Abstract
Endarterectomy is generally recommended for symptomatic high-grade (70 to 99%) stenosis of the internal carotid artery, but whether this procedure is beneficial among patients with chronic kidney disease (CKD) is unknown. In this re-analysis of data from the North American Symptomatic Carotid Endarterectomy Trial, we included patients with symptomatic stenosis and either stage 3 CKD (n = 524) or preserved kidney function (n = 966; estimated GFR ≥ 60). For medically treated patients with high-grade stenosis, risk for ipsilateral stroke at 2 yr was significantly higher in patients with CKD than in those with preserved renal function (31.6 versus 19.3%; P = 0.042); carotid endarterectomy significantly reduced this risk by 82 and 51%, respectively. To prevent one ipsilateral stroke, the number needed to treat by endarterectomy was four for patients with CKD and 10 for patients with preserved renal function. Compared with patients with preserved renal function, those with CKD had similar rates of perioperative stroke and death but higher rates of cardiac events. In conclusion, patients with stage 3 CKD and symptomatic high-grade carotid stenosis gain a large benefit in stroke risk reduction after endarterectomy.
Approximately 795,000 people in North America have a stroke every year, 185,000 of whom have recurrent strokes.1 As a secondary prevention strategy, current practice guidelines recommend carotid endarterectomy for patients with symptomatic high grade (70 to 99%) carotid stenosis.2 Endarterectomy is also offered to patients with moderate-grade (50 to 69%) stenosis, after considering factors such as age, gender, comorbidities, and severity of recent symptoms.2
It is estimated that >15 million Americans have chronic kidney disease (CKD), and the prevalence is increasing.3 Patients with CKD are at high risk for cardiovascular4–6 and cerebrovascular disease, including stroke7,8; however, patients with CKD are less likely than others to undergo invasive procedures to reduce this risk,9 a phenomenon sometimes called “renalism.”10 Although patients with CKD are prone to adverse events from procedures and treatments,11,12 in some situations patients with CKD derive larger absolute benefits than others because of their high baseline risk.13–16
Little is known about the effectiveness and safety of carotid endarterectomy in patients with CKD. No randomized trial has exclusively focused on endarterectomy in the CKD population, and it remains unlikely that such a trial will ever be conducted. The next best approach is to re-analyze previously conducted trials. Three large randomized trials have examined the benefit of carotid endarterectomy, namely the Asymptomatic Carotid Atherosclerosis Study (ACAS),17 the European Carotid Surgery Trial (ECST),18 and the North American Symptomatic Carotid Endarterectomy Trial (NASCET).19 Of the three trials, only NASCET collected baseline serum creatinine for all trial participants. The aim of this study was to examine the outcomes of patients with and without CKD among those enrolled in NASCET.
Results
Among the 1517 patients who had ≥50% internal carotid artery (ICA) stenosis, 25 did not have a baseline serum creatinine recorded and two had implausible values. The remaining 1490 (98.2%) patients were analyzed in this study. Because there were no differences in baseline characteristics between patients assigned to the medical (n = 740) and surgical (n = 750) arms in both the high-grade and moderate-grade stenosis categories, the baseline characteristics are reported in aggregate (Table 1). Patients with CKD had a mean age of 69 yr and a mean estimated GFR (eGFR) of 49 ml/min per 1.73 m2 compared with 64 yr and 79 ml/min per 1.73 m2 among patients without CKD. In addition to being older, patients with CKD were more likely to be female and to have a history of hypertension and myocardial infarction (Table 1).
Table 1.
