Abstract
An increase in the number of blood γδ T cells follows cytomegalovirus (CMV) infection in kidney transplant recipients. These cells react against CMV-infected cells and tumor epithelial cells in vitro. We hypothesized that these CMV-induced γδ T cells play a protective role against cancer in kidney transplant recipients. We performed a longitudinal case-control study involving 18 recipients who developed cancer between 2 and 6 yr after transplantation and 45 recipients who did not. The median percentage of γδ T cells among total lymphocytes in patients with malignancies was significantly lower compared with that in control patients at 6, 12, and 18 mo before the diagnosis of cancer. Patients with a γδ T cell percentage of more than 4% were protected from cancer. An increase of the Vδ2neg γδ T cell subset significantly associated with lower incidence of cancer only in recipients who experienced pre- or postgraft CMV infection. Finally, a retrospective follow-up of 131 recipients for 8 yr revealed that CMV-naive recipients had an approximately 5-fold higher risk of cancer compared with CMV-exposed patients. In summary, these results suggest a protective role of CMV exposure against cancer in kidney transplant recipients.
Currently, the rates of cancer in kidney transplant recipients (KTRs) are similar to those of nontransplant recipients that are 20 to 30 yr older, and the risk of cancer among this population is between 2.5 and 4 times greater than in the general population.1,2 Elevated age at transplantation, Caucasian origin, long-term exposure to immunosuppressant therapy, and the presence of cancer before graft are risk factors for cancer after transplant, whereas diabetes mellitus and return to dialysis significantly reduce risk of subsequent malignancy.1,3 Twenty years after the graft, 80% of patients develop at least one nonmelanoma skin cancer, which is the most common type of malignancy in KTRs.4 Other cancers that have the highest standardized incidence ratio are lymphoma, cancer of the lip, vulvovaginal tumors, and kidney cancers.2,3
Many possible explanations for this increased incidence of cancer in KTRs have been proposed. Immunosuppressive agents may cause DNA damage and promote tumor formation. Some cancer types have been linked to viral infection. However, drug-mediated impairment of immune surveillance, which ordinarily prevents the development of malignancies, is also a major contributing factor. The concept of cancer immunosurveillance is well characterized in mice, in which recombinase-activating gene knockout mice that lack functional lymphocytes exhibit increased tumor incidence.5 In humans, immunosurveillance has also been demonstrated. Healthy Japanese subjects whose whole blood lymphocytes exhibited a high degree of natural cytotoxicity had a significantly lower risk of cancer than subjects whose lymphocytes exhibited a low degree of cytotoxicity.6 More recently, in patients suffering of colorectal cancer, the presence of a high level of tumor-infiltrating effector memory T cells was correlated with the absence of early metastatic invasion signs.7 Much is known about the antitumor role of αβ T cells, and in fact, in vitro expansion of autologous CD4+ and CD8+ T cell clones with specificity for melanoma antigens is used in treating patients with melanoma.8,9
In addition to αβ T cells, γδ T cells that function in the innate immune system are also involved in antitumor immunity.10 Indeed, γδ T cell receptor (TCR) knockout mice develop more skin cancers than wild-type mice.11 In humans, the major population of circulating γδ T cells expresses a TCR displaying the Vδ2 chain (Vδ2pos γδ T cells), and these cells are able to kill myeloma and carcinoma cell lines in vitro.10 An additional population expresses a TCR composed of the Vδ1 or Vδ3 chain (Vδ2neg γδ T cells). These cells, which reside in the epithelia, are retrieved from the infiltrating cells of many carcinomas and exert a strong cytotoxicity against carcinoma cells in vitro.12,13 Circulating Vδ2neg γδ T cells have also been found to be increased in few other diseases by other groups (HIV, HHV-8, systemic sclerosis, and Crohn disease).14–17 In contrast to αβ T cells, the development of intraepithelial γδ T cells seems to be resistant to cyclosporin A in mice,18 rendering these cells especially interesting for KTRs.
