Abstract
Hereditary hypouricemia may result from mutations in the renal tubular uric acid transporter URAT1. Whether mutation of other uric acid transporters produces a similar phenotype is unknown. We studied two families who had severe hereditary hypouricemia and did not have a URAT1 defect. We performed a genome-wide homozygosity screen and linkage analysis and identified the candidate gene SLC2A9, which encodes the glucose transporter 9 (GLUT9). Both families had homozygous SLC2A9 mutations: A missense mutation (L75R) in six affected members of one family and a 36-kb deletion, resulting in a truncated protein, in the other. In vitro, the L75R mutation dramatically impaired transport of uric acid. The mean concentration of serum uric acid of seven homozygous individuals was 0.17 ± 0.2 mg/dl, and all had a fractional excretion of uric acid >150%. Three individuals had nephrolithiasis, and three had a history of exercise-induced acute renal failure. In conclusion, homozygous loss-of-function mutations of GLUT9 cause a total defect of uric acid absorption, leading to severe renal hypouricemia complicated by nephrolithiasis and exercise-induced acute renal failure. In addition to clarifying renal handling of uric acid, our findings may provide a better understanding of the pathophysiology of acute renal failure, nephrolithiasis, hyperuricemia, and gout.
In most mammals, uric acid (UA) is oxidized by the hepatic enzyme uricase to highly soluble allantoin. In humans, however, this enzyme is inactive as a result of mutational silencing,1 making UA the end product of purine metabolism. Serum UA concentration depends on both UA production and UA removal by the kidneys and intestinal tract and is high in humans compared with other mammals. Elevation of serum UA levels has been associated with various diseases, including gout, hypertension, and cardiovascular and renal disease.2 Conversely, it has been suggested that UA has a beneficial role as a natural antioxidant, and low serum UA levels have been linked to several neurologic diseases.2
Studies of renal handling of UA in humans have provided evidence for a historical model of urinary UA excretion, which consists of four components: Free glomerular filtration, tubular absorption, secretion, and postsecretion reabsorption. The location and molecular physiology of the three tubular transport components, however, have not been completely clarified.3
The first renal UA transporter, URAT1, was identified in 2002 by Enomoto et al.4 The significance of URAT1 in the handling of UA was demonstrated by genetic analysis of Japanese patients with hereditary renal hypouricemia.4,5 These patients were characterized by very low levels of serum UA, high fractional excretion of UA, and attenuated response of urinary urate excretion to pyrazinamide and probenecid.5 Most of these patients were asymptomatic, but some had nephrolithiasis or were predisposed to exercise-induced acute renal failure (EIARF). The Japanese patients were found to possess homozygous or compound heterozygous loss-of-function mutations in the gene SLC22A12 coding for human URAT1; most of them carry at least one allele with the truncation mutation W258X.4–6
Mutations in SLC22A12 seem to be very rare outside Japan. A mutation analysis of renal hypouricemia in Korea showed that three of four patients with URAT1 mutations carried the W258X mutation.7 We previously described hereditary hypouricemia as a result of a homozygous SLC22A12 missense mutation (R496C) in three Israeli families of Iraqi origin.8 Although serum UA level and fractional excretion of UA were similar to those of the Japanese patients, none of our patients developed EIARF.
A recent meta-analysis of 14 genome-wide association scans in Europe demonstrated significant association of serum UA concentration with several other genes, including SLC22A11 coding for organic anion transporter 4 (OAT4), SLC17A1 coding for NPT4, the ATP-binding cassette transporter ABCG2, and SLC2A9 coding for the glucose-facilitated transporter GLUT9.9 OAT4,10 NPT1,11 ABCG2,12 and GLUT913–16 have been shown to be expressed in renal tubular cells and to transport UA in vitro. Recently, heterozygous mutations of GLUT9 were shown to cause renal hypouricemia.16
In this report, we show that homozygous mutations of GLUT9 cause severe hereditary hypouricemia complicated by nephrolithiasis and EIARF. Our findings provide further evidence for the key role played by GLUT9 in renal UA handling.
Results
Clinical Characteristics
Family 1.
The index patient (IV6; Figure 1A) was a previously healthy 18-yr-old man, who presented with ARF after physical exertion and required hemodialysis for 3 wk. One month after hospital discharge, serum urea and creatinine were normal and serum UA level was 0.1 mg/dl. The clinical course of the patient was described in detail in our previous report.17
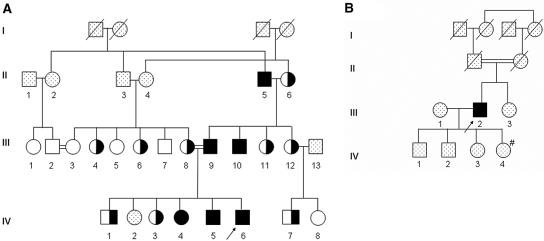
Figure 1.
Pedigrees of two unrelated consanguineous families with severe renal hypouricemia and SLC2A9 mutations. (A) Pedigree of family 1. (B) Pedigree of family 2. Solid symbols denote affected family members, open symbols denote unaffected family members, half-solid denote heterozygous family members, and dotted symbols denote family members who were not available for examination. Circles represent female family members, squares represent male family members, and crosses represent dead family members. Arrows indicate index patients. #Low serum UA (1.5 mg/dl), not available for genetic evaluation.
