Abstract
Ingestion of aristolochic acid (AA) can cause AA nephropathy (AAN), in which excessive death of tubular epithelial cells (TECs) characterize the acute phase. AA forms adducts with DNA, which may lead to TEC apoptosis via p53-mediated signaling. We tested this hypothesis both by studying p53-deficient mice and by blocking p53 in TECs with its inhibitor pifithrin-α. AA induced acute AAN in wild-type mice, resulting in massive apoptotic and necrotic TEC death and acute renal failure; p53 deficiency or pharmacologic inhibition attenuated this injury. In vitro, AA induced apoptotic and necrotic death of TEC in a time- and dosage-dependent manner, with apoptosis marked by a 10-fold increase in cleaved caspase-3 and terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling–positive/Annexin V-positive propidium iodide–negative TECs (all P < 0.001). AA induced dephosphorylation of STAT3 and the subsequent activation of p53 and TEC apoptosis. In contrast, overexpression of STAT3, p53 inhibition, or p53 knockdown with small interfering RNA all attenuated AA-induced TEC apoptosis. Taken together, these results suggest that AA induces TEC death via apoptosis by dephosphorylation of STAT3 and posttranslational activation of p53, supporting the hypothesis that p53 promotes renal injury in acute AAN.
Chinese herb nephropathy was first reported in Belgium in patients with prolonged intake of Chinese herbs during a slimming regimen and is recognized as one of the most severe complications caused by traditional Chinese medicine.1–3 It is now clear that the major substance that causes Chinese herb nephropathy is the plant nephrotoxin aristolochic acid and its metabolism products.4–6 Thus, the term aristolochic acid nephropathy (AAN), instead of Chinese herbal nephropathy, is used today.7,8 AAN has emerged as an important cause of drug-associated renal failure worldwide.9
Patients with AAN exhibit a rapidly progressive renal deterioration, resulting in acute renal failure that could lead to ESRD.1–3,10,11 A similar clinical course was observed in experimental animals treated with AA.12,13 Pathologically, chronic AAN is characterized by extensive interstitial fibrosis with atrophy and loss of renal tubules.1–3,10–13 The lesions of chronic AAN are mainly in the cortex involving proximal tubular epithelial cells (TECs)10–13; glomeruli are relatively spared with minimal inflammation.9–12 In contrast, progressive TEC death occurs early in the clinical course with an absence of renal fibrosis and inflammation in experimental models and patients with acute AAN.10,14,15 Although apoptosis is an important pathologic feature in in vivo and in vitro studies of acute AAN,16–18 the underlying mechanisms remain unclear.
In considering the genotoxic effect of AA with the formation of AA-DNA adducts and the importance of the p53 signaling pathway in DNA damage and cell apoptosis,19–21 we hypothesized that TEC apoptosis in acute AAN is dependent on p53 signaling. We investigated this by inducing acute AAN in p53 knockout (KO) and p53 wild-type (WT) mice and by blocking the p53 activities with a pharmacologic inhibitor. We further studied the toxicity of AA on TEC apoptosis by examining a panel of apoptotic biomarkers. The mechanism that AA induced TEC apoptosis by activating p53 via a STAT3-dependent posttranslational modification was identified.
Results
Mice Lacking the p53 Gene Are Protected from the Development of Acute AAN
We first tested the hypothesis that AA induced acute AAN via a p53-dependent mechanism by examining acute AAN induced in p53 WT and p53 KO mice. Histologically, compared with saline control mice, p53 WT mice developed severe acute AAN with focal and severe “shrinkage necrosis” and coagulative necrosis in TECs in the renal cortex at day 4 after AA injection, affecting up to 25 to 30% of cortical tubules (Figure 1). Most of the nonviable TECs exhibited typical apoptotic cell morphology, including cytoplasmic and nuclear condensation, fragmentation, and vacuolization (Figure 1, C and E). The TECs were detached from the tubular basement leading to naked basement membrane, a typical pathologic feature of AAN. The p53 WT mice treated with AA developed moderate to severe renal impairment with a three-fold increase in urinary protein excretion (Figure 1H), a two-fold increase in serum creatinine (Figure 1I), and a two-fold reduction of creatinine clearance (Figure 1J). In contrast, the KO mice lacking p53 were resistant to the development of AA-induced acute AAN with normal levels of urinary protein excretion, serum creatinine, and creatinine clearance. Only a small proportion of tubules demonstrated coagulative necrosis with a swollen, rather than condensed, necrotic morphology, but naked basement membrane change was not observed (Figure 1, D through J). Glomerular morphology in both WT and KO mice was normal as compared with saline control mice. Infiltration of inflammatory cells was not observed in kidneys with acute AAN (Figure 1, A through D).
Figure 1.
AA-induced acute AAN at day 4 is prevented in p53 KO mice. (A) Representative histology from a normal p53 WT mouse treated with saline. (B) Representative histology from a normal p53 KO mouse treated with saline. (C) Representative histology from a p53 WT mouse with acute AAN. Focal tubular shrinkage necrosis (outlined by a dashed line) is apparent in the cortex. (D) Representative histology from a p53 KO mouse with acute AAN. Note that, in contrast to the shrinkage necrosis, there are only a few tubules with coagulative necrosis (outlined by a dashed line). (E) Characterization of apoptotic cell morphology from a p53 WT mouse with acute AAN. Note that there are many TECs with typical apoptotic morphologies, including nuclear condensation (arrowheads), fragmentation (arrows), and vacuolization. Shrinkage of TEC with detachment from the tubular basement results in the typical pathologic feature of AAN with the naked basement membrane changes. (F) Characterization of coagulative necrosis in a p53 KO mouse with acute AAN. Note that typical coagulative necrosis morphology, including swollen granular cytoplasm and nuclei, is found in many TECs with a few apoptotic cells (arrowhead) and without the naked basement change (*). (G) Quantitative analysis of tubular death. (H) Measurement of proteinuria. (I) Measurement of serum creatinine. (J) Measurement of creatinine clearance. Data are means ± SEM for groups of six animals. **P < 0.01, ***P < 0.001 versus normal mice treated with saline; #P < 0.05, ###P < 0.001 versus p53 WT mice with acute AAN. Sections are stained with periodic acid-Schiff. Magnifications: ×200 in A through D; ×400 in E through F.