Comparison of baseline patient characteristics
| Characteristic | eGFR <60(n = 524) | eGFR ≥60(n = 966) | P |
|---|---|---|---|
| Age | <0.001 | ||
| >65 yr (%) | 73.3 | 44.4 | |
| mean (range) | 69 (38 to 89) | 64 (31 to 85) | |
| Female gender (%) | 37.6 | 27.1 | <0.001 |
| Black race (%) | 1.5 | 3.8 | 0.01 |
| Qualifying (presenting) ischemic event (%) | |||
| stroke (not TIA) | 41.2 | 38.8 | 0.37 |
| hemispheric (not retinal) | 72.1 | 71.3 | 0.74 |
| History of (%) | |||
| TIA or stroke within past 6 moa | 54.8 | 55.7 | 0.73 |
| hypertension | 73.7 | 54.3 | <0.001 |
| diabetes | 22.5 | 21.0 | 0.50 |
| MI | 24.0 | 18.0 | 0.006 |
| CHF | 3.8 | 2.0 | 0.03 |
| arrhythmia | 4.2 | 5.3 | 0.36 |
| hyperlipidemia | 35.7 | 34.5 | 0.64 |
| intermittent claudication | 17.0 | 13.8 | 0.10 |
| Smoking within past 12 mo (%) | 34.5 | 50.2 | <0.001 |
| Systolic BP >160 mmHg (%) | 23.5 | 16.7 | 0.001 |
| Diastolic BP >90 mmHg (%) | 13.0 | 12.0 | 0.59 |
| Glucose >7 mmol/L (>126 mg/dl) (%) | 20.0 | 21.2 | 0.59 |
| Degree of ICA stenosis 70 to 99% (%) | 41.4 | 44.5 | 0.25 |
| eGFR (ml/min per 1.73 m2; mean [range]) | 49 (19 to 60) | 79 (60 to 153) | – |
| Serum creatinine (μmol/L; mean [range]) | 127 (86 to 292) | 87 (39 to 133) | – |
| Serum creatinine (mg/dl; mean [range]) | 1.5 (0.9 to 3.3) | 1.0 (0.4 to 1.5) | – |
Characteristics were compared using a χ2 test. TIA, transient ischemic attack; ICA, internal carotid artery stenosis; MI, myocardial infarction.
aIn addition to the qualifying (presenting) event.
Of the 750 patients assigned to receive endarterectomy, six did not have surgery. Among the remaining 744 patients, 98.4% had their surgery within 30 d of randomization with a median time of 2 d. Of the 740 patients assigned to the medical arm, 6.9% received endarterectomy during follow-up. The overall median patient follow-up was 2 yr. Log binomial regression was used to calculate risk ratios for treatment group, eGFR category, and the cross-product, before and after adjustment for all baseline characteristics listed in Table 1, except for race, history of congestive heart failure (CHF), and history of arrhythmia. The prevalence of the last three characteristics was too low to include without destabilizing the regression model. The corresponding magnitudes of the unadjusted and adjusted risk ratios all were within 12% of each other, supporting an absence of confounding (Supplemental Tables 1 and 2).20
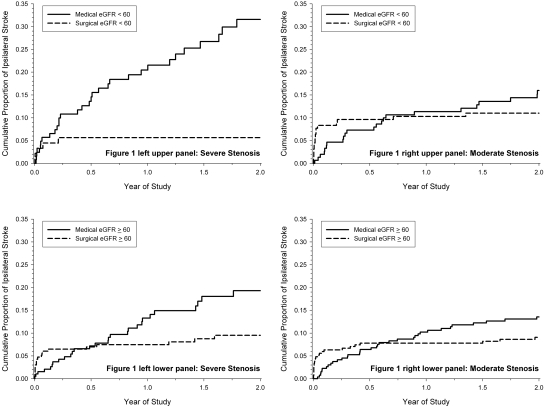
For medically treated patients with high-grade stenosis, the 2-yr risk for ipsilateral stroke was higher in patients with CKD than those without CKD (31.6 versus 19.3%; P = 0.042). With surgery, there was a statistically significant relative risk reduction of 82.3% (95% confidence interval [CI] 54.5 to 93.1%) in the 2-yr risk for ipsilateral stroke for patients with CKD and 50.8% (95% CI 12.6 to 72.3%) for patients without CKD (Table 2). The number needed to treat by surgery to prevent one ipsilateral stroke within 2 yr was four for patients with CKD and 10 for patients without CKD. The difference in prognosis of patients with and without CKD is graphically illustrated in the left upper and lower panels of Figure 1.
Table 2.