This patient is a member of a highly consanguineous Israeli-Arab family (Figure 1A). We evaluated all available family members and found five additional individuals with extremely low hypouricemia and fractional excretion of UA >150%; two had a history of EIARF (III9 and IV5), and two reported renal stones (II5 and III10). Clinical features and data related to UA handling of all individuals are shown in Table 1.
Table 1.
Clinical data and SLC2A9 mutations of the patients with renal hypouricemia and their family members
| Patient | Gender | Age (yr) | History | SLC2A9 Mutation | Serum UA (mg/dl) | Urine UA (mg/dl) | Serum Creatinine (mg/dl) | Urine Creatinine (mg/dl) | Fractional excretion of UA (%) |
|---|---|---|---|---|---|---|---|---|---|
| F1 | |||||||||
| II5 | M | 67 | Nephrolithiasis, CKD | L75R/L75R | 0.67 | 67.9 | 1.53 | 88.2 | >150.0 |
| II6 | F | 64 | L75R/WT | 4.50 | 47.2 | 0.89 | 172.9 | 5.4 | |
| III1 | F | 31 | WT | 4.00 | 93.5 | 0.74 | 186.6 | 9.3 | |
| III2 | M | 46 | WT | 7.90 | NA | 1.23 | NA | NA | |
| III3 | F | 46 | WT | 4.30 | 69.9 | 0.93 | 119.5 | 12.6 | |
| III4 | F | 28 | L75R/WT | 2.00 | 80.4 | 0.78 | 114.3 | 21.7 | |
| III5 | F | 35 | WT | 3.40 | 30.7 | 0.70 | 56.4 | 11.2 | |
| II6 | F | 38 | L75R/WT | 2.20 | 69.3 | 0.85 | 136.8 | 19.6 | |
| III7 | M | 44 | WT | 5.90 | 78.6 | 0.93 | 214.4 | 5.8 | |
| III8 | F | 48 | L75R/WT | 3.40 | 23.1 | 0.70 | 63.6 | 7.5 | |
| III9 | M | 46 | EIARF | L75R/L75R | 0.20 | 80.3 | 0.88 | 174.8 | >150.0 |
| III10 | M | 36 | Nephrolithiasis | L75R/L75R | 0.04 | 19.0 | 0.79 | 74.1 | >150.0 |
| III11 | F | 40 | L75R/WT | 3.70 | 50.5 | 0.65 | 118.7 | 7.4 | |
| III12 | F | 44 | L75R/WT | 3.10 | 55.6 | 0.84 | 121.4 | 12.4 | |
| IV1 | M | 5 | L75R/WT | 2.60 | NA | NA | NA | NA | |
| IV3 | F | 15 | L75R/WT | 2.40 | NA | NA | NA | NA | |
| IV4 | F | 10 | L75R/L75R | 0.01 | 34.8 | 0.51 | 74.4 | >150.0 | |
| IV5 | M | 24 | EIARF | L75R/L75R | 0.20 | 45.8 | 0.93 | 114.9 | >150.0 |
| IV6 | M | 19 | EIARF | L75R/L75R | 0.10 | 92.1 | 0.93 | 281.7 | >150.0 |
| IV7 | M | 16 | L75R/WT | 2.00 | 41.0 | 0.60 | 71.6 | 17.0 | |
| IV8 | F | 19 | WT | 6.40 | 83.9 | 0.70 | 287.3 | 3.2 | |
| F2 | |||||||||
| III2 | M | 69 | Nephrolithiasis | delExon7/delExon7 | 0.10 | 11.4 | 1.14 | 49.5 | >150.0 |
CKD, chronic kidney disease.
Family 2.
The index (III2) is a 69-yr-old Ashkenazi-Jewish man whose parents are first cousins (Figure 1B). He was found to have extremely low serum UA levels in routine repeated examinations in the past 5 yr (0.0 to 0.1 mg/dl). He had one episode of renal colic and confirmed nephrolithiasis approximately 30 yr ago but no history of renal failure. One of his daughters had hypouricemia (serum UA of 1.5 mg/dl). She and the other family members were unavailable for genetic studies.
Molecular Analysis
We first excluded mutations in the URAT1-encoding gene, SLC22A12, in the index patients of families 117 and 2. Family 1 consists of three generations of patients with hereditary hypouricemia (Table 1, Figure 1A). This pattern could suggest an autosomal dominant inheritance; however, because both families described here are consanguineous and some family members have intermediate serum UA levels (Table 1), compatible with heterozygosity, the disease was more consistent with an autosomal recessive mode of inheritance. Therefore, we chose to perform a genome-wide screening of family 1 under a hypothesis of homozygosity by descent for an ancestral mutation.