Role of p53 in Tubular Cell Apoptosis in a Murine Model of Acute AAN
We further defined the pathogenetic role of a p53-dependent apoptosis pathway in acute AAN. Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling–positive (TUNEL+) cells were occasionally detected in control WT and KO mice treated with saline (Figure 2A, i and ii). In contrast, there was a marked increase in the number of TUNEL+ apoptotic cells in WT mice treated with AA, particularly profound in areas with severe and focal tubular shrinkage necrosis (Figure 2Aiii). Nonetheless, only a few TUNEL+ cells around the necrotic tubules were detected in p53 KO mice treated with AA (Figure 2Aiv). Semiquantitative analysis of TUNEL+ cell population suggested that, in addition to necrosis, apoptosis contributed to acute tubular cell death in acute AAN (Figure 2C). The importance of a p53-dependent apoptotic pathway in acute AAN was further suggested by another biomarker, cleaved caspase-3. The renal expression of cleaved caspase-3 was increased in p53 WT mice treated with AA, but such increase was attenuated in p53 KO mice under an identical experimental condition (Figure 2, B and D).
Figure 2.
AA-induced tubular apoptosis in acute AAN at day 4 is blocked in p53 KO mice. (A) Representative TUNEL-stained sections from a p53 WT (i and iii) or a p53 KO (ii and iv) mouse treated with either saline (i and ii) or AA (iii and iv) at day 4. Note that many TUNEL+ apoptotic cells are found in the area with cortical tubular necrosis (*) in a p53 WT mouse with acute AAN as compared with a few TUNEL+ apoptotic cells noted in the area round a necrotic tubulus (*) in p53 KO mouse after AA treatment. (B) Western blot showing an increase in cleaved caspase-3 is noted in p53 WT but not in p53 KO mice with acute AAN. Each lane represents one mouse kidney. (C) Quantitative analysis of TUNEL+ cells. (D) Ratio between cleaved caspase-3 and GAPDH measured from Western blots. Data are means ± SEM for groups of six animals. *P < 0.05, ***P < 0.001 versus normal mice treated with saline; #P < 0.05, ##P < 0.01 versus p53 WT mice with acute AAN. Magnification, ×400.
We next explored whether a p53 inhibitor, pifithrin-α, exerted any therapeutic potential in acute AAN. Pifithrin-α at a dosage of 4.4 mg/kg per d was administered intraperitoneally for 3 d to p53 WT mice pretreated with AA. p53 blockade reduced TEC death (Figure 3, A through E) and the rise of serum creatinine (Figure 3G) with no significant attenuation of proteinuria (Figure 3F) or improvement of creatinine clearance (Figure 3H). Correspondingly, the histology and biomarker for apoptosis in AA-induced AAN were improved after pifithrin-α treatment (Figure 4).
Figure 3.
AA-induced acute AAN in p53 WT mice at day 4 is attenuated by treatment with a p53 inhibitor, pifithrin-α. (A) Representative histology from a p53 WT mouse with acute AAN treated with DMSO. Note that focal tubular necrosis (outlined by a dashed line) is apparent in the cortex. (B) Representative histology from a p53 WT mouse with acute AAN treated with pifithrin-α. Note that only a few tubules with necrotic changes (outlined by a dashed line) are found. (C) Characterization of apoptotic cell morphology from a p53 WT mouse with acute AAN treated with DMSO. Note that many TECs with typical apoptotic morphologies including nuclear condensation (arrowheads), fragmentation (arrows), vacuolization, and shrinkage are found with detachment from the tubular basement, resulting in the typical AAN pathology with the naked basement membrane change. (D) Characterization of coagulative necrosis from an acute AAN p53 KO mouse treated with a pifithrin-α. Note that treatment with the pifithrin-α inhibits TEC apoptosis (arrowheads) but not coagulative necrosis (*). (E) Quantitative analysis of tubular death. (F) Measurement of proteinuria. (G) Measurement of serum creatinine. (H) Measurement of creatinine clearance. Data are means ± SEM for groups of six animals. *P < 0.05, **P < 0.01, ***P < 0.001 versus normal mice treated with saline; #P < 0.05, ##P < 0.01 versus p53 WT mice with acute AAN treated with DMSO control. Sections are stained with periodic acid-Schiff. Magnifications: ×200 in A and B; ×400 in C and D.
Figure 4.
AA-induced tubular apoptosis in acute AAN in p53 WT mice at day 4 is blocked by a p53 inhibitor, pifithrin-α. (A) Representative TUNEL-stained sections from a normal p53 WT mouse treated with saline (i), a p53 WT mouse with acute AAN treated with DMSO (ii), and a p53 WT mouse with acute AAN treated with pifithrin-α (iii) and quantitative analysis of TUNEL+ cells (iv). Note that treatment with a p53 inhibitor, pifithrin-α (4.4 mg/kg per d), significantly attenuates the number of TUNEL+ apoptotic cells in acute AAN. (B) Western blot showing an increase in cleaved caspase-3 in p53 WT mice with acute AAN is inhibited by treatment with pifithrin-α. Data are means ± SEM for groups of six animals. ***P < 0.001 versus normal mice treated with saline; ##P < 0.01, ###P < 0.001 versus DMSO treatment in the p53 WT mice with acute AAN. Magnification, ×400.