Two-year risks for outcomes by treatment and CKD status (eGFR, ml/min per 1.73 m2) in patients with high-grade internal carotid artery stenosis (70 to 99%)
| Parameter | Medical Risk (%)(n = 126)a(n = 197)b | Surgical Risk (%)(n = 91)a(n = 233)b | RRR (%) | Absolute Risk Difference (%; 95% CI) | P | Interaction Test (P) |
|
|---|---|---|---|---|---|---|---|
| Risk Difference | RRR | ||||||
| Ipsilateral strokec | |||||||
| eGFR <60 | 31.6 | 5.6 | 82.3 | 26.0 (15.1 to 36.8) | <0.001 | 0.018 | 0.051 |
| eGFR ≥60 | 19.3 | 9.5 | 50.8 | 9.8 (1.9 to 17.7) | 0.047 | ||
| Death (any cause) | |||||||
| eGFR <60 | 9.4 | 9.7 | −3.2 | −0.3 (−11.1 to 10.5) | 0.94 | 0.83 | 0.78 |
| eGFR ≥60 | 5.9 | 4.9 | 16.9 | 1.0 (−4.4 to 6.4) | 0.59 | ||
| Any stroke, MI, or vascular death | |||||||
| eGFR <60 | 40.7 | 17.5 | 57.0 | 23.2 (9.6 to 36.7) | 0.016 | 0.07 | 0.26 |
| eGFR ≥60 | 23.0 | 15.0 | 34.8 | 8.0 (−1.0 to 17.0) | 0.13 | ||
Medical and surgical groups were compared using a log-rank test. RRR, relative risk reduction.
aeGFR <60 ml/min per 1.73 m2.
beGFR ≥60 ml/min per 1.73 m2.
cTo provide a more conservative estimate of the benefits of endarterectomy, the outcome “ipsilateral stroke” includes any stroke and any death within 32 d of randomization (30 d postoperatively; median time from randomization to surgery was 2 d).
Figure 1.
Cumulative proportion of ipsilateral stroke from Kaplan-Meier analyses by degree of ICA stenosis, eGFR level (ml/min per 1.73 m2), and treatment group is shown.
Among patients with moderate-grade stenosis, the 2-yr medical risk of ipsilateral stroke in patients with CKD was approximate half of those with high grade stenosis (Table 3). Among patients with moderate-grade stenosis, the effectiveness of surgery was less and not statistically significant, with less separation between the medical and surgical cumulative outcome curves (Figure 1, right). A similar pattern of results was observed for the composite outcome of any stroke, MI, or vascular death, although they were not statistically significant for patients without CKD. Although the 2-yr risk for death for both the high-grade and moderate-grade stenosis was twice as high among patients with CKD compared with those without CKD, endarterectomy had no effect in reducing overall mortality (Tables 2 and 3). In considering perioperative risk, patients with CKD did not have higher rates of perioperative stroke or death when compared with those without CKD (Table 4). Patients with CKD did, however, have a significantly higher relative risk for perioperative cardiac events (MI, CHF, or arrhythmia; Table 4) even after adjustment for cardiac-related patient characteristics (Table 5).
Table 3.
Two-year risks of outcomes by treatment and CKD status (eGFR, ml/min per 1.73 m2) in patients with moderate-grade ICA stenosis (50 to 69%)
| Parameter | Medical Risk (%)(n = 151)a(n = 266)b | Surgical Risk (%)(n = 156)a(n = 270)b | RRR (%) | Absolute Risk Difference (%; 95% CI) | P | Interaction Test (P) |
|
|---|---|---|---|---|---|---|---|
| Risk Difference | RRR | ||||||
| Ipsilateral strokec | |||||||
| eGFR <60 | 16.0 | 11.0 | 31.2 | 5.0 (−2.8 to 12.8) | 0.34 | 0.92 | 0.94 |
| eGFR ≥60 | 13.6 | 9.1 | 33.1 | 4.5 (−1.0 to 9.9) | 0.15 | ||
| Death (any cause) | |||||||
| eGFR <60 | 10.7 | 10.4 | 2.8 | 0.3 (−6.7 to 7.3) | 0.99 | 0.89 | 0.88 |
| eGFR ≥60 | 5.1 | 5.3 | −3.9 | −0.2 (−4.1 to 3.6) | 0.88 | ||
| Any stroke, MI, or vascular death | |||||||
| eGFR <60 | 29.9 | 19.7 | 34.1 | 10.2 (−1.3 to 18.4) | 0.08 | 0.26 | 0.35 |
| eGFR ≥60 | 21.5 | 18.2 | 15.3 | 3.3 (−2.7 to 11.1) | 0.45 | ||
Medical and surgical groups were compared using a log-rank test. RRR, Relative Risk Reduction.
aeGFR <60 ml/min per 1.73 m2.
beGFR ≥60 ml/min per 1.73 m2
cTo provide a more conservative estimate of the benefits of endarterectomy, the outcome “ipsilateral stroke” includes any stroke and any death within 32 d of randomization (30 d postoperatively; median time from randomization to surgery was 2 d).