The genome-wide search identified five genomic intervals of homozygosity of >4 Mb on chromosomes 4, 5, 8, 11, and 17. To limit the candidate genomic regions, we performed genetic analysis of additional family members as well as patient III2 of family 2, using microsatellite markers located in these regions (see the Supplemental Appendix for a complete list of microsatellite markers used in this study). By this method, we were able to exclude all but one interval on chromosome 4 between markers D4S403 and D4S419 (20.1 Mb), which contains the gene SLC2A9. SLC2A9 encodes for two variants of GLUT9: Short (GLUT9S) and long (GLUT9L).13
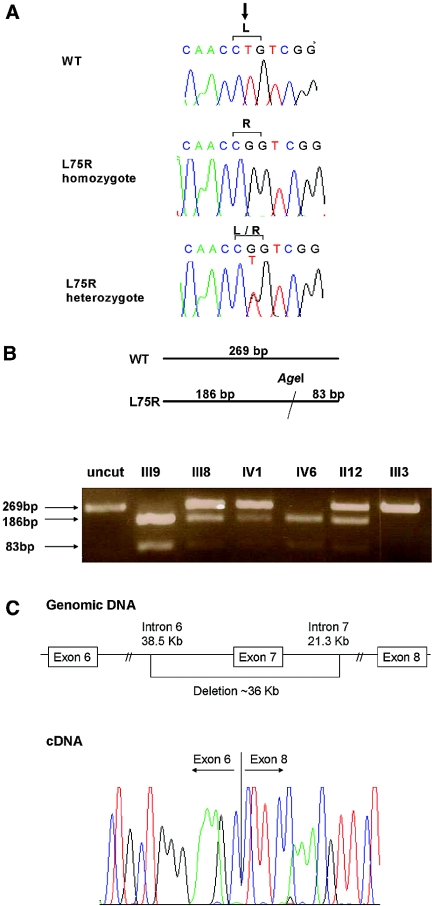
DNA sequencing of SLC2A9 in the index patient of family 1 (Figure 2A) identified a novel homozygous missense mutation, GLUT9L-L75R/GLUT9S-L46R. The L75R/L46R mutation creates an Age1 restriction site. Restriction enzyme analysis using Age1 of all family members detected six patients bearing homozygous L75R/L46R SLC2A9 mutation, nine heterozygous carriers, and six members with the wild type (WT) SLC2A9 gene (Figure 2B). The presence of homozygous or heterozygous L75R/L46R mutation was also confirmed in all affected members by direct sequencing. This mutation was absent in a control group of 150 unrelated normal control subjects (300 alleles), including 100 Israeli-Arabs.
Figure 2.
SLC2A9 mutations, found in two families with severe hereditary hypouricemia. (A) Missense mutation (c.T224G, p.L75R) found in the index patient of family 1 as compared with the sequences in a heterozygous family member and a healthy control subject (WT). (B) Restriction enzyme analysis of family 1, showing individuals with WT, homozygote, and heterozygote SLC2A9. The L75R mutation creates an AgeI recognition site that does not exist in the WT sequence. Digestion fragments of 269 and 83 bp are seen in mutated alleles. (C) Schematic presentation of the 36-kb deletion identified in the index patient of family 2 (top) and cDNA sequence of the transition from exon 6 to exon 8 (bottom).
Whereas the Leu75 (or Leu46) residue is conserved in all known GLUT9 orthologs, it is present only in seven of 14 SLC2 family paralogues (SLC2A1 through 14; data not shown).
DNA sequencing of SLC2A9 in patient III2 (family 2) identified only two common polymorphisms: G25R and P350L. However, exon 7 was not detected in the genomic DNA. We therefore sequenced SLC2A9 transcript and demonstrated skipping of the entire exon 7 and a direct transition from exon 6 to 8 causing a translational frameshift introducing a premature termination codon after 14 amino acids, resulting in a truncated protein of 231 amino acids instead of 540. PCR of genomic DNA showed a rearrangement with a deletion of approximately 36 kb including parts of intron 6, exon 7, and part of intron 7 (Figure 2C).
Genotype–Phenotype Correlations
In family 1 we detected six individuals with a homozygous SLC2A9-L75R mutation, nine with heterozygous mutation, and six normal individuals. Mean serum UA level in homozygous individuals was 0.17 ± 0.20 mg/dl. The highest serum UA level (0.67 mg/dl) was found in a 67-yr-old man with chronic kidney disease (serum creatinine 1.53 mg/dl). Mean serum UA level in heterozygous individuals was 2.88 ± 0.87 mg/dl compared with 5.10 ± 1.88 mg/dl in family members with a WT SLC2A9 gene. Fractional excretion of UA was >150% in all homozygous individuals, compared with 13.00 ± 6.74% in heterozygous and 8.40 ± 3.88% in nonaffected members of the family.
Functional and Expression Studies in Oocytes
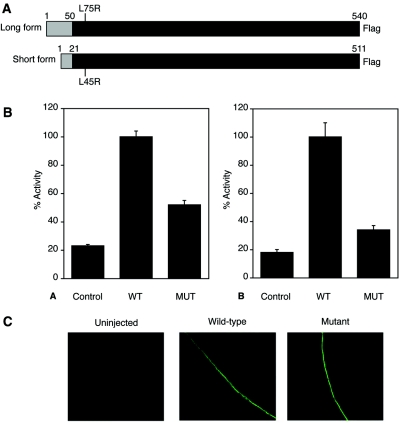
To determine whether the mutated SLC2A9 gene encodes a compromised UA transporter, we measured [8-14C]UA transport in oocytes injected with WT or mutant SLC2A9 compared with a control mRNA. As described previously,14 GLUT9S efficiently transported urate compared with oocytes injected with the control mRNA, averaging 0.041 ± 0.005 (SEM) pmol oocyte/min. The GLUT9S-L46R mutant-injected oocytes showed reduced uptake compared with WT GLUT9S (19.4% of WT uptake; Figure 3B, B).