Immunity to tumors may be acquired during events that have no clear relationship to cancer. For example, certain febrile infectious childhood diseases (measles, mumps, rubella, pertussis, and chicken pox) are associated with a reduced risk of many cancers in adulthood.19 Moreover, history of severe infectious disease is associated with a reduced risk of melanoma.20 Human cytomegalovirus (CMV), a virus that establishes a lifelong viral persistence in immunocompetent individuals, is responsible for opportunistic infection after transplantation. Recent evidence indicated that the genome and antigens of CMV were frequently present in certain malignant tumors, such as colon cancer and malignant glioma,21,22 suggesting a relationship between CMV and some cancers. Interestingly, we have previously observed a major and specific increase of blood Vδ2neg γδ T cell levels after CMV infection in KTRs,23 and this expansion was correlated with the resolution of CMV infection.24 We have also demonstrated that reactive Vδ2neg γδ T cell clones or cell lines displayed a specific TCR-dependent crossreactivity against CMV-infected cells and tumor epithelial cells, suggesting the recognition of a common surface molecular pattern on infected and transformed cells.25 Moreover, we recently reported on the ability of human CMV-specific Vδ2neg γδ T cells to inhibit tumor cell development in vivo in a model of human tumor xenograft in immunodeficient mice.26
Taken together, our previous findings have suggested that the γδ T cells that were amplified during CMV infection may also be involved in tumor surveillance. In this report, we have examined the relationship between CMV-induced γδ T cells and in vivo cancer occurrence in KTRs. This population allowed us to test his hypothesis because KTRs typically develop CMV infection before graft or during the first year posttransplantation, whereas cancer development occurs much later.
Results
Association between Elevated Blood γδ T Cells and a Diminished Occurrence of Cancer in KTRs
We performed a case versus control study to longitudinally analyze the putative link between blood γδ T cell percentages and malignancy incidence in KTRs. Eighteen patients who developed cancer (12 skin cancers and 6 solid cancers) between 2 and 6 yr after transplantation (median 3 yr) were compared with 45 control KTRs. Patients from the malignancy group were slightly older than patients from the control group (mean age 54 ± 6.5 yr versus 49 ± 9 yr, P = 0.03). No statistical differences were observed between the malignancy patient group and the control patient group for the sex ratio (12/6 versus 31/14 men/women, P = 0.9), use of anti-thymocyte globulin (ATG) (56% versus 49%, P = 0.8), CMV status (R+: 72% versus 69%, D+R−: 6% versus 17%, D−R−: 22% versus 13%, P = 0.5), HLA mismatches (MMs) (0 to 1 MM: 37.5% versus 20%, 2 to 4 MMs: 62.5% versus 76%, 5 to 6 MMs: 0% versus 4%, P = 0.5), and acute rejection (22% versus 13%, P = 0.1). Patients with skin types I/II, III/IV, and V/VI (Fitzpatrick classification) represented 18% and 21%, 73% and 67%, and 9% and 12% in patients with cutaneous cancer and their matched controls, respectively (P = 0.9).
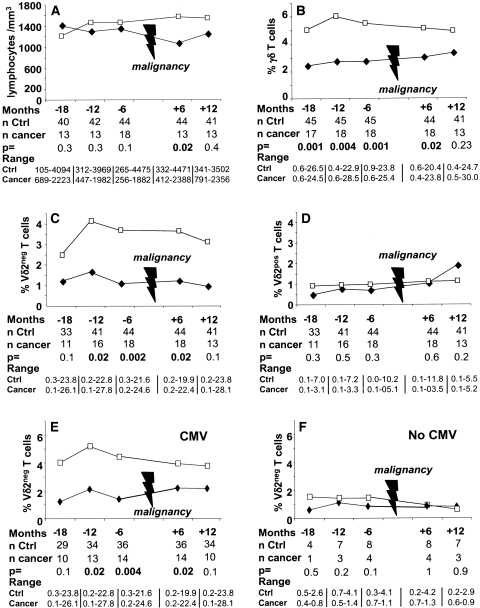
We did not find any statistical differences between the cancer patient group and the control patient group for the median number of total blood lymphocytes at 18 [(M-18) 1401 versus 1200/mm3, P = 0.3], 12 [(M-12) 1294 versus 1455/mm3, P = 0.3], and 6 [(M-6) 1347 versus 1458/mm3, P = 0.1] mo before cancer diagnosis (Figure 1A). By contrast, the median γδ T cell percentage among total lymphocytes in patients with malignancies was significantly lower than that of control patients at M-18 (2.4% versus 5%, P < 0.001), M-12 (2.7% versus 6%, P < 0.004), and M-6 (2.7% versus 5.5%, P < 0.001) (Figure 1B). Similar results were only obtained when skin cancer patients were compared with their matched controls (data not shown).