In Vitro Study of Cultured TECs Exposed to AA
To understand better the signaling pathway mediating the p53-dependent apoptosis in acute AAN, we performed in vitro studies on a normal rat TEC line (NRK52E). We confirmed that AA caused TEC necrosis at 24 h in a dosage-dependent manner (Figure 5A). This was also associated with apoptosis, shown by a 10-fold increase in both TUNEL+ and Annexin V+ propidium iodide–negative (PI−) TECs (Figure 5, B through E) and in cleaved caspase-3 (Figure 6). Notably, higher dosages of AA (>10 μg/ml) caused significant necrosis as a result of the cytotoxicity of AA demonstrated by Trypan blue exclusion (Figure 5F), lactate dehydrogenase (LDH) release assay (Figure 5G), and the 3-(4, 5-Dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay (Figure 5H). Nonetheless, apoptosis remained the most important mechanism of TEC death in acute AAN because this occurred even at a dosage with undetectable cytotoxicity (<5 μg/ml).
Figure 5.
AA induces TEC apoptosis in a dosage-dependent manner. TECs were cultured with AA at various dosages for 24 h. TEC apoptosis or cytotoxicity was analyzed as described in the Concise Methods section. (A) Morphologic analysis by inverted microscope. (B) TUNEL staining. (C) Annexin V/PI flow cytometry analysis. The percentage of apoptotic cells is identified with Annexin V+PI− as shown in 4. (D) Quantitative analysis of TUNEL+ TECs. (E) Quantitative analysis of Annexin V+PI− TECs by flow cytometry. (F) Cell death rate determined by Trypan blue. (G) Cytotoxicity by LDH assay. (H) MTT assay. Each graph represents results from at least three independent experiments, and each bar or data point represents means ± SEM for three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus dosage 0. Magnification, ×400.
Figure 6.
Western blot analysis showing AA induces cleaved caspase-3 in a time- and dosage-dependent manner. (A) AA at a dosage of 5 μg/ml increases cleaved caspase-3 in a time-dependent manner. (B) AA (5 μg/ml) increases cleaved caspase-3 at 24 h in a dosage-dependent manner. Each bar represents the mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01 versus time or dosage 0.
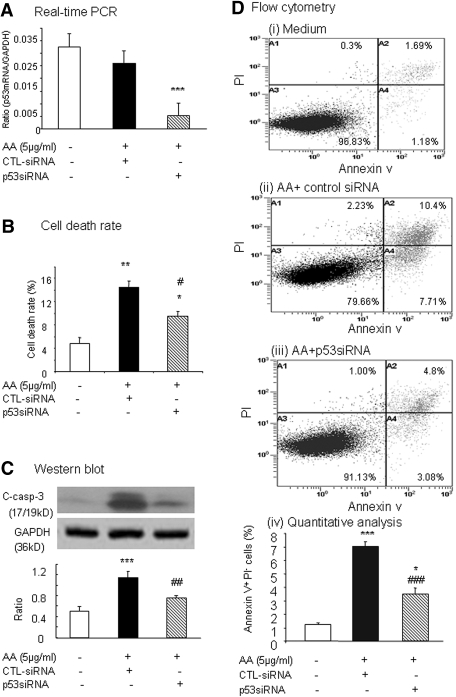
The therapeutic effect of p53 blockade in AA-induced TEC apoptosis was confirmed in vitro with a p53 pharmacologic inhibitor, pifithrin-α, and p53 small interfering RNA (siRNA). The p53 inhibitor (20 μM) inhibited AA-induced (5 μg/ml) TEC death (Figure 7A) and apoptosis as determined by TUNEL+ TECs (60% reduced; Figure 7, B and D), Annexin V+PI− TECs (50% reduced; Figure 7, C and E), and cleaved caspase-3 (40% reduced; Figure 7, F and G). The addition of p53 siRNA (25 nM) but not a control siRNA (25 nM) reduced p53 mRNA expression by >80% (Figure 8A). This was associated with the inhibition of AA-induced (5 μg/ml) TEC death detected by Trypan blue exclusion (Figure 8B) and apoptosis detected by cleaved caspase-3 (Figure 8C) and Annexin V+PI− TECs (Figure 8D).
Figure 7.
AA-induced TEC apoptosis is blocked by a p53 inhibitor, pifithrin-α. TECs were cultured with AA (5 μg/ml) in the presence or absence of pifithrin α (20 μM) or control DMSO (20 μM) for 24 h. TEC apoptosis was analyzed as described in the Concise Methods section. (A) Morphologic analysis by inverted microscope. (B) TUNEL staining. (C) Annexin V/PI flow cytometry analysis. The percentage of apoptotic cells is identified with Annexin V+PI− as shown in 4. (D) Quantitative analysis of TUNEL+ TECs. (E) Quantitative analysis of Annexin V+PI− TEC by flow cytometry. (F) Western blot analysis of cleaved caspase-3. (G) Semiquantitative analysis of cleaved caspase-3 from Western blots (F). Each graph represents results from at least three independent experiments, and each bar represents the mean ± SEM for three repeat experiments. *P < 0.05, **P < 0.001 versus AA and AA+DMSO. Magnification, ×400.
Figure 8.