Figure 3.
(A) Schematic representation of the variants GLUT9L and GLUT9S showing the location of the mutation (L75R, L45R) found in family 1. (B) Reduced [8-14C]UA transport activity in oocytes injected with L75R mutant (MUT; A) or L46R mutant (MUT; B) compared with WT SLC2A9 mRNA. Oocytes were injected with control, WT, or mutant mRNA, and transport assays were performed at room temperature for 1 h, 2 d after injection. Activity is expressed as counts per minute of [8-14C]UA uptake, as a percentage of WT. The average of either three or four experiments is shown, and error bars represent SEs. (C) The L46R mutation does not significantly impair transport of GLUT9S to the plasma membrane. Immunodetection with an anti-GLUT9S antibody shows that the WT and L46R proteins are expressed at the plasma membrane, whereas fluorescence levels were undetectable in control oocytes or in the absence of GLUT9S antibody (data not shown).
The GLUT9L-L75R mutation also showed reduced uptake (37.8% of WT GLUT9L uptake; Figure 3B, A), although the difference was less than with GLUT9S. The reduced UA transport by the mutant transporter cannot be explained by failure to reach the plasma membrane, because both the WT and mutant proteins are similarly expressed at the oocyte plasma membrane (Figure 3C). GLUT9S-positive staining was scored at the plasma membrane, and expression of the WT and L46R mutant at the plasma membrane was shown to be identical. The data were derived from a minimum of 40 oocytes per experimental point from three different animals.
Discussion
Hereditary hypouricemia complicated by nephrolithiasis and EIARF has been reported so far only in patients with loss-of-function URAT1 mutations. Most of the patients were of Japanese origin and carried the truncation mutation W258X.4–6,18,19 This study shows that a similar but not identical syndrome is caused by homozygous loss-of-function mutations in the SLC2A9 gene encoding GLUT9.
We detected a homozygous SLC2A9 missense mutation, L75R, in six members of an Israeli-Arab consanguineous family with severe renal hypouricemia (family 1). Expression studies in oocytes demonstrated that the GLUT9L-L75R/GLUT9S-L46R mutation markedly reduced the UA transport activity of both GLUT9 variants (Figure 3B). These findings confirm the loss-of-function nature of the GLUT9-L75R/L46R mutation. The results of oocyte transport studies did not show a complete loss of UA transport as might have been expected from the severe clinical findings. This discrepancy may perhaps be explained by the combined effects of reduced UA transport and impaired trafficking and membrane targeting of the mutant GLUT9 in humans that cannot be demonstrated in oocytes (Figure 3).
In one patient of a consanguineous Ashkenazi-Jewish family (family 2), who exhibited clinical characteristics very similar to those of family 1, we found an approximately 36-kb deletion in the SLC2A9 gene. Because the deletion results in a truncated 231–amino acid protein, we assume that it leads to loss of function.
Although patients with homozygous GLUT9 mutations can present with clinical features resembling hereditary hypouricemia as a result of URAT1 mutations, we note an important difference: Patients with loss of function of GLUT9 (as a result of either the L46/75R mutation or the truncating deletion mutation) have much lower serum UA levels (near 0) compared with patients with loss of URAT1 function (0.5 to 1.0 mg/dl).5,6 Furthermore, renal excretion of UA in these patients is markedly higher: Fractional excretion of UA in all homozygous members was >150%, compared with 13% in those with heterozygous GLUT9 mutations and 40 to 90% in patients with homozygous loss of URAT1.5,6,8
The human SLC2A9 gene, cloned in 2000,20 encodes two isoforms of GLUT9—long and short (Figure 3A)—through the use of alternative promoters. In the kidney, GLUT9L is localized to the basolateral membrane of proximal tubular epithelial cells, whereas GLUT9S is localized to the apical membrane of these cells.21 On the basis of sequence similarity to other members of the facilitative sugar transporter family, GLUT9 was first predicted to be a sugar transporter. Uptake studies in Xenopus oocytes demonstrated that GLUT9 exhibits glucose transport activity.21 Surprising, recent population studies showed significant association between SLC2A9 genotype and serum UA concentrations.9,13,22 Moreover, two rare variants, including one null allele, were found to be associated with low serum UA.13 Analysis of GLUT9 expression in Xenopus oocytes provided strong evidence for its role as a UA transporter.13,15 UA uptake for GLUT9-expressing oocytes was 31-fold higher versus control and seven-fold higher versus URAT1-expressing oocytes.13 Increased UA uptake by overexpression of GLUT9 was also confirmed in transfected human and mouse cells.14 The significance of GLUT9 function for human UA handling was further supported by the recent report of Matsuo et al.,16 who described three individuals, including a mother and a son, with hypouricemia caused by loss-of-function heterozygous GLUT9 mutations. Their report led to the definition of hereditary renal hypouricemia type 2 (RHUC2; OMIM 612076; http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=612076) in addition to hereditary renal hypouricemia type 1, caused by URAT1 mutations (RHUC1; OMIM 220150; http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=220150). Interestingly, mutations in canine SLC2A9 were recently found to affect UA handling by the kidney and the liver of Dalmatian dogs, which exhibit hyperuricosuria and relative hyperuricemia.23
Our findings provide definitive proof of the pivotal role played by GLUT9 in UA renal absorption in human. Ichida et al.5 concluded their survey of renal hypouricemia in Japan with the statement that the ability of a UA transporter other than URAT1 to regulate serum UA levels should not be greater than that of URAT1. We show that the impact of GLUT9 deficiency on UA renal excretion and serum UA levels exceeds that of URAT1.