Figure 1.
Evolution of γδ T cells. Evolution of the median (A) number of lymphocytes, (B) percentage of γδ T cells, (C) Vδ2neg γδ T cells, and (D) Vδ2pos γδ T cells among total T cells before and after cancer occurrence in KTRs with cancer (♦) and their matched controls (□). (E, F) Evolution of the medians of Vδ2neg γδ T cell percentage before and after cancer occurrence in KTRs with cancer (♦) and their matched controls (□) according to history of CMV. (E) Patients who have been in contact with CMV (before or after transplantation). (F) Patients naïve for CMV infection.
Next, we determined a blood γδ T cell percentage threshold, which would predict protection of KTRs from malignancy occurrence. Using a conditional logistic model, we determined that patients with a γδ T cell percentage above 4.1% at M-18 [odds ratio (OR) = 0.06, Akaike's information criterion (AIC) = 32.7, P = 0.008], 4% at M-12 (OR = 0.11, AIC = 35.9, P = 0.006), and 3.5% at M-6 (OR = 0.06, AIC = 32.8, P = 0.007) displayed significantly less neoplasia than patients with a lower γδ T cell percentage. The number of patients who developed a cancer with the risk phenotype of a γδ T cell percentage below 4% was 14 of 32 at M-18 (44%), 15 of 32 at M-12 (47%), and 14 of 33 at M-6 (42%). Conversely, the number of patients who developed a cancer with a γδ T cell percentage above 4% was only 3 of 30 at M-18 (10%, P = 0.004), 3 of 31 at M-12 (10%, P = 0.002), and 4 of 30 at M-6 (13%, P = 0.01).
Vδ2neg γδ T Cells Are Associated with Diminished Cancer Occurrence in KTRs
As previously mentioned, Vδ2pos and Vδ2neg γδ T cells have been shown to exhibit antitumor functions; thus, we determined which of these two subsets was associated with lower cancer occurrence in KTRs. The median Vδ2neg γδ T cell percentage in patients with malignancies was lower than that of cancer-free KTRs at M-18 (1.2% versus 2.5%, P = 0.1), M-12 (1.7% versus 4.1%, P = 0.02), and M-6 (1.1% versus 3.7%, P = 0.002), whereas no differences in the median percentage of Vδ2pos γδ T cells were observed between the two groups (Figure 1, C and D).
Six and 12 mo after the cancer occurrence, total γδ T cell and Vδ2neg γδ T cell percentages in the cancer group were lower than those of the control group, but this difference was statistically significant only at 6 mo (3% versus 5%, P = 0.02 and 1.2% versus 3.6%, P = 0.02, respectively).
Only CMV-Induced Vδ2neg γδ T Cells Are Associated with Lower Cancer Occurrence
CMV is the only factor reported to date to be responsible for the long-term expansion of the peripheral blood Vδ2neg γδ T cell population in KTRs23 and healthy individuals.27 Therefore, we next determined whether these Vδ2neg γδ T cells, which had increased after the CMV infection, were associated with lower cancer occurrence in KTRs. We separated the patients who have never experienced CMV infection from those who had been in contact with CMV either before (pregraft CMV infection, determined through CMV seropositivity at the day of the graft) or after transplantation (postgraft CMV infection, determined through pp65 antigenemia). A significant association between an elevated number of blood Vδ2neg γδ T cells and a lower cancer occurrence was only found in KTRs who experienced pre- or postgraft CMV infection (n = 51; Figure 1, E and F). This result strongly supports a link between CMV infection-induced high blood Vδ2neg γδ T cell percentages and protection against subsequent cancer development.
