial oxygen level; WES Weighted effect size; WMD Weighted mean difference
Methodological quality of relevant systematic reviews
Using a previously outlined scoring method (26–28), the mean score for the 11 reviews was 6.36/7.0 (SD 0.67). The minimum score was 5/7, and the maximum score was 7/7 (Table 1); the six Cochrane reviews scored 7/7. All 11 reviews were rated as having either minimal or minor flaws. Five of these 11 highly rated reviews evaluated pharmacological pain interventions (Table 1) and six evaluated nonpharmacological interventions (Table 2).
Pharmacological pain interventions
Bellù et al (31) assessed whether opioid analgesics were effective in reducing pain intensity for ventilated preterm and term infants in 13 RCTs. Although pain scores were significantly reduced in four studies using the validated Premature Infant Pain Profile (PIPP) scale (40), the authors did not consider the results to be clinically significant. Heterogeneity in the type and doses of opioids used, as well as in the outcomes and reporting of results prevented the authors from recommending opioids to reduce pain in mechanically ventilated newborns.
In 35 RCTs of preterm and term infants, Brady-Fryer et al (32) compared dorsal penile nerve block (DPNB) with placebo, EMLA (AstraZeneca Canada, Inc) and sucrose for pain during circumcision. DPNB demonstrated statistically and clinically significant reductions in heart rate compared with placebo, EMLA and sucrose. In addition, DPNB significantly decreased crying time compared with placebo and sucrose. Limitations of the studies included differences in the characteristics of the study participants, lack of double-blinding in almost one-half of the studies, variable wait times after the DPNB was administered, heterogeneity in pain interventions and differences in the reporting of outcomes across studies, limited use of validated pain scales and incomplete data reported in the studies. The authors concluded that DPNB and EMLA can be recommended over no treatment for attenuation of circumcision pain, with DPNB demonstrating greater effectiveness than EMLA. Although both DPNB and EMLA are considered safe to use in newborn infants, based on the limitations of the studies included in the review, the authors recommended that the results of the meta-analysis be interpreted with caution.
In their review of seven RCTs and two cohort studies of pre-medication for endotracheal intubation in preterm and term infants who were mechanically ventilated, Shah and Ohlsson (38) found that premedication of infants using anticholinergics, analgesics, anesthetics, muscle relaxants, sedatives and amnesic medications reduced individual physiological pain indicators and intubation times in some studies. The most common medications used in combination were atropine, fentanyl and succinylcholine. None of the studies used validated composite measures to assess pain. Further research was recommended to examine the safety and effectiveness of drugs as premedication for endotracheal intubation, and to evaluate pain using validated pain measures. The authors suggested pre-medication for intubation, because intubation while awake is not appropriate in infants.
Stevens et al (22) assessed whether sucrose was efficacious and effective in reducing procedural pain in hospitalized and preterm and term infants in a review of 21 RCTs. In three studies, there was a statistically and clinically significant reduction in physiological and behavioural indicators of pain and composite PIPP (40) pain scores. However, heterogeneity in study interventions and outcomes, and the lack of reported results in the primary studies prevented meta-analysis. The authors advised that sucrose may be used safely in doses ranging from 0.012 g to 0.12 g for single heel lances and venipunctures. The repeated use of sucrose was not included in the review and, consistent with current clinical practice, is a major question that requires investigation in future systematic reviews.
Taddio et al (29,30) evaluated the use of lidocaineprilocaine cream (EMLA) in treating pain from circumcision, heel lance, venipuncture, arterial puncture, lumbar puncture and percutaneous venous catheter placement in preterm and term infants. A total of nine RCTs, three of which were double-blinded, and two non-RCTs indicated that EMLA was more effective than placebo for treating circumcision, as indicated by changes in physiological and behavioural pain indicators. Some evidence was provided for the use of EMLA in relieving pain during venipuncture, arterial puncture and placement of percutaneous venous catheters; however, results remain inconclusive. Limitations of the studies included small sample sizes, heterogeneity in the EMLA dosages and pain outcomes precluding meta-analyses on all outcomes. The authors concluded that EMLA was safe and efficacious for neonatal circumcision pain, but not heel lance, and recommended further evaluations on the effectiveness of other forms of analgesia for circumcision, such as DPNB.
Nonpharmacological pain interventions
Cepeda et al (33) evaluated the efficacy of music on acute, chronic or cancer pain using measures of pain intensity, pain relief and requirements for analgesics in 51 studies, of which eight studies focused on children. Of the eight pediatric RCTs, four addressed reducing pain in infants for circumcision or heel lance pain. Because benefits of this intervention were statistically small, clinical significance was considered inconclusive.