Table 4.
30-Day perioperative risks of outcomes by CKD status (eGFR, ml/min per 1.73 m2) for patients assigned to carotid endarterectomy (n = 744)
| Parameter | eGFR <60 (%; n = 245) | eGFR ≥60 (%; n = 499) | Risk Difference (%; 95% CI) | P |
|---|---|---|---|---|
| Any stroke or death | 6.9 | 6.4 | 0.5 (−3.3 to 4.4) | 0.79 |
| Any nonfatal stroke | 4.9 | 5.6 | −0.7 (−4.1 to 2.7) | 0.68 |
| Death (any cause) | 2.0 | 0.8 | 1.2 (−0.7 to 3.2) | 0.15 |
| MI, CHF, or arrhythmia | 6.5 | 1.2 | 5.3 (2.1 to 8.6) | <0.001 |
| MI | 2.0 | 0.6 | 1.4 (−0.5 to 3.3) | 0.07 |
| CHF | 1.6 | 0.2 | 1.4 (−0.2 to 3.1) | 0.025 |
| Arrhythmia | 3.6 | 0.6 | 3.0 (0.6 to 5.5) | 0.002 |
Includes patients who had moderate and severe stenoses at time of randomization and actually had surgery. Patients with and without CKD were compared using a χ2 test.
Table 5.
30-Day perioperative unadjusted and adjusted risk ratios for patients assigned to carotid endarterectomy (n = 744) with CKD compared to those without CKD
| Parameter | Unadjusted Risk Ratio (95% CI) | P | Adjusted Risk Ratio (95% CI) | P |
|---|---|---|---|---|
| Any stroke or death | 1.08 (0.61 to 1.90) | 0.79 | 1.30 (0.73 to 2.33) | 0.37 |
| Any nonfatal stroke | 0.87 (0.45 to 1.69) | 0.68 | 1.09 (0.54 to 2.20) | 0.81 |
| Death (any cause) | 2.55 (0.69 to 9.40) | 0.16 | 2.67 (0.91 to 7.84) | 0.07 |
| MI, CHF, or arrhythmia | 5.43 (2.15 to 13.71) | <0.001 | 3.98 (1.60 to 9.90) | 0.003 |
| MI | 3.39 (0.82 to 14.09) | 0.09 | 3.14 (0.81 to 12.23) | 0.10 |
| CHF | 8.14 (0.92 to 72.4) | 0.06 | 5.82 (0.77 to 44.07) | 0.09 |
| Arrhythmia | 6.11 (1.67 to 22.37) | 0.006 | 3.99 (1.17 to 13.65) | 0.027 |
Includes patients who had moderate and severe stenoses at time of randomization and actually had surgery. Patients with and without CKD were compared using log-binomial regression. Patients without CKD served as the referent group. Risk ratios were adjusted for age, gender, and a history of hypertension, MI, or smoking.
Discussion
After a comprehensive literature search and communication with carotid endarterectomy trialists, we conclude NASCET is the first and only large randomized trial of carotid endarterectomy in which renal function was assessed in trial participants. Our results show patients with mild to moderate CKD and symptomatic high-grade carotid stenosis have at least a similar and perhaps a larger absolute benefit in stroke reduction after endarterectomy, compared with those without CKD. The risk for perioperative death was similar between patients with and without CKD; however, rates of perioperative cardiac complications (MI, CHF, and arrhythmias) were higher. This study provides some of the best evidence for physicians and patients to help them make decisions about the appropriateness of endarterectomy.
In those with high-grade carotid stenosis, it is true the benefits of endarterectomy on the prevention of ipsilateral stroke were observed to be greater in those with CKD than in those with preserved kidney function (absolute risk difference P = 0.018, relative risk reduction P = 0.052). In several large trials, P < 0.10 has been interpreted as a significant test for treatment effect modification in subgroup analysis (interaction), despite increasing the chance of type I error,21,22 yet cautious interpretation of the interaction terms is required for the NASCET results. The seeming benefit may be in part due to chance variation in the rates of stroke at 2 yr among surgically treated patients with CKD compared with those with preserved renal function (5.6 versus 9.5% respectively; Table 2). Nonetheless, all would agree that patients with mild to moderate CKD benefit at least equally from carotid endarterectomy compared with those without CKD, with no observed increase in the risk for perioperative death or stroke.