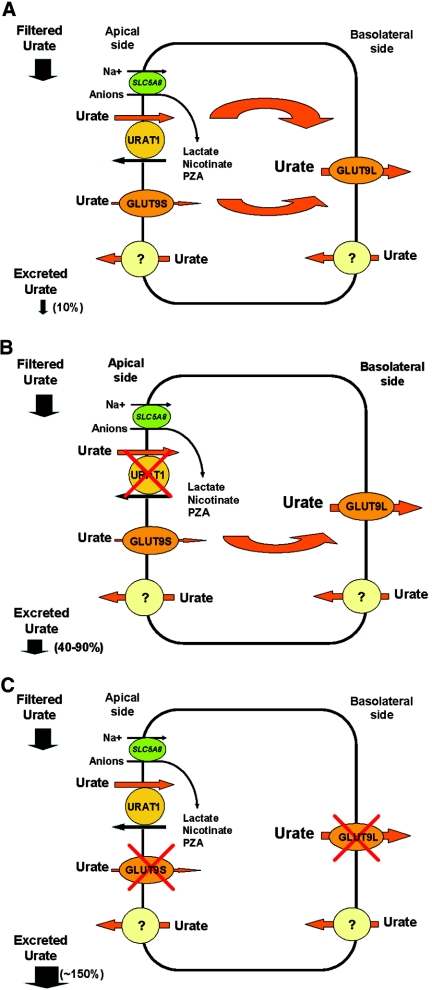
On the basis of our and others' findings, we speculate that UA efflux is mediated solely by basolateral GLUT9L. In contrast, UA absorption from the tubular lumen is carried out not only by URAT1 but also by GLUT9S and possibly other apical transporters (Figure 4A). Thus, in RHUC1, the loss of URAT1 function produces a partial UA absorption defect (fractional excretion of UA of 40 to 90%; Figure 4B). In contrast, the loss of function of GLUT9 in RHUC2 precludes UA absorption by all of the apical transporters (including URAT1) through complete blocking of UA efflux, resulting in a total UA reabsorption defect (Figure 4C). The finding of a fractional excretion of UA of >100% (>150% in our patients) confirms the existence of a functioning UA excretion pathway, yet to be defined.
Figure 4.
A simplified model of UA handling by the proximal renal tubular cell, modified after Anzai et al.15 and Matsuo et al.16 (A) Normal physiology. (B) Loss-of-function of URAT1. (C) Loss-of-function of GLUT9 as a result of homozygous mutations.
Three of seven patients with homozygous SLC2A9 mutations had experienced one or more episodes of renal colic. Urolithiasis is a known complication of hereditary hypouricemia as a result of URAT1 mutations with a prevalence of 8.5%, compared with 2.0 to 3.0% in the general population.6 The high prevalence of urolithiasis is attributed to increased urine UA concentration. Because the renal UA absorption defect is more severe in SLC2A9-associated hypouricemia than in URAT1-associated disease, a higher prevalence of urolithiasis might be expected.
The most severe complication of hereditary renal hypouricemia is EIARF. This disorder was previously described in patients bearing the Japanese URAT1 mutation (W258X).4–6,24 We show here that EIARF occurs also in hereditary renal hypouricemia as a result of homozygous SLC2A9 mutations. Thus, predisposition to EIARF seems to be related to a reduced renal UA absorption, regardless of the genetic or environmental background.
Two mechanisms have been previously proposed to explain the mechanism of EIARF. The first is acute urate nephropathy, caused by increased production of UA during physical exercise and leading to increased urinary UA excretion and resulting in renal UA precipitation.3 Acute urate nephropathy occurs in conditions of rapid increase in serum UA levels such as the tumor lysis syndrome. It is characterized by typical histologic findings in kidney biopsy25 that have not been detected in affected patients with hereditary hypouricemia, making this explanation very unlikely.
The second explanation is ischemic kidney injury secondary to vasoconstriction of renal vessels mediated by an exercise-induced increase in oxygen free radicals.3 UA is the most abundant aqueous antioxidant in humans and has been shown to preserve endothelial dilation in the face of oxidative stress.26 It is thus possible that the lack of UA in plasma of patients with hereditary hypouricemia predisposes them to ischemic acute renal failure. This hypothesis is supported by reported imaging results27 as well as biopsy results of patients with EIARF showing mainly acute tubular necrosis28; however, EIARF has never been described in patients with xanthinuria, whose serum UA levels are as low as those of patients with renal hypouricemia. We propose a third mechanism for EIARF: Reduced clearance of urate-coupled anions by URAT1 as a result of loss-of-function mutations of either URAT1 or GLUT9 may exert toxic effects on renal proximal tubules, leading to toxic acute tubular necrosis.