Cignacco et al (34) comprehensively reviewed nonpharmacological pain interventions (non-nutritive sucking [NNS], music, swaddling, positioning, olfactory and multisensorial stimulation, kangaroo care, maternal touch) to relieve procedural pain in infants in 13 RCTs and two meta-analyses (36,39) for preterm and term infants. Based on methodologically sound studies, the authors reported that NNS, swaddling and facilitative tucking interventions were effective, to some extent, in reducing pain in infants undergoing single painful procedures. Although Cignacco et al (34) cited the review by Prasopkittikun and Tilokskulchai (36), they did not include the use of positioning, maternal holding or touching in their summary of recommended strategies. Two additional studies did not support the use of positioning for procedural pain (41,42). Maternal touching and holding were effective in term infants only and the effects of this intervention dropped off rapidly (36). Limitations of the studies included the lack of blinding of assessors, small sample sizes in the studies, lack of standardization of interventions and the use of unidimensional indicators of pain, rather than validated multidimensional or composite measures of pain. The authors emphasized the importance of not relying solely on nonpharmacological interventions when acute pain is more severe. Furthermore, they recommended that future researchers evaluate the efficacy of nonpharmacological interventions used alone and in combination with pharmacological interventions, taking contextual factors, such as gestational age, and varying levels of severity of illness and chronic pain states into consideration.
Pinelli et al (35) also evaluated the role of NNS in reducing pain in preterm and term high-risk infants using validated pain indicators in four randomized crossover trials. In one study (42), NNS was effective in reducing pain assessed by the PIPP (40). Two studies reported statistically significant, but not clinically significant, effects of NNS for painful procedures. Limitations included randomization and outcome assessors that were not blinded. The authors recommended NNS for pain management in high-risk infants.
Prasopkittikun and Tilokskulchai (36) reported that swaddling, maternal holding, touching and positioning were effective nonpharmacological interventions that reduced pain using validated pain assessment measures in preterm and term infants undergoing a heel lance. The authors advised the use of a combination of these interventions because their effectiveness may vary across infants.
Shah et al (37) evaluated the effectiveness of breastfeeding or supplemental administration of breast milk in reducing procedural pain in preterm and term infants in 11 RCTs or quasi-RCTs. Breastfeeding significantly reduced physiological pain indicators (ie, heart rate, crying) and PIPP scores compared with placebo or positioning. Evidence in one study suggested that supplemental administration of breast milk resulted in fewer changes in facial expression compared with infants who received no intervention; however, results from studies in the review varied. Supplemental breast milk resulted in higher increases in changes in heart rate and duration of crying compared with sucrose groups; however, these results were based on single studies. Limitations included heterogeneity across studies on previous exposure to breastfeeding or administration of supplemental breast milk, methods used to assess pain, and the use of different control interventions. Breastfeeding or the administration of supplemental breast milk was recommended as an effective pain-relieving intervention for infants undergoing single painful events.
Shiao et al (39) reported, in nine experimental or quasi-experimental designs, that NNS was effective in influencing physiological pain indicators (ie, heart rate and transcutaneous oxygen levels). The authors reported that only two of the studies used independent treatment and control groups in the analysis, and the remaining studies were within-subjects designs. Only unidimensional measures of pain were used to assess pain. Despite these limitations, NNS was recommended during procedural pain.
DISCUSSION
Because little quality assessment has been conducted on systematic reviews in acute pain in infants, our goal was to identify methodologically sound systematic reviews that would provide clinicians with the best evidence of effective strategies for minimizing acute procedural pain and the development of immediate and long-term consequences. A validated rating tool (26–28) employed in other studies (27,43) was used to rate the scientific quality of systematic reviews.
Given that recommendations are abundant in hospital pain procedures and clinical guidelines, it was most striking that only a few pharmacological interventions were supported by high-quality evidence for acute pain management. Effective pharmacological interventions were limited to DPNB and EMLA cream for circumcision (29,30,32), and sucrose for single painful procedures (22). Nonpharmacological pain interventions, including the use of pacifiers or NNS (34,35,39), swaddling (34,36), facilitated tucking (34), and breast milk or breastfeeding (37) had higher levels of support for reducing pain during single painful events. However, the crucial issue of whether these interventions could be used repeatedly was not addressed in existing reviews.