For patients with moderate-grade ICA stenosis (50 to 69% stenosis), NASCET results originally demonstrated the 5-yr rate of any ipsilateral stroke was 15.7% among patients who were treated surgically and 22.2% among those who were treated medically (absolute risk reduction of 6.5%; P = 0.045). Our results did not capture this modest but significant benefit of surgery in patients with CKD and moderate-grade stenosis. This may be due to the attenuated 2-yr period of patient follow-up used in our analysis. Thus, when considering endarterectomy in a patients with CKD and moderate-grade stenosis, physicians should consider current American Heart Association guidelines: “Recommendation for surgery should be based on clinical judgment, comorbid patient characteristics, and severity of initial cerebrovascular symptoms.”2
Our study has several strengths. We used an intention-to-treat analysis with patient follow-up of 2 yr. Nearly all patients in our analysis remained in their randomized arm, with minimal crossover and no loss to follow-up. We adhered to published guidelines for subgroup analysis to guard against spurious results.20,23,24 We prespecified the analysis including the statistical test for treatment effect modification, and we reduced false-positive results by avoiding multiple subgroup analyses. We also reported the same primary outcomes as the original trial; however, several limitations of this study merit consideration. First, serum creatinine was measured in the laboratory of each NASCET participating center and was not standardized for the application of modern eGFR estimating equations.25 Given the historical nature of the study, we were also unable to attain more information about the methods and quality control measures used to assay serum creatinine in each laboratory. Although this could slightly change the number of NASCET patients with CKD, it does not alter the clinical importance of our results. Second, the NASCET trial excluded patients with a serum creatinine greater than twice the upper limit of normal. Among the 524 patients with CKD analyzed in this study, the mean eGFR was 49 ml/min per 1.73 m2, with a minimum of 19 ml/min per 1.73 m2. Thus, our results apply directly to patients with stage 3 CKD but cannot be generalized to patients with more severe CKD or those who have kidney failure and require dialysis. Finally, NASCET was published before the widespread use of new medical and surgical techniques for secondary stroke prevention. Only 14% of patients were receiving lipid-lowering therapy at time of enrollment, and aspirin was the only recommended antiplatelet agent. Current guidelines for secondary stroke prevention include stringent control of BP and lipids and antiplatelet therapy with clopidogrel or extended-release dipyramidole and aspirin.2 Compared with risks reported in NASCET, improved medical care has certainly reduced the risk for stroke irrespective of the receipt of endarterectomy.
CKD often coexists with older age, hypertension, and a history of atherosclerotic disease (Table 1). Each of these conditions is an important risk factor for arterial vascular disease; however, a greater benefit of endarterectomy for high-grade carotid stenosis in patients with CKD was observed even after accounting for baseline differences to those without CKD. Our rationale for studying the subgroup of patients with CKD was to establish the effectiveness and safety of endarterectomy in this “highest risk” group for recurrent vascular events, as highlighted in an American Heart Association scientific statement.25
Our results provide strong evidence for the benefit of carotid endarterectomy in patients with stage 3 CKD; however, a higher risk for perioperative mortality in patients with CKD compared with those without CKD has been described in several previous studies.26–28 These studies followed patients with severe CKD, including those who were undergoing dialysis. Our previous meta-analysis confirms a graded increase in the risk for postoperative mortality with declining eGFR.11 Thus, a risk-benefit profile that seems favorable for patients with stage 3 CKD may be less so in those with more advanced kidney failure. A recent review of the literature confirmed the need for additional large-scale, multicenter, prospective trials to identify high-risk patients and quantify their risk better.29
Carotid stenting was recently studied as an alternative therapy to endarterectomy in the patient at high risk for surgical complications.30–32 It is not known whether carotid stenting is better than endarterectomy for stroke prevention with less perioperative complications. American Heart Association guidelines currently describe carotid stenting as “not inferior” to endarterectomy in symptomatic patients with high-grade stenosis and difficult surgical access or medical conditions that greatly increase risk for surgery.2 Thus, carotid stenting could be considered in patients who have CKD and symptomatic ICA stenosis and pose a high operative risk.
To generalize our results to individual patient care, physicians must also be aware of local perioperative complication rates. Patient selection and surgical expertise at NASCET study centers resulted in low rates of perioperative complications from carotid endarterectomy. Variability in these rates could alter the benefits of carotid endarterectomy demonstrated in this study.