We show here that loss-of-function mutations of GLUT9 cause severe hypouricemia in homozygous individuals and moderately low serum UA levels in heterozygous carriers. Interestingly, several polymorphisms in the same gene were found to be associated with hyperuricemia and gout in large population studies.13,22,29,30 This may suggest that the pathogenesis of hyperuricemia and gout involves increased tubular UA reabsorption as a result of a gain of function of GLUT9, rather than reduced UA secretion, as postulated previously.31 Similar transport-related mirror-image diseases caused by different mutations in the same molecules were described in the epithelial sodium channel in which a loss of function mutation causes pseudohypoaldosteronism with hypotension type 1A, and a gain-of-function mutation causes Liddle syndrome with hypertension.32
In summary, this study describes the clinical and molecular characteristics of a severe type of hereditary renal hypouricemia (RHUC2), caused by homozygous loss-of-function mutations in the SLC2A9 gene, coding for the UA transporter GLUT9. Our clinical and molecular findings may contribute to the understanding of the physiology of renal UA handling and the pathogenesis of common diseases such as ARF, nephrolithiasis, hyperuricemia, and gout.
Concise Methods
Clinical Analysis
Individuals were evaluated for clinical history of exercise-induced acute renal injury, renal stones, or other renal diseases. Blood and spot urine samples were collected for measurement of UA and creatinine levels and for genetic analysis. The study was approved by the institutional and Ministry of Health review boards for human experimentation. All participants gave written informed consent. Parental consent for children who were younger than 18 yr was obtained.
Molecular Analysis
DNA and RNA extraction and sequencing.
Genomic DNA was isolated from peripheral blood cells using the ArchivePure DNA Blood Kit (5 PRIME, USA) according to the manufacturer's instructions. Total RNA was extracted from whole blood with TRIzol Reagent (Life Technologies-BRL, Paisley, UK) according to the manufacturer's instructions.
The coding areas and splice-sites of SLC22A12 and SLC2A9 were amplified by PCR, using intronic primers (see the Supplemental Appendix for primer sequences). All PCR products were sequenced directly (ABI Prism 3100; Applied Biosystems, Foster City, CA). RNA from blood leukocytes of patient III2, family 2, was reverse-transcribed using the Reverse-iT 1st strand Synthesis Kit (ABgene, Surrey, UK). cDNA of exons 6 through 8 of SLC2A9 was sequenced using coding area primers. For better characterization of the deletion of exon 7, primer pairs were designed to amplify short amplicons (approximately 200 bp) on each side of the exon in a stepwise manner. An appropriate WT control was used in every reaction. For primer sequences, see Supplemental Appendix Table 1D.
Restriction Enzyme Analysis (Family 1).
Exon 3 of SLC2A9 was amplified using flanking intronic primers and digested with the restriction enzyme AgeI. The digested fragments were detected using gel electrophoresis.
Microarray Studies.
DNA of three individuals with severe renal hypouricemia (II5, III9, and III10; Figure 1A), assumed to be carrying a homozygous mutation in the candidate gene, were analyzed using GeneChip 500 K Mapping Arrays (Affymetrix, Santa Clara, CA) according to the manufacturer's protocol. For detection of regions that are homozygous in all three individuals, the GeneChip genotyping analysis software was used to run dynamic model mapping analysis.
Haplotype Analysis.
Haplotype analysis for identifying homozygous regions in all members of families 1 and 2 was used to narrow the regions of homozygosity detected by GeneChip analysis. Genotyping was performed at the Center for Genomic Technologies at the Hebrew University of Jerusalem, Israel, using microsatellite markers from the Genethon human linkage map (Applied Biosystems). PCR product electrophoresis and detection were performed using the 3700 Automated DNA Analyzer (Applied Biosystems). Sizing and genotyping were performed using GENESCAN and GENOTYPER software (Applied Biosystems). Haplotypes were constructed and compared between family members.
Molecular/Functional Studies
Plasmids.
Plasmid pLuc-MS2 has been previously described.33 For oocyte transport and immunohistochemistry studies, plasmids pSLC2A9_S and pSLC2A9_L were created as follows. Human SLC2A9 (long form L and short form S) cDNAs were PCR-amplified with forward primer 5′-GATGGCAAGGAAACAAAATAGG-3′ (SLC2A9_L) or 5′-GATGAAGCTCAGTAAAAAGGAC-3′ (SLC2A9_S) and with the reverse primer 5′-GTTAAGGCCTTCCATTTATCTTACC-3′ (both forms). The PCR products were ligated into pGEM-Teasy vector (Promega). The mutant form of L75R in SLC2A9_L and of L46R in SLC2A9_S were generated (CTG to CGG) by site-directed mutagenesis following the manufacturer's protocol (Stratagene). All constructs were verified by sequencing.
Antibody Production.
The GLUT9S antibody was made by expression of amino acids 1 through 22 from GLUT9S (NP_001001290; MKLSKKDRGEDEESDSAKKKLD) in rabbits using Genomic Antibody Technology.
Immunohistochemistry.