Higher Frequency of Malignancies in KTRs Who Have Never Been Exposed to CMV
The above data, together with our previous in vitro studies,25 suggested a new antitumor function of CMV-reactive Vδ2neg γδ T cells. Therefore, an indirect positive effect of CMV infection on a diminished cancer occurrence in KTRs may be envisioned. To examine this unexpected link, we retrospectively analyzed a cohort of 105 consecutive long-term immunosuppressed KTRs. Twenty-three of these patients developed at least one malignancy (13 cutaneous, 8 solid, and 2 lymphomas) from 1 to 9 yr after transplantation (median 5 yr). Three patients died as a result of their cancer. Eighty-two patients without cancer were included in the control group.
Baseline characteristics of these patients are summarized in Table 1. Using univariate analysis, we failed to show any significant association between cancer occurrence and dialysis duration, age at time of graft, sex, calcineurin inhibitor use, induction treatment with ATG, HLA MMs, delayed graft function, or acute rejection (Table 2). The 13 skin cancer patients had the same skin type distribution as the control group patients (P = 0.8 by χ2 test). Taken separately, neither pregraft CMV infection nor postgraft CMV infection were associated with cancer occurrence; however, patients who never experienced CMV infection (absence of pre- or postgraft CMV infection) displayed an increased risk of cancer (OR = 4.3, P = 0.009). The use of azathioprine (versus mycophenolate mofetil) was also associated with an increased risk of cancer (OR = 3.2, P = 0.02), as described previously.3 Patients receiving ganciclovir displayed the same rate of cancer (9 of 44 cancers) than those who did not receive ganciclovir (14 of 61 cancers) (P = 0.8).
Table 1.
Descriptive analysis of KTRs
| Malignancy Group (n = 23) | Control Group (n = 82) | |
|---|---|---|
| Dialysis duration (mo) | 51.7 ± 71.7 | 36.4 ± 40.3 |
| Age at the graft (yr) | 52.6 ± 8 | 49.6 ± 7.3 |
| Gender (male/female) | 15/8 | 51/31 |
| Mean time of study (yr) | 8.5 ± 0.9 | 8.2 ± 1 |
| Immunosuppressive treatment | ||
| cyclosporin/tacrolimus | 12/11 | 38/44 |
| azathioprine/mycophenolate mofetil | 14/9 | 27/55 |
| ATG/ anti-IL-2 receptor antibody/no induction | 8/1/14 | 21/5/56 |
| HLA MMs | ||
| 0 to 1 MM (%) | 7 (30) | 11 (14) |
| 2 to 4 MMs (%) | 16 (70) | 69 (85) |
| 5 to 6 MMs (%) | 0 | 1 (1) |
| CMV status | ||
| D+R− (%) | 3 (13) | 18 (22) |
| D−R− (%) | 7 (30) | 4 (5) |
| R+ (%) | 13 (56) | 60 (73) |
| Postgraft CMV infection (%) | 7 (30) | 32 (39) |
| Pre- or postgraft CMV infection (%) | 15 (65) | 73 (89) |
| Delayed graft function (%) | 11 (48) | 47 (57) |
| Acute rejection | 3 (13) | 13 (16) |
| Acute rejection treatment (steroid/ATG) | 1/2 | 3/14 |
Table 2.