Finally, in clinical practice, it is common for beneficial invasive therapies to be withheld from patients with CKD because of concern for adverse events.10,12 This phenomenon is sometimes called “renalism.”10 Patients with CKD are frequently excluded from large, randomized trials, and few subgroup analyses for such patients are ever reported.33 In this broader context, this study is important to help reverse this practice.
Concise Methods
The design of NASCET has been described elsewhere.19 Briefly, this was a randomized clinical trial to determine the efficacy and safety of carotid endarterectomy in patients with symptomatic ICA stenosis. Patients were eligible for randomization when they had a transient ischemic attack or nondisabling stroke in the preceding 180 d attributable to a carotid artery stenosis on selective angiography. Exclusion criteria were renal impairment defined as greater than twice the normal serum urea nitrogen or serum creatinine value as assessed in the laboratory of each participating center, significant liver disease, diseases likely to cause death within 5 yr, cardiac conditions likely to cause cardioembolism, and intracranial stenosis more significant than the carotid artery lesion. Eligible patients were randomly assigned to endarterectomy plus best medical care or best medical care alone, stratified by center and by degree of stenosis. Neurologists assessed all patients at entry; at 1, 3, 6, 9, and 12 mo; and every 4 mo thereafter. A patient's serum creatinine was recorded as part of the baseline data collection process. A blinded external committee adjudicated all primary, secondary, and perioperative clinical outcomes.
The timeline of events after the initiation of NASCET is as follows. NASCET began enrollment in December 1987 and stratified patients by degree of ICA stenosis. High-grade stenosis was defined as 70 to 99%, and moderate-grade stenosis was defined as <70%. The measurement of carotid stenosis was strictly defined by the NASCET protocol. Stenosis was calculated using the ratio of the luminal diameter of the narrowest segment of the diseased portion of the artery to the diameter of the artery beyond any poststenotic dilation, determined using angiography.34 By February 1991, 659 patients with high-grade stenosis had been enrolled. Endarterectomy in this group was associated with a significant reduction in ipsilateral stroke (absolute risk reduction of 17%; 95% CI 10 to 24%). Enrollment into this arm of the study ceased, and these early NASCET results were published.34 Eighteen percent of patients who had high-grade stenosis and were randomly assigned to medical therapy then received an endarterectomy. NASCET continued to enroll patients with moderate-grade stenosis and followed all patients until trial end in December 1997. Final NASCET results were published in 1998.19 To compare reasonably the treatment groups before the large patient crossover for patients enrolled with high-grade stenosis, we counted in this analysis only outcomes that occurred before February 1991.
Consistent with National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) current recommendations,35 we defined CKD by a GFR <60 ml/min per 1.73 m2. We used the Modification of Diet in Renal Disease (MDRD) equation to estimate GFR, where eGFR in ml/min per 1.73 m2 = 186 × (serum creatinine [in mg/dl]−1.154) × (age)−0.203 × (0.742 if female) × (1.210 if black).36 In a supportive analysis, we defined a GFR <60 ml/min per 1.73 m2 by a serum creatinine >137 μmol/L (1.5 mg/dl) in men and >104 μmol/L (1.2 mg/dl) in women.37
We analyzed the same perioperative and long-term outcomes as in the original NASCET publications.19,38 We reported the outcomes of patients with high-grade (70 to 99%) carotid stenosis separately from those with moderate-grade (50 to 69%) carotid stenosis. The two primary outcomes, analyzed separately, were ipsilateral stroke and death (from any cause) within 2 yr of randomization. The ipsilateral stroke outcome included all strokes (at any location) and all deaths (from any cause) during the 30-d postoperative period in the surgical arm and during the 32-d period after randomization in the medical arm, because median time from randomization to surgery was 2 d. Including these early events in the 2-yr outcome of ipsilateral stroke provides a more conservative estimate of the benefits of endarterectomy. The secondary outcome was a composite of any stroke, MI, and vascular death. Thirty-day perioperative outcomes included death, stroke, MI (based on electrocardiogram and cardiac enzyme change), CHF, and arrhythmia requiring treatment with antiarrhythmic medication.