Two days after injection, oocytes were fixed in Dents (80%/methanol, 20% DMSO), paraffin-embedded, and sectioned at 5 μm. Sections were antigen-retrieved in a pressure cooker using 0.01 M citrate (pH 6) for 5 min followed by a 20-min stand in hot buffer. Slides were incubated in 3% H2O2 in methanol for 30 min at room temperature. After a 30-min incubation in 20% normal goat serum in Tris-buffered saline (0.05 M [pH 7.4] and 0.85% NaCl), sections were incubated with a GLUT9S antibody (1:5000) at 4°C overnight, washed twice in TBS, and incubated in goat anti-rabbit peroxidase Fab secondary antibody (1:500). After two additional washes, sections were incubated with Tyramide Cy5 (NEN) for 10 min. Sections had two final washes in TBS before coverslipping. The sections were visualized under a Zeiss LSM 510 confocal laser scanning microscope, using a HeNe 633 laser and dichroics and a long-pass 650 emission filter.
Transport Studies.
Plasmids were linearized with BglII (pLuc-MS2) or XbaI (pSLC2A9_S/pSLC2A9_S_L46R and pSLC2A9_L/pSLC2A9_L_L75R), and mRNAs were transcribed, adenylated, and purified as described previously.13 Ten or 40 ng of mRNA was injected, as stated, into defolliculated stage VI Xenopus laevis oocytes, and 2 d after injection, [8-14C]UA uptake assays were carried out as described previously13 for 60 min using 25 μM [8-14C]UA (American Radiolabeled Chemicals). Three or four pools of five oocytes were collected per experimental point, and statistical analyses were done using Microsoft Excel software.
Disclosures
None.
Acknowledgments
We thank Evgenia Valsamidou for help with the homology modeling, Rotem Ron for technical assistance, and Eithan Friedman for providing DNA samples of Israeli-Arabs.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Wu XW, Lee CC, Muzny DM, Caskey CT: Urate oxidase: Primary structure and evolutionary implications. Proc Natl Acad Sci U S A 86: 9412–9416, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feig DI, Kang DH, Johnson RJ: Uric acid and cardiovascular risk. N Engl J Med 359: 1811–1821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperling O: Hereditary renal hypouricemia. Mol Genet Metab 89: 14–18, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H: Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417: 447–452, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Ichida K, Hosoyamada M, Hisatome I, Enomoto A, Hikita M, Endou H, Hosoya T: Clinical and molecular analysis of patients with renal hypouricemia in Japan: Influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol 15: 164–173, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Ichida K, Hosoyamada M, Kamatani N, Kamitsuji S, Hisatome I, Shibasaki T, Hosoya T: Age and origin of the G774A mutation in SLC22A12 causing renal hypouricemia in Japanese. Clin Genet 74: 243–251, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Cheong HI, Kang JH, Lee JH, Ha IS, Kim S, Komoda F, Sekine T, Igarashi T, Choi Y: Mutational analysis of idiopathic renal hypouricemia in Korea. Pediatr Nephrol 20: 886–890, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Dinour D, Gafter U, Knecht A, Rachamimov R, Serban I, Holtzman EJ: Novel missense mutations in the Urat1 gene are associated with renal hypouricemia in Iraqui-Jews [Abstract]. J Am Soc Nephrol 15: 89A, 2004 [Google Scholar]
- 9.Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, Mangino M, Albrecht E, Wallace C, Farrall M, Johansson A, Nyholt DR, Aulchenko Y, Beckmann JS, Bergmann S, Bochud M, Brown M, Campbell H, Connell J, Dominiczak A, Homuth G, Lamina C, McCarthy MI, Meitinger T, Mooser V, Munroe P, Nauck M, Peden J, Prokisch H, Salo P, Salomaa V, Samani NJ, Schlessinger D, Uda M, Volker U, Waeber G, Waterworth D, Wang-Sattler R, Wright AF, Adamski J, Whitfield JB, Gyllensten U, Wilson JF, Rudan I, Pramstaller P, Watkins H, Doering A, Wichmann HE, Spector TD, Peltonen L, Volzke H, Nagaraja R, Vollenweider P, Caulfield M, Illig T, Gieger C: Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet 5: e1000504, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagos Y, Stein D, Ugele B, Burckhardt G, Bahn A: Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol 18: 430–439, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Uchino H, Tamai I, Yamashita K, Minemoto Y, Sai Y, Yabuuchi H, Miyamoto K, Takeda E, Tsuji A: p-Aminohippuric acid transport at renal apical membrane mediated by human inorganic phosphate transporter NPT1. Biochem Biophys Res Commun 270: 254–259, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, Kottgen M: Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A 106: 10338–10342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, Knott SA, Kolcic I, Polasek O, Graessler J, Wilson JF, Marinaki A, Riches PL, Shu X, Janicijevic B, Smolej-Narancic N, Gorgoni B, Morgan J, Campbell S, Biloglav Z, Barac-Lauc L, Pericic M, Klaric IM, Zgaga L, Skaric-Juric T, Wild SH, Richardson WA, Hohenstein P, Kimber CH, Tenesa A, Donnelly LA, Fairbanks LD, Aringer M, McKeigue PM, Ralston SH, Morris AD, Rudan P, Hastie ND, Campbell H, Wright AF: SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 40: 437–442, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Caulfield MJ, Munroe PB, O'Neill D, Witkowska K, Charchar FJ, Doblado