Blockade of p53 with siRNA prevents AA-induced TEC apoptosis as determined by cleaved caspase-3 and Annexin V. (A) Real-time PCR showing TECs treated with p53 siRNA (25 nM) but not control siRNA (25 nM) significantly inhibits p53 mRNA expression. (B) Cell death rate determined by Trypan blue analysis. (C) Western blot analysis showing TECs treated with p53 siRNA (25 nM) but not control siRNA (25 nM) are able to prevent AA-induced (5 μg/ml) cleaved caspase-3. (D) Flow cytometry showing blockade of p53 with siRNA prevents AA-induced (5 μg/ml) TEC apoptosis: Medium alone (i), AA treatment (5 μg/ml for 24 h) after control siRNA (25 nM) transfection (ii), AA treatment (5 μg/ml for 24 h) after p53 siRNA transfection (iii), and quantitative analysis (iv). Each bar represents the mean ± SEM for three repeat experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus medium alone; ##P < 0.01, ###P < 0.001 versus control siRNA treatment.
AA Activates p53 by Posttranslational Modification
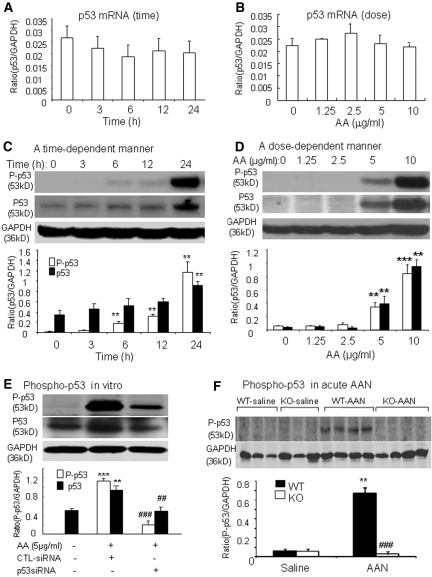
We next investigated the mechanism by which AA activated the p53 pathway during TEC apoptosis by examining p53 mRNA expression transcriptionally and posttranslational modification by phosphorylation of p53 protein. Real-time PCR showed that the addition of AA did not significantly induce p53 mRNA expression in a time- and dosage-dependent manner (Figure 9, A and B); however, Western blot analysis revealed that AA was able to activate the p53 pathway posttranslationally by phosphorylation, because we detected high levels of phosphorylated p53 (Ser15) in a time- and dosage-dependent manner (Figure 9, C and D). This resulted in a 130-fold increase in the phosphorylated p53 at 24 h after stimulation by AA (5 μg/ml; Figure 9C) that was associated with an increase in total p53 protein in a time- and dosage-dependent manner (Figure 9, C and D).
Figure 9.
AA activates p53 by phosphorylation in vitro and in vivo. (A) Real-time PCR showing no change in p53 mRNA in TECs cultured with AA (5 μg/ml) for various periods. (B) Real-time PCR showing expression of tubular p53 mRNA at 3 h is not significantly altered by addition of AA in a dosage-dependent manner. (C) AA at a dosage of 5 μg/ml induces phosphorylation of p53 in a time-dependent manner, resulting in a 130-fold increase in phosphorylated p53 by 24 h, which is associated with a marked upregulation of p53 protein. (D) Addition of AA for 24 h induces phosphorylation of p53 in a dosage-dependent manner, which is also associated with a marked upregulation of p53 protein. (E) Western blot analysis showing TECs treated with p53 siRNA (25 nM) but not control siRNA (25 nM) are able to prevent AA-induced (5 μg/ml for 24 h) p53 phosphorylation and p53 protein expression. (F) Western blot analysis showing administration of AA (10 mg/kg per d for 3 d) causes a marked phosphorylation of p53 in the diseased kidney in p53 WT mice, not in p53 KO mice. Each lane represents one mouse kidney, and each bar represents the mean ± SEM from three independent experiments for in vitro study (A through E) or a group of six animals (F). **P < 0.01, ***P < 0.001 versus medium- or saline-treated controls; ##P < 0.01, ###P < 0.001 versus control siRNA treatment (E) or p53 WT mice with acute AAN (F).
To determine further the functional importance of phosphorylated p53 in AA-induced apoptosis, we examined whether blockade of p53 inhibited AA-induced TEC apoptosis in vitro and in vivo. Knockdown of p53 with the p53 siRNA resulted in a marked inhibition of AA-induced (5 μg/ml) p53 protein phosphorylation (>80% reduced) and accumulation (60% reduced; Figure 9E). In parallel, this was associated with a marked inhibition of TEC apoptosis in response to AA shown by measuring cleaved caspase-3 and Annexin V (Figure 8, C and D). The functional importance of phosphorylated p53 in AA-induced apoptosis was better documented in acute AAN induced in p53 WT and KO mice (Figure 9F).
AA Induces Phosphorylation of p53 via a STAT3-Dependent Mechanism
To explore further the upstream signaling mechanism by which AA activated the p53 pathway, we examined the activation of STAT3 by phosphorylation because phospho-STAT3 mediated the suppression of p53 function.22 Addition of AA (5 μg/ml) caused dephosphorylation of phospho-STAT3 (Tyr705), thereby reducing STAT3 protein expression in a time- and dosage-dependent manner (Figure 10). The functional role of phospho-STAT3 in protecting AA-mediated TEC apoptosis via the p53 pathway was further demonstrated by the finding that overexpression of STAT3 in TECs rescued AA-induced (5 μg/ml) dephosphorylation of STAT3, thereby inhibiting AA-induced p53 phosphorylation and p53 protein accumulation and p53-mediated apoptosis demonstrated by cleaved caspase-3 and TUNEL+ TECs (Figure 11).
Figure 10.