The intention-to-treat principle guided all of our analyses. We compared baseline characteristics for patients with and without CKD using χ2 tests. We assessed the effect of endarterectomy using a log-rank test from Kaplan-Meier analyses and plotted cumulative outcome curves. From these curves, we derived the risks for outcome within 2 yr of randomization. We calculated SEs for the absolute and relative risk reductions and 95% CIs using Greenwood's formula.39 We also calculated the number of patients who would need to be treated with endarterectomy to prevent one additional outcome within 2 yr after the procedure. We used recommended methods for subgroup analysis to determine whether the risk differences and relative risk reductions differed among patients with and without CKD.23,24,40,41 Because the statistical test of treatment effect modification is known to be underpowered, P < 0.10 was considered to be statistically significant.40 χ2 tests were used to compare perioperative risks for outcomes between patients with and without CKD. We repeated these analyses using log binomial regression modeling to assess whether any of the baseline patient characteristics influenced the findings. We avoided Cox proportional hazards modeling because the hazard assumption was not expected to be met because of perioperative adverse events among surgical patients (compared with medical patients). All analyses were performed using SAS 9.1.3 (SAS Institute, Cary, NC).
Disclosures
None.
Acknowledgments
This analysis was supported by a research grant from the Physicians Services Incorporated Foundation. The NASCET trial was supported by grant R01-NS-24456 from the National Institute of Neurological Disorders and Stroke. M.E. was supported by the Alberta Heritage Foundation for Medical Research, P.J.D. was supported by a New Investigator Award from the Canadian Institutes of Health Research, and A.X.G. was supported by a Clinician Scientist Award from the Canadian Institutes of Health Research.
We acknowledge Dr. Brian Haynes and the NASCET collaborators for help and support.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Management of Symptomatic Carotid Stenosis in Individuals with CKD,” on pages 9–11.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y: Heart disease and stroke statistics: 2009 update—A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119: e21–e181, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick TL: Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: Co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke 37: 577–617, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Tonelli M, Jose P, Curhan G, Sacks F, Braunwald E, Pfeffer M, Cholesterol and Recurrent Events (CARE) Trial Investigators : Proteinuria, impaired kidney function, and adverse outcomes in people with coronary disease—Analysis of a previously conducted randomised trial. BMJ 332: 1426, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Zuo L, Wang F, Wang M, Wang S, Lv J, Liu L, Wang H: Cardiovascular disease in early stages of chronic kidney disease in a Chinese population. J Am Soc Nephrol 17: 2617–2621, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Koren-Morag N, Goldbourt U, Tanne D: Renal dysfunction and risk of ischemic stroke or TIA in patients with cardiovascular disease. Neurology 67: 224–228, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Charytan DM, Setoguchi S, Solomon DH, Avorn J, Winkelmayer WC: Clinical presentation of myocardial infarction contributes to lower use of coronary angiography in patients with chronic kidney disease. Kidney Int 71: 938–945, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Chertow GM, Normand SL, McNeil BJ: “Renalism”: Inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol 15: 2462–2468, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Mathew A, Devereaux PJ, O'Hare A, Tonelli M, Thiessen-Philbrook H, Nevis IF, Iansavichus AV, Garg AX: Chronic kidney disease and postoperative mortality: A systematic review and meta-analysis. Kidney Int 73: 1069–1081, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Ix JH, Mercado N, Shlipak MG, Lemos PA, Boersma E, Lindeboom W, O'Neill WW, Wijns W, Serruys PW: Association of chronic kidney disease with clinical outcomes after coronary revascularization: The Arterial Revascularization Therapies Study (ARTS). Am Heart J 149: 512–519, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Solomon SD, Rice MM, A Jablonski K, Jose P, Domanski M, Sabatine M, Gersh BJ, Rouleau J, Pfeffer MA, Braunwald E, Prevention of Events with ACE inhibition (PEACE) Investigators : Renal function and effectiveness of angiotensin-converting enzyme inhibitor therapy in patients with chronic stable coronary disease in the Prevention of Events with ACE inhibition (PEACE) trial. Circulation 114: 26–31, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Shepherd J, Kastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A, Wenger NK, TNT (Treating to New Targets) Investigators : Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease: The TNT (Treating to New Targets) study. J Am Coll Cardiol 51: 1448–1454, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S: Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE randomized trial. Ann Intern Med 134: 629–636, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, Ménard J, Rahn KH, Wedel H, Westerling S: Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet 351: 1755–1762, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study: Endarterectomy for asymptomatic carotid artery stenosis. JAMA 273: 1421–1428, 1995 [PubMed] [Google Scholar]