M, Evans S, Eyheramendy S, Onipinla A, Howard P, Shaw-Hawkins S, Dobson RJ, Wallace C, Newhouse SJ, Brown M, Connell JM, Dominiczak A, Farrall M, Lathrop GM, Samani NJ, Kumari M, Marmot M, Brunner E, Chambers J, Elliott P, Kooner J, Laan M, Org E, Veldre G, Viigimaa M, Cappuccio FP, Ji C, Iacone R, Strazzullo P, Moley KH, Cheeseman C: SLC2A9 is a high-capacity urate transporter in humans. PLoS Med 5: e197, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anzai N, Ichida K, Jutabha P, Kimura T, Babu E, Jin CJ, Srivastava S, Kitamura K, Hisatome I, Endou H, Sakurai H: Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem 283: 26834–26838, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Matsuo H, Chiba T, Nagamori S, Nakayama A, Domoto H, Phetdee K, Wiriyasermkul P, Kikuchi Y, Oda T, Nishiyama J, Nakamura T, Morimoto Y, Kamakura K, Sakurai Y, Nonoyama S, Kanai Y, Shinomiya N: Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet 83: 744–751, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahat H, Dinour D, Ganon L, Feldman L, Holtzman EJ, Goldman M: Non-urate transporter 1-related renal hypouricemia and acute renal failure in an Israeli-Arab family. Pediatr Nephrol 24: 999–1003, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Iwai N, Mino Y, Hosoyamada M, Tago N, Kokubo Y, Endou H: A high prevalence of renal hypouricemia caused by inactive SLC22A12 in Japanese. Kidney Int 66: 935–944, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Wakida N, Tuyen DG, Adachi M, Miyoshi T, Nonoguchi H, Oka T, Ueda O, Tazawa M, Kurihara S, Yoneta Y, Shimada H, Oda T, Kikuchi Y, Matsuo H, Hosoyamada M, Endou H, Otagiri M, Tomita K, Kitamura K: Mutations in human urate transporter 1 gene in presecretory reabsorption defect type of familial renal hypouricemia. J Clin Endocrinol Metab 90: 2169–2174, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Phay JE, Hussain HB, Moley JF: Cloning and expression analysis of a novel member of the facilitative glucose transporter family, SLC2A9 (GLUT9). Genomics 66: 217–220, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH: Identification and characterization of human glucose transporter-like protein-9 (GLUT9): Alternative splicing alters trafficking. J Biol Chem 279: 16229–16236, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Doring A, Gieger C, Mehta D, Gohlke H, Prokisch H, Coassin S, Fischer G, Henke K, Klopp N, Kronenberg F, Paulweber B, Pfeufer A, Rosskopf D, Volzke H, Illig T, Meitinger T, Wichmann HE, Meisinger C: SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet 40: 430–436, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Bannasch D, Safra N, Young A, Karmi N, Schaible RS, Ling GV: Mutations in the SLC2A9 gene cause hyperuricosuria and hyperuricemia in the dog. PLoS Genet 4: e1000246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komoda F, Sekine T, Inatomi J, Enomoto A, Endou H, Ota T, Matsuyama T, Ogata T, Ikeda M, Awazu M, Muroya K, Kamimaki I, Igarashi T: The W258X mutation in SLC22A12 is the predominant cause of Japanese renal hypouricemia. Pediatr Nephrol 19: 728–733, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Ejaz AA, Mu W, Kang DH, Roncal C, Sautin YY, Henderson G, Tabah-Fisch I, Keller B, Beaver TM, Nakagawa T, Johnson RJ: Could uric acid have a role in acute renal failure? Clin J Am Soc Nephrol 2: 16–21, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Waring WS, McKnight JA, Webb DJ, Maxwell SR: Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes 55: 3127–3132, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa I: Acute renal failure with severe loin pain and patchy renal ischemia after anaerobic exercise in patients with or without renal hypouricemia. Nephron 91: 559–570, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Ohta T, Sakano T, Igarashi T, Itami N, Ogawa T: Exercise-induced acute renal failure associated with renal hypouricaemia: Results of a questionnaire-based survey in Japan. Nephrol Dial Transplant 19: 1447–1453, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Li S, Sanna S, Maschio A, Busonero F, Usala G, Mulas A, Lai S, Dei M, Orru M, Albai G, Bandinelli S, Schlessinger D, Lakatta E, Scuteri A, Najjar SS, Guralnik J, Naitza S, Crisponi L, Cao A, Abecasis G, Ferrucci L, Uda M, Chen WM, Nagaraja R: The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet 3: e194, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark K, Reinhard W, Neureuther K, Wiedmann S, Sedlacek K, Baessler A, Fischer M, Weber S, Kaess B, Erdmann J, Schunkert H, Hengstenberg C: Association of common polymorphisms in GLUT9 gene with gout but not with coronary artery disease in a large case-control study. PLoS ONE 3: e1948, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taniguchi A, Kamatani N: Control of renal uric acid excretion and gout. Curr Opin Rheumatol 20: 192–197, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Schild L: The ENaC channel as the primary determinant of two human diseases: Liddle syndrome and pseudohypoaldosteronism. Nephrologie 17: 395–400, 1996 [PubMed] [Google Scholar]
- 33.Gray NK, Coller JM, Dickson KS, Wickens M: Multiple portions of poly(A)-binding protein stimulate translation in vivo. EMBO J 19: 4723–4733, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]