AA induces dephosphorylation of STAT3 in NRK52 TECs. (A) Western blot analysis showing addition of AA (5 μg/ml) induces dephosphorylation of phospho-STAT3 and reduces STAT3 protein accumulation in a time-dependent manner. (B) Western blot analysis showing AA induces dephosphorylation of phospho-STAT3 and reduces STAT3 protein accumulation at 24 h in a dosage-dependent manner. Each bar represents the mean ± SEM for three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus time 0 and medium control.
Figure 11.
Overexpression of STAT3 rescues AA-induced desphosphorylation of STAT3 and prevents AA-induced p53 activation and apoptosis. (A) Western blot analysis showing that compared with control pcDNA3, dephosphorylation of phospho-STAT3 and reduction of STAT3 protein induced by AA (5 μg/ml) are prevented in NRK52E cells stably expressing STAT3, which results in inhibition of AA-induced p53 phosphorylation and protein accumulation, and cleaved caspase-3 expression. (B) Quantitative analysis showing an inhibitory effect of overexpressing STAT3 on AA-induced dephosphorylation of phospho-STAT3, STAT3 protein expression, phospho-p53, p53 protein accumulation, cleaved caspase-3, and TUNEL+ cells. Each bar represents the mean ± SEM for three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus medium control; #P < 0.05, ##P < 0.01, ###P < 0.001 versus pcDNA3 control.
Discussion
Emerging evidence shows that AA is a key mediator of AAN characterized by progressive TEC death and renal scarring that ultimately result in acute or chronic renal failure.1–3,10–13 In animals and humans with acute AAN, progressive TEC death with patchy necrosis, primarily affecting proximal TECs in the renal cortex, is the major pathologic feature.3,10,11,14,15,23 Increased apoptosis has been observed in AA-associated cancers24–26 and in cultured tubular cells exposed to AA.19,27,28 A calcium-dependent mitochondria stress mechanism has been proposed.17,27,28 In this study, we provide new evidence to support the role of p53 in AA-induced acute AAN by demonstrating that mice null for p53 are protected against the development of acute AAN. These animals exhibited reduced TEC death through apoptosis with less renal injury. Furthermore, pharmacologic blockade of p53 using a p53 inhibitor, pifithrin-α, partially protected AA-induced acute AAN with reduced TEC apoptosis and blunt rise of serum creatinine. The inability of pifithrin-α to improve proteinuria and creatinine clearance in acute AAN may be related to the necrotic feature of AAN and also the specificity, dosage, and individual response to pifithrin-α treatment. Interestingly, p53 blockade with specific siRNA inhibited the AA-induced TEC apoptosis that further supported the pathogenic role for p53 in the development of acute AAN, similar to that of p53 in cisplatin-induced nephrotoxicity.29
p53 is a tumor suppressor that induces growth arrest, senescence, and apoptosis in response to various cellular stresses, including exposure to DNA-damaging agents and hypoxia.21 A stress signal is transmitted to the p53 protein by posttranslational modifications, including phosphorylation, acetylation, methylation, ubiquitination, and sumoylation.28,30,31 Activation of the p53 protein initiates downstream programs, including cell-cycle arrest, cellular senescence, and apoptosis.28,30–32 Phosphorylation of p53 enhances the p53 activity through stabilization of the p53 protein, thereby preventing p53 from degradation.31 Once activated, p53 can induce apoptosis through a transcriptional-dependent or -independent mechanism. The latter acts through increased permeabilization of the mitochondrial outer membrane, which may account for AA-induced TEC apoptosis via the calcium-dependent mechanism.17,27,28 AA is a genotoxic agent, as evidenced by the formation of AA-DNA adducts in TECs and urothelial cells in AAN.14,20 AA-related DNA adducts are detected as early as day 1, reach a steady state level at day 2, and remain almost unchanged up to 5 wk.14 Although the potential impact of AA-DNA adducts on tubular damage in acute AAN is largely unknown, its genotoxic stress is able to activate p53 by posttranslational modifications.32,33 Hence, AA-DNA adducts formed in TECs exert the genotoxic stress,14,20 which may represent a mechanism by which AA induces p53 phosphorylation as shown in this study.
In this study, we had found that TEC apoptosis induced by AA is mediated through the p53 pathway by posttranslational modification via a STAT3-dependent mechanism. We also demonstrated that AA is capable of inducing dephosphorylation of phospho-STAT3 in TECs, which resulted in a marked increase of phosphorylation of p53 (130-fold), increased accumulation of p53 protein, and enhanced TEC apoptosis. The functional importance of STAT3 in AA-induced p53-dependent TEC apoptosis was strongly supported by the finding that overexpressing STAT3 prevented AA-induced p53 phosphorylation and p53-dependent TEC apoptosis. This is similar to a previous finding that IL-24 induces apoptosis of chronic lymphocytic leukemia B cells through a mechanism of dephosphorylation of STAT3 and stabilization of p53 expression by phosphorylating p53 (Ser15).22 In this study, AA induced TEC apoptosis by activating the p53 pathway via inactivation of the p53 repressor STAT3. Addition of AA also induces the dephosphorylation of STAT3, leading to stabilization of p53 by increasing p53 (Ser15) phosphorylation and protein accumulation. Hence, dephosphorylation of STAT3 may be an important mechanism by which AA activates the p53-dependent pathway to mediate TEC apoptosis. Because of the complexity of p53 stabilization and activation in various disease conditions,22,29,30–35 further studies to understand fully how AA activates p53 signaling pathways are warranted.