- 18.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 351: 1379–1387, 1998 [PubMed] [Google Scholar]
- 19.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE, Spence JD: Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 339: 1415–1425, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Maldonado G, Greenland S: Simulation study of confounder-selection strategies. Am J Epidemiol 138: 923–936, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Ziegler D, Pritchett YL, Wang F, Desaiah D, Robinson MJ, Hall JA, Chappell AS: Impact of disease characteristics on the efficacy of duloxetine in diabetic peripheral neuropathic pain. Diabetes Care 30: 664–669, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Furberg CD, Vittinghoff E, Davidson M, Herrington DM, Simon JA, Wenger NK, Hulley S: Subgroup interactions in the Heart and Estrogen/Progestin Replacement Study: Lessons learned. Circulation 105: 917–922, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM: Statistics in medicine: Reporting of subgroup analyses in clinical trials. N Engl J Med 357: 2189–2194, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Oxman AD, Guyatt GH: A consumer's guide to subgroup analyses. Ann Intern Med 116: 78–84, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M, Eckfeldt JH, National Kidney Disease Education Program Laboratory Working Group : Recommendations for improving serum creatinine measurement: A report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 52: 5–18, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Ayerdi J, Sampson LN, Deshmukh N, Farid A, Gupta SK: Carotid endarterectomy in patients with renal insufficiency: Should selection criteria be different in patients with renal insufficiency? Vasc Surg 35: 429–435, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Debing E, Van den Brande P: Chronic renal insufficiency and risk of early mortality in patients undergoing carotid endarterectomy. Ann Vasc Surg 20: 609–613, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Stoner MC, Abbott WM, Wong DR, Hua HT, Lamuraglia GM, Kwolek CJ, Watkins MT, Agnihotri AK, Henderson WG, Khuri S, Cambria RP: Defining the high-risk patient for carotid endarterectomy: An analysis of the prospective National Surgical Quality Improvement Program database. J Vasc Surg 43: 285–295, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Govindarajan G, Saab G, Whaley-Connell A: Outcomes of carotid revascularization in patients with chronic kidney disease. Adv Chronic Kidney Dis 15: 347–354, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Mas JL, Trinquart L, Leys D, Albucher JF, Rousseau H, Viguier A, Bossavy JP, Denis B, Piquet P, Garnier P, Viader F, Touzé E, Julia P, Giroud M, Krause D, Hosseini H, Becquemin JP, Hinzelin G, Houdart E, Hénon H, Neau JP, Bracard S, Onnient Y, Padovani R, Chatellier G, EVA-3S investigators : Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: Results up to 4 years from a randomised, multicentre trial. Lancet Neurol 7: 885–892, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Ansel G, Strickman NE, Wang H, Cohen SA, Massaro JM, Cutlip DE, SAPPHIRE Investigators : Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med 358: 1572–1579, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, Hennerici M, Stingele R, Fiehler J, Zeumer H, Jansen O: Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: A multinational, prospective, randomised trial. Lancet Neurol 7: 893–902, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Coca SG, Krumholz HM, Garg AX, Parikh CR: Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA 296: 1377–1384, 2006 [DOI] [PubMed] [Google Scholar]
- 34.North American Symptomatic Carotid Endarterectomy Trial Collaborators: Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 325: 445–453, 1991 [DOI] [PubMed] [Google Scholar]
- 35.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 36.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Couchoud C, Pozet N, Labeeuw M, Pouteil-Noble C: Screening early renal failure: Cut-off values for serum creatinine as an indicator of renal impairment. Kidney Int 55: 1878–1884, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Paciaroni M, Eliasziw M, Kappelle LJ, Finan JW, Ferguson GG, Barnett HJ: Medical complications associated with carotid endarterectomy. North American Symptomatic Carotid Endarterectomy Trial (NASCET). Stroke 30: 1759–1763, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Greenwood M: A report on the natural duration of cancer. Reports on Public Health and Medical Subjects 33: 1–26, 1926 [Google Scholar]
- 40.Selvin S: Variation and bias. In: Statistical Analysis of Epidemiologic Data, 3rd Ed., Oxford, Oxford University Press, 2004, pp 54–69 [Google Scholar]
- 41.Lagakos SW: The challenge of subgroup analyses: Reporting without distorting. N Engl J Med 354: 1667–1669, 2006 [DOI] [PubMed] [Google Scholar]