Systematic reviews and meta-analyses focus on reducing sources of bias by ensuring that the search strategies are thorough, threats to internal validity of the individual studies are addressed and results of the studies in the review are appropriately combined (27). All Cochrane reviews included in the present appraisal were rated as having minimal flaws and received the highest quality ratings. Cochrane reviews generally report higher levels of methodological quality because they follow specific guidelines to minimize bias (44). Although the methodological quality of the reviews were considered to have minimal flaws, there were common methodological limitations noted across studies in all of the reviews (including the Cochrane reviews). These limitations included small sample sizes, and the heterogeneity of study participants, interventions and outcomes, including the lack of validated pain measures to assess pain. Results from primary studies often did not include sufficient details of the outcomes measured or did not use common metrics to report results. These reporting inadequacies precluded meta-analyses from being conducted on all studies.
Because few pain-relieving strategies for infants have been rigorously evaluated, and methodological limitations of these reviews persist, clinicians are left to ponder the evidence needed to support their practice. Ongoing reviews on the use of individual infant pain interventions (both for single and repeated use) and combined pharmacological and nonpharmacological interventions (both for single and repeated use) are required to minimize acute pain as a result of repeated exposure to painful procedures (9). A small number of systematic reviews on infant pain management were identified in the literature. Furthermore, methodological limitations that exist within the primary studies included in these reviews highlight the need for more high-quality studies that evaluate individual and combined pharmacological and non-pharmacological infant pain interventions using validated measures of pain.
Research is only one source of evidence that can influence practice changes; other sources that should be considered when planning practice changes include the clinical experience of health professionals, patient preferences and experiences, and the use of local data and information to inform practice changes (45). Furthermore, the existence of high-quality evidence is only the preliminary step in a chain of events for improving clinical management of procedural pain in infants. Translating recommendations from high-quality systematic reviews into practice requires a complex interactive process.
CONCLUSIONS
Selected pharmacological and nonpharmacological interventions to treat pain were identified based on high-quality systematic reviews. Recommendations for these selected interventions were limited due to the heterogeneity of the methods of the primary studies included in the reviews. Despite high-quality systematic reviews of pain interventions in infants, there is a lack of good-quality evidence that supports many of the interventions currently described in clinical practice guidelines. Future studies that use validated pain measures can contribute to the generation of high-quality systematic reviews. Furthermore, the application of standard methods of reporting randomized trials (using the Consolidated Standards of Reporting Trials [CONSORT; 46]) and systematic reviews (using the Quality of Reporting of Meta-analyses [QUOROM; 25]) will contribute to the quality of systematic reviews on pain interventions in infants. Recommendations from these systematic reviews will be integral to both clinicians and policy makers in planning practice changes that could ultimately contribute to improved patient-and system-related outcomes.
Acknowledgments
Funding is acknowledged from the Canadian Institutes of Health Research (CIHR) (CTP-79854). The authors thank the CIHR Team in Children’s Pain for providing thoughtful feedback on earlier versions of the present manuscript, and the Signy Hildur Eaton Chair in Paediatric Nursing Research at the Hospital for Sick Children (Toronto, Ontario) and the Samuel Lunenfeld Research Summer Student Program for providing financial support to Dr Stevens and the research assistants. Janet Yamada’s work is supported by a CIHR fellowship. Dr Stinson’s work is supported by a CIHR postdoctoral fellowship. We acknowledge Elizabeth Uleryk ba mls and Thomasin Adams-Webber ma mls cas for their assistance with the electronic searches.