It should be pointed out that although apoptosis is a mechanism of AA-induced acute AAN, TEC necrosis also occurs in acute AAN; a small proportion of tubules with typical changes of coagulative necrosis, rather than shrinkage necrosis, is found in p53 KO mice, and addition of AA at a higher dosage (>10 μg/ml) also causes significant cytotoxicity in TECs in vitro. We also recognize that TEC apoptosis induced by AA in both in vitro and in vivo settings is a dynamic process, and, hence, analysis of apoptosis at certain time points may underestimate the proportion of apoptotic cells. Furthermore, only a few TUNEL+ apoptotic cells are found in p53 KO mice with acute AAN despite that we have demonstrated an important role of p53 in AA-induced TEC apoptosis. These findings suggest other mechanisms independent of p53 may also participate in the process of apoptosis induced by AA.
Concise Methods
Animal Model of Acute AAN
p53 KO mice were a gift from Prof. Lawrence A. Donehower (Baylor College of Medicine).36 This p53 mutant mouse was bred on a C57BL/6-Tyrc background and tagged with a tyrosinase coat color minigene that makes mouse genotype distinguishable by their coat color. Acute AAN was induced in genetically identical littermates of p53 WT and KO mice (n = 6, male, aged 8 to 12 wk) with AA at a dosage of 10 mg/kg per d in 200 μl of saline injected intraperitoneally for 3 d. For examination of the therapeutic effect of blocking p53 on acute AAN, a p53 pharmacologic inhibitor, pifithrin-α, at an optimal dosage of 4.4 mg/kg per d was dissolved in DMSO and injected intraperitoneally into a group of six male p53 WT mice at the same time with AA (10 mg/kg per d) for 3 d. The dosage used for this study was determined by dosage-dependent studies of acute AAN induced in p53 WT mice. Control animals received the same amount of AA but saline-containing DMSO. Groups of six mice were killed on day 4 after AA injection with or without pifithrin-α treatment. In addition, groups of six normal, aged-matched WT and KO mice receiving a daily intraperitoneal injection of saline served as negative controls. Urine and kidney tissues were collected for further investigations as detailed next. p53 KO mice suspected of tumor were excluded. The experimental procedures were approved by the committee on the use of live animals for teaching and research at the University of Hong Kong.
Measurements of Proteinuria and Renal Function
All mice were housed in metabolic cages for 16 h before and after AA injection on days 0 and 3. Urinary protein excretion was determined using Coomassie Blue method and expressed as mg against urinary creatinine (mg), whereas creatinine in serum and urine was detected by QuantiChrom Creatinine Assay Kit (BioAssay Systems, Hayward, CA) as based on the improved Jaffe method.
Histology
Kidney sections (3 μm) from p53 WT and KO mice embedded in methyl Carnoy's fixed paraffin and stained with periodic acid-Schiff was examined. Tubular necrosis in the entire cortex and tubulointerstitium (a cross-section of the kidney) was determined using the Image J program (National Institutes of Health). Briefly, the cortical area with tubular necrosis was identified and outlined. Ten fields (×10) of cortical tissues were scored, and the percentage of necrotic area in the examined renal cortex was then measured. Data were expressed as a percentage of cortical area examined.
Cell Culture
Normal rat kidney TEC line (NRK52E) was grown in DMEM/F12 (Hyclone, Logan, UT) in a 5% CO2 atmosphere at 37°C, containing 5% FBS, 60 μg/ml penicillin, and 100 μg/ml streptomycin. AA sodium salt (mixture of AAI [65%] and AAII [27%]; Sigma-Aldrich, St. Louis, MO) in various dosages (0.00, 1.25, 2.50, 5.00, and 10.00 μg/ml) was added to the culture for 24 h. For a time-dependent experiment, a dosage of AA at 5 μg/ml was added to the culture for periods of 0, 3, 6, 12, and 24 h. For blocking the p53 activity pharmacologically, a p53 inhibitor, pifithrin-α (20 μM; Calbiochem, Darmstadt, Germany), or DMSO as control was added to the culture 1 h before addition of AA (5 μg/ml). Parallel blocking experiment was conducted by transfecting p53 siRNA (25 nM) or control siRNA (25 nM; Ambion, Austin, TX) into NRK52E using lipofectamine 2000 (Invitrogen, Carlsbad, CA) for 72 h before addition of AA (5 μg/ml) for 24 h. At least three independent experiments were performed throughout the study. For determination of cell survival in response to AA, the p53 inhibitor, and the p53 siRNA, dead cells (identified by 0.4% Trypan blue [Sigma-Aldrich]) were counted at 24 h, and the cell death rate was expressed as percentage of Trypan blue–positive cells.
For investigation of the protective role of STAT3 in AA-induced TEC apoptosis via the p53-dependent mechanism, a stable TEC line (NRK52E) expressing WT STAT3 was established by transfecting cells with a STAT3 WT or a control empty plasmid (pcDNA3) with lipofectamine 2000 (Invitrogen). Cells stably expressing STAT3 were selected with addition of G418 at a dosage of 100 mg/ml and thereafter were maintained in G418 at 50 mg/ml. The STAT3 plasmid was a gift from Prof. Akihiko Yoshimura (Kyushu University, Japan).37
Cytotoxicity Analysis with LDH Assay and MTT Assay
NRK52E were seeded in 96-well plates for confluence. Then AA in various dosages (1.25, 2.5, 5.0, 10.0, and 20.0 μg/ml) was added for 24 h. The supernatants were collected for cytotoxicity analysis by LDH (TOX-7 kit; Sigma-Aldrich) and MTT assay (celltiter 96 Nonradioactive Cell Proliferation Assay; Promega, Madison, WI) according to the manufacturers' instructions. The LDH release rate was calculated as a percentage of total LDH.