Factors associated with the occurrence of cancer in KTRs
| OR | P | |
|---|---|---|
| Univariate analysis | ||
| dialysis duration (mo) | 1.01 | 0.2 |
| age at the graft (yr) | 1.7 | 0.09 |
| female (versus male) | 0.89 | 0.82 |
| tacrolimus (versus cyclosporin) | 0.79 | 0.62 |
| azathioprine (versus mycophenolate mofetil) | 3.2 | 0.02 |
| induction with ATG | 1.55 | 0.39 |
| HLA mismatches 2 to 4 versus 0 to 1 | 0.37 | 0.07 |
| CMV R+ (versus D+ R− and D−R−) | 0.47 | 0.13 |
| postgraft CMV infection | 0.68 | 0.45 |
| no pre- and postgraft CMV infection | 4.3 | 0.009 |
| delayed graft function | 0.68 | 0.42 |
| acute rejection | 1.25 | 0.74 |
| acute rejection treatment (ATG versus steroid) | 0.43 | 0.54 |
| Multivariate analysis | ||
| azathioprine (versus mycophenolate mofetil) | 3.76 | 0.01 |
| no pre- or postgraft CMV infection | 5.28 | 0.006 |
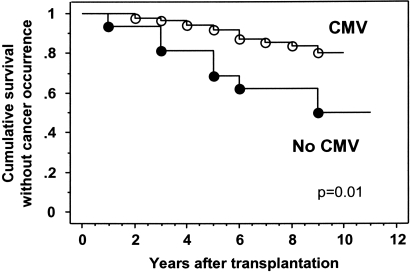
Using multivariable analysis, the same variables remained significantly correlated to the occurrence of cancer. Patients naïve for CMV exhibited a risk of cancer 5.28 times greater than patients who had been exposed to CMV (P = 0.006). Finally, patient survival without cancer development was decreased in KTRs who never encountered CMV than in those patients who had been infected with CMV (68.8% versus 92% at 5 yr and 62.5% versus 83.6% at 8 yr, respectively; P = 0.01 by log-rank test) (Figure 2).
Figure 2.
Cumulative survival without cancer development in KTRs stratified according to history of CMV infection (pre- or postgraft CMV infection). Kaplan–Meier analysis was used. Comparison was made using the log-rank test.
In Vitro Antitumor Reactivity of CMV-Induced Vδ2neg γδ T Lymphocytes
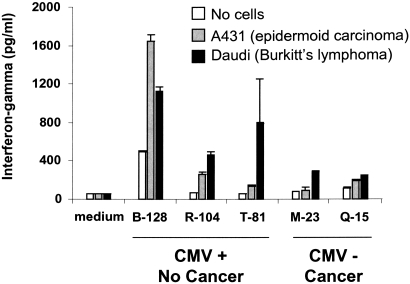
We next tested whether γδ T cells from CMV-infected KTR patients had a better antitumor potential than γδ T cells from patients who had never been exposed to CMV. Among the 105 patients of the cohort study, we selected either patients who developed CMV-infection and did not display cancer (CMV+ cancer-free) or patients who did not develop any CMV infection and displayed a cancer (CMV-free cancer+). Because peripheral blood mononuclear cells (PBMCs) had to be harvested 1 yr posttransplantation (after CMV-infection and before cancer occurrence), we only found available frozen PBMCs for ten patients. After sorting of γδ T cells from all ten patients, γδ T cell lines from only five patients grew. Those γδ T cells were co-cultured with the A431 (epidermoid carcinoma) and the Daudi (Burkitt's lymphoma) cell lines, two tumors occurring frequently after kidney transplantation. In accordance with our hypothesis, Vδ2neg γδ T cell lines from the three CMV+ cancer-free KTRs produced more IFN-γ than those from the two CMV-free cancer+ KTRs when cultured with the tumor cells (Figure 3).
Figure 3.
In vitro antitumor reactivity of CMV-induced Vδ2neg γδ T cells. γδ T cells from either patients who developed CMV-infection and did not display cancer (CMV+ cancer-free) or patients who did not develop any CMV infection but displayed a cancer (CMV-free cancer+) were sorted from PBMCs. γδ T cells were then expanded in culture RPMI medium supplemented with 10% human serum, 1000 U/ml rIL-2, 15 ng/ml rIL-15, and irradiated autologous PBMCs. After 1 mo, those γδ T cell lines were incubated with the A431 (epidermoid carcinoma) and the Daudi (Burkitt's lymphoma) cell lines for 24 h in the presence of rIL-12 and rInterferon-α. IFN-γ released into the supernatant was quantified by ELISA.
Discussion
For several years, our group has been committed to the study of γδ T cell response in KTRs, and longitudinal monitoring of γδ T cells for all patients has been routine practice in our center for 10 yr. This unique opportunity has allowed us to make a long-term retrospective analysis to observe that