REFERENCES
- 1.Simons SH, van Dijk M, Anand KS, Roofthooft D, van Lingen RA, Tibboel D. Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch Pediatr Adolesc Med. 2003;157:1058–64. doi: 10.1001/archpedi.157.11.1058. [DOI] [PubMed] [Google Scholar]
- 2.Stevens B, McGrath P, Gibbins S, et al. Procedural pain in newborns at risk for neurologic impairment. Pain. 2003;105:27–35. doi: 10.1016/s0304-3959(03)00136-2. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate. 2000;77:69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- 4.Bartocci M, Bergqvist LL, Lagercrantz H, Anand KJ. Pain activates cortical areas in the preterm newborn brain. Pain. 2006;122:109–17. doi: 10.1016/j.pain.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Slater R, Cantarella A, Gallella S, et al. Cortical pain responses in human infants. J Neurosci. 2006;26:3662–6. doi: 10.1523/JNEUROSCI.0348-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunau RE, Holsti L, Peters JW. Long-term consequences of pain in human neonates. Semin Fetal Neonatal Med. 2006;11:268–75. doi: 10.1016/j.siny.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Taddio A, Gurguis MG, Koren G. Lidocaine-prilocaine cream versus tetracaine gel for procedural pain in children. Ann Pharmacother. 2002;36:687–92. doi: 10.1345/aph.1A138. [DOI] [PubMed] [Google Scholar]
- 8.McClain BC, Kain ZN. Procedural pain in neonates: The new millennium. Pediatrics. 2005;115:1073–5. doi: 10.1542/peds.2005-0204. [DOI] [PubMed] [Google Scholar]
- 9.Stevens BJ, Pillai Riddell R. Looking beyond acute pain in infancy. Pain. 2006;124:11–2. doi: 10.1016/j.pain.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 10.Anand KJ, International Evidence-Based Group for Neonatal Pain Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155:173–80. doi: 10.1001/archpedi.155.2.173. [DOI] [PubMed] [Google Scholar]
- 11.Stevens B. Pain in infants and children: Assessment and management strategies within the context of professional guidelines, standards, and roles. In: Giamberardino MA, editor. Pain 2002 – An Updated Review Refresher Course Syllabus. Seattle: IASP Press; 2002. pp. 315–26. [Google Scholar]
- 12.McKechnie L, Levene M. Procedural pain guidelines for the newborn in the United Kingdom. J Perinatol. 2008;28:107–11. doi: 10.1038/sj.jp.7211822. [DOI] [PubMed] [Google Scholar]
- 13.Berry PH, Dahl JL. The new JCAHO pain standards: Implications for pain management nurses. Pain Manag Nurs. 2000;1:3–12. doi: 10.1053/jpmn.2000.5833. [DOI] [PubMed] [Google Scholar]
- 14.Accreditation Canada. <http://www.cchsa.ca/default.aspx> (Version current at June 30, 2008).
- 15.Prevention and management of pain and stress in the neonate. American Academy of Pediatrics. Committee on Fetus and Newborn. Committee on Drugs Section on Anesthesiology. Section on Surgery. Canadian Paediatric Society. Fetus and Newborn Committee. Pediatrics. 2000;105:454–61. [PubMed] [Google Scholar]
- 16.American Academy of Pediatrics Committee on Fetus and Newborn. American Academy of Pediatrics Section on Surgery. Canadian Paediatric Society Fetus and Newborn Committee. Batton DG, Barrington KJ, Wallman C. Prevention and management of pain in the neonate: An update. Pediatrics. 2006;118:2231–41. doi: 10.1542/peds.2006-2277. [DOI] [PubMed] [Google Scholar]
- 17.McGrath P, Unruh AM. Neonatal and infant pain in a social context. In: Anand KJS, Stevens BJ, McGrath PJ, editors. Pain in Neonates and Infants. 3rd edn. Toronto: Elsevier; 2007. [Google Scholar]
- 18.Anand KJ, Aranda JV, Berde CB, et al. Summary proceedings from the neonatal pain-control group. Pediatrics. 2006;117:S9–22. doi: 10.1542/peds.2005-0620C. [DOI] [PubMed] [Google Scholar]
- 19.National Association of Neonatal Nurses. Position statement #3019: Pain Management in Infants. <http://www.nann.org/membership/sigs/post_stmnts.html> (Version current at May 23, 2008).
- 20.Guideline statement: Management of procedure-related pain in children and adolescents. J Paediatr Child Health. 2006;42:S1–29. doi: 10.1111/j.1440-1754.2006.00798_1.x. [DOI] [PubMed] [Google Scholar]
- 21.Scott-Findlay S, Estabrooks CA. Knowledge translation and pain management. In: Finley GA, editor. Bringing Pain Relief to Children: Treatment Approaches. Totowa: Humana Press; 2006. pp. 199–226. [Google Scholar]
- 22.Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. 2004:CD001069. doi: 10.1002/14651858.CD001069.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Anand KJ, Johnston CC, Oberlander TF, Taddio A, Lehr VT, Walco GA. Analgesia and local anesthesia during invasive procedures in the neonate. Clin Ther. 2005;27:844–76. doi: 10.1016/j.clinthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: The QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 26.Oxman AD, Guyatt GH. Validation of an index of the quality of review articles. J Clin Epidemiol. 1991;44:1271–8. doi: 10.1016/0895-4356(91)90160-b. [DOI] [PubMed] [Google Scholar]