Western Blot Analysis
Proteins from cultured cells or renal tissues were extracted with RIPA lysis buffer and analyzed by Western blot as described previously.38 Briefly, after blocking nonspecific binding, the membranes were incubated with primary antibodies against p53, STAT3, phospho-STAT3 (Tyr705; Santa Cruz Biotechnology, Santa Cruz, CA), phospho-p53 (Ser15), cleaved-caspase-3 (Asp175; Cell Signaling, Beverly, MA), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Chemicon, Temecula, CA) overnight at 4°C. After extensive wash, the membranes were then incubated with IRDye 800–conjugated secondary antibodies (Rockland, Gilbertsville, PA) for 1 h before detection with Odyssey Infrared Imaging System. Positive immunoreactive bands were quantified and normalized by GAPDH using the Image J program (National Institutes of Health).
FACS Assay with Annexin V/PI Staining
Cells under various experimental conditions were first harvested with PBS containing 0.25% trypsin and 2% EDTA before processing with Annexin V/PI staining kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions and finally analyzed in Cytomics FC 500 Flow Cytometer (Beckman Coulter, Fullerton, CA) for apoptosis detection. Annexin V+PI− cells were recorded as apoptotic cells.
TUNEL Staining for Apoptosis
NRK52E cells were cultured in eight-chamber glass slides under various experimental conditions as described already. After fixing with 4% paraformaldehyde for 1 h, slides were stained with TUNEL kit (in situ cell death detection kit, POD; Roche Applied Science) following the manufacturer's instructions. Positive cells were counted from 500 cells on each slide chamber and expressed as a percentage of total cells. For detection of apoptotic cells in mouse kidney tissues, 3-μm tissue sections were stained with TUNEL kit. TUNEL+ cells were counted under high-power fields (×400) by means of a 0.025-mm2 graticule fitted in the eyepiece of the microscope and expressed as cells per mm2.
Real-Time PCR
RNA was collected from cells or renal tissues and purified by the RNeasy kit according to the manufacturer's instructions (Qiagen Inc., Valencia, CA). The cDNA was synthesized, and real-time PCR was run with the Opticon real-time PCR machine (MJ Research, Waltham, MA) as described previously.38 The specificity of real-time PCR was confirmed by the melting-curve analysis. Housekeeping gene GAPDH was used as an internal standard. The primers used in this study are as follows: p53, forward 5′-ACATGACTGAGGTCGTGAGA and reverse 5′-TTTCCTTCCACCCGGATAAG; GAPDH, forward 5′-CCTGGAGAAACCTGCCAAGTATGA and reverse 5′-TTGAAGTCACAGGAGACAACCTGG.
Statistical Analysis
Data obtained from this study are expressed as means ± SEM. Statistical analyses were performed using one-way ANOVA followed by the Newman-Keuls as post hoc tests from GraphPad Prism 4.0 (GraphPad Software, San Diego, CA).
Disclosures
None.
Acknowledgments
This study was supported by grants from Key Project of Science and Technology Bureau of Sichuan Province of China (2006z09-026) and Research Grants Council of Hong Kong (RGC, GRF 768207, and 767508)
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Vanherweghem JL, Depierreux M, Tielemans C, Abramowicz D, Dratwa M, Jadoul M, Richard C, Vandervelde D, Verbeelen D, Vanhaelen-Fastre R: Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. Lancet 341: 387–391, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Yang CS, Lin CH, Chang SH, Hsu HC: Rapidly progressive fibrosing interstitial nephritis associated with Chinese herbal drugs. Am J Kidney Dis 35: 313–318, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Nortier JL, Vanherweghem JL: For patients taking herbal therapy: Lessons from aristolochic acid nephropathy. Nephrol Dial Transplant 22: 1512–1517, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Vanhaelen M, Vanhaelen-Fastre R, But P, Vanherweghem JL: Identification of aristolochic acid in Chinese herbs. Lancet 343: 174, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Chen SM, Fan MY, Tseng CC, Ho Y, Hsu KY: Pharmacokinetics and nephrotoxicity of aristolochic acid in rabbits. Toxicon 50: 180–188, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Wen YJ, Su T, Tang JW, Zhang CY, Wang X, Cai SQ, Li XM: Cytotoxicity of phenanthrenes extracted from Aristolochia contorta in human proximal tubular epithelial cell line. Nephron Exp Nephrol 103: e95–e102, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Chen HY, Ma B-Y, Grant A, Lampert N: Time to abandon the term “Chinese herbs nephropathy.” Kidney Int 60: 2039–2040, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Gillerot G, Jadoul M, Arlt VM, van Ypersele De Strihou C, Schmeiser HH, But PP, Bieler CA, Cosyns JP: Aristolochic acid nephropathy in a Chinese patient: Time to abandon the term “Chinese herbs nephropathy”? Am J Kidney Dis 38: E26, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Debelle FD, Vanherweghem JL, Nortier JL: Aristolochic acid nephropathy: A worldwide problem. Kidney Int 74: 158–169, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Li X, Wang H: Possible mechanisms explaining the tendency towards interstitial fibrosis in aristolochic acid-induced acute tubular necrosis. Nephrol Dial Transplant 22: 445–456, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Depierreux M, Van Damme B, Vanden Houte K, Vanherweghem JL: Pathologic aspects of a newly described nephropathy related to the prolonged use of Chinese herbs. Am J Kidney Dis 24: 172–180, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Debelle FD, Nortier JL, De Prez EG, Garbar CH, Vienne AR, Salmon IJ, schodt-Lanckman MM, Vanherweghem JL: Aristolochic acids induce chronic renal failure with interstitial fibrosis in salt-depleted rats. J Am Soc Nephrol 13: 431–436, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Cosyns JP, Dehoux JP, Guiot Y, Goebbels RM, Robert A, Bernard AM, van Ypersele de SC: Chronic aristolochic acid toxicity in rabbits: A model of Chinese herbs nephropathy? Kidney Int 59: 2164–2173, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Lebeau C, Debelle FD, Arlt VM, Pozdzik A, De Prez EG, Phillips DH, Deschodt-Lanckman MM, Vanherweghem JL, Nortier JL: Early proximal tubule injury in experimental aristolochic acid nephropathy: Functional and histological studies. Nephrol Dial Transplant 20: 2321–2332, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Sato N, Takahashi D, Chen SM, Tsuchiya R, Mukoyama T, Yamagata S, Ogawa M, Yoshida M, Kondo S, Satoh N, Ueda S: Acute nephrotoxicity of aristolochic acids in mice. J Pharm Pharmacol 56: 221–229, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Liu MC, Maruyama S, Mizuno M, Morita Y, Hanaki S, Yuzawa Y, Matsuo S: The nephrotoxicity of Aristolochia manshuriensis in rats is attributable to its aristolochic acids. Clin Exp Nephrol 7: 186–194, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Gao R, Zheng F, Liu Y, Zheng D, Li X, Bo Y, Liu Y: Aristolochic acid I-induced apoptosis in LLC-PK1 cells and amelioration of the apoptotic damage by calcium antagonist. Chin Med J (Engl) 113: 418–424, 2000 [PubMed] [Google Scholar]
- 18.Balachandran P, Wei F, Lin RC, Khan IA, Pasco DS: Structure activity relationships of aristolochic acid analogues: Toxicity in cultured renal epithelial cells. Kidney Int 67: 1797–1805, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Liu Z, Guo X, Shu J, Chen Z, Li L: Aristolochic acid I-induced DNA damage and cell cycle arrest in renal tubular epithelial cells in vitro. Arch Toxicol 80: 524–532, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Lebeau C, Arlt VM, Schmeiser HH, Boom A, Verroust PJ, Devuyst O, Beauwens R: Aristolochic acid impedes endocytosis and induces DNA adducts in proximal tubule cells. Kidney Int 60: 1332–1342, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Fridman JS, Lowe SW: Control of apoptosis by p53. Oncogene 22: 9030–9040, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Sainz-Perez A, Gary-Gouy H, Gaudin F, Maarof G, Marfaing-Koka A, de Revel T, Dalloul A: IL-24 induces apoptosis of chronic lymphocytic leukemia B cells engaged into the cell cycle through dephosphorylation of STAT3 and stabilization of p53 expression. J Immunol 181: 6051–6060, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Pozdzik AA, Salmon IJ, Debelle FD, Decaestecker C, Van den Branden C, Verbeelen D, Deschodt-Lanckman MM, Vanherweghem JL, Nortier JL: Aristolochic acid induces proximal tubule apoptosis and epithelial to mesenchymal transformation. Kidney Int 73: 595–607, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Cosyns JP, Jadoul M, Squifflet JP, Wese FX, van Ypersele de Strihou C: Urothelial lesions in Chinese-herb nephropathy. Am J Kidney Dis 33: 1011–1017, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Simões ML, Hockley SL, Schwerdtle T, Gamboa da Costa G, Schmeiser HH, Phillips DH, Arlt VM: Gene expression profiles modulated by the human carcinogen aristolochic acid I in human cancer cells and their dependence on TP53. Toxicol Appl Pharmacol 232: 86–98, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Chang HR, Lian JD, Lo CW, Huang HP, Wang CJ: Aristolochic acid-induced cell cycle G1 arrest in human urothelium SV-HUC-1 cells. Food Chem Toxicol 45: 396–402, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Qi X, Cai Y, Gong L, Liu L, Chen F, Xiao Y, Wu X, Li Y, Xue X, Ren J: Role of mitochondrial permeability transition in human renal tubular epithelial cell death induced by aristolochic acid. Toxicol Appl Pharmacol 222: 105–110, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Hsin YH, Cheng CH, Tzen JT, Wu MJ, Shu KH, Chen HC: Effect of aristolochic acid on intracellular calcium concentration and its links with apoptosis in renal tubular cells. Apoptosis 11: 2167–2177, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z: Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol 293: F1282–2891, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris SL, Levine AJ: The p53 pathway: Positive and negative feedback loops. Oncogene 24: 2899–2908, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Lavin MF, Gueven N: The complexity of p53 stabilization and activation. Cell Death Differ 13: 941–950, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Sagaguchi T, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, Anderson CW, Appella E: DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev 12: 2831–2841, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apella E, Anderson CW: Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem 268: 2764–2772, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Pabla N, Huang S, Mi QS, Daniel R, Dong Z: ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem 283: 6572–6583, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Jiang M, Wei Q, Wang J, Du C, Yu J, Zhang L, Dong Z: Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25: 4056–4066, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Zheng B, Vogel H, Donehower LA, Bradley A: Visual genotyping of a coat color tagged p53 mutant mouse line. Cancer Biol Ther 1: 433–435, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Yoshida T, Hanada T, Tokuhisa T, Kosai K, Sata M, Kohara M, Yoshimura A: Activation of STAT3 by the hepatitis C virus core protein leads to cellular transformation. J Exp Med 196: 641–653, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu P, Liu F, Wang W, Huang XR, Entman ML, Schwartz RJ, Wei L, Lan HY: Signaling mechanism of renal fibrosis in unilateral ureteral obstructive kidney disease in ROCK1 knockout mice. J Am Soc Nephrol 17: 3105–3114, 2006 [DOI] [PubMed] [Google Scholar]