- 27.Jadad AR, McQuay HJ. Meta-analyses to evaluate analgesic interventions: A systematic qualitative review of their methodology. J Clin Epidemiol. 1996;49:235–43. doi: 10.1016/0895-4356(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 28.Oxman AD, Guyatt GH, Singer J, et al. Agreement among reviewers of review articles. J Clin Epidemiol. 1991;44:91–8. doi: 10.1016/0895-4356(91)90205-n. [DOI] [PubMed] [Google Scholar]
- 29.Taddio A, Ohlsson K, Ohlsson A. Lidocaine-prilocaine cream for analgesia during circumcision in newborn boys. Cochrane Database Syst Rev. 2000:CD000496. doi: 10.1002/14651858.CD000496. [DOI] [PubMed] [Google Scholar]
- 30.Taddio A, Ohlsson A, Einarson TR, Stevens B, Koren G. A systematic review of lidocaine-prilocaine cream (EMLA) in the treatment of acute pain in neonates. Pediatrics. 1998;101:E1. doi: 10.1542/peds.101.2.e1. [DOI] [PubMed] [Google Scholar]
- 31.Bellù R, de Waal KA, Zanini R. Opioids for neonates receiving mechanical ventilation. Cochrane Database Syst Rev. 2005:CD004212. doi: 10.1002/14651858.CD004212.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Brady-Fryer B, Wiebe N, Lander JA. Pain relief for neonatal circumcision. Cochrane Database Syst Rev. 2004:CD004217. doi: 10.1002/14651858.CD004217.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cepeda MS, Carr DB, Lau J, Alvarez H. Music for pain relief. Cochrane Database Syst Rev. 2006:CD004843. doi: 10.1002/14651858.CD004843.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Cignacco E, Hamers JP, Stoffel L, et al. The efficacy of non-pharmacological interventions in the management of procedural pain in preterm and term neonates. A systematic literature review. Eur J Pain. 2007;11:139–52. doi: 10.1016/j.ejpain.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Pinelli J, Symington A, Ciliska D. Non-nutritive sucking in high-risk infants: Benign intervention or legitimate therapy? J Obstet Gynecol Neonatal Nurs. 2002;31:582–91. doi: 10.1111/j.1552-6909.2002.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 36.Prasopkittikun T, Tilokskulchai F. Management of pain from heel stick in neonates: An analysis of research conducted in Thailand. J Perinat Neonatal Nurs. 2003;17:304–12. doi: 10.1097/00005237-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Shah PS, Aliwalas LI, Shah V. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database Syst Rev. 2006:CD004950. doi: 10.1002/14651858.CD004950.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Shah V, Ohlsson A. The effectiveness of premedication for endotracheal intubation in mechanically ventilated neonates. A systematic review. Clin Perinatol. 2002;29:535–54. doi: 10.1016/s0095-5108(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 39.Shiao SY, Chang YJ, Lannon H, Yarandi H. Meta-analysis of the effects of nonnutritive sucking on heart rate and peripheral oxygenation: Research from the past 30 years. Issues Compr Pediatr Nurs. 1997;20:11–24. doi: 10.3109/01460869709026874. [DOI] [PubMed] [Google Scholar]
- 40.Stevens B, Johnston C, Petryshen P, Taddio A. Premature Infant Pain Profile: Development and initial validation. Clin J Pain. 1996;12:13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Grunau RE, Linhares MB, Holsti L, Oberlander TF, Whitfield MF. Does prone or supine position influence pain responses in preterm infants at 32 weeks gestational age? Clin J Pain. 2004;20:76–82. doi: 10.1097/00002508-200403000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevens B, Johnston C, Franck L, Petryshen P, Jack A, Foster G. The efficacy of developmentally sensitive interventions and sucrose for relieving procedural pain in very low birth weight neonates. Nurs Res. 1999;48:35–43. doi: 10.1097/00006199-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Furlan AD, Clarke J, Esmail R, Sinclair S, Irvin E, Bombardier C. A critical review of reviews on the treatment of chronic low back pain. Spine. 2001;26:E155–62. doi: 10.1097/00007632-200104010-00018. [DOI] [PubMed] [Google Scholar]
- 44.Jadad AR, Cook DJ, Jones A, et al. Methodology and reports of systematic reviews and meta-analyses: A comparison of Cochrane reviews with articles published in paper-based journals. JAMA. 1998;280:278–80. doi: 10.1001/jama.280.3.278. [DOI] [PubMed] [Google Scholar]
- 45.McCormack B, Kitson A, Harvey G, Rycroft-Malone J, Titchen A, Seers K. Getting evidence into practice: The meaning of ‘context’. J Adv Nurs. 2002;38:94–104. doi: 10.1046/j.1365-2648.2002.02150.x. [DOI] [PubMed] [Google Scholar]
- 46.CONSORT: Transparent Reporting of Trials. <http://www.consort-statement.org/> (Version current at June 30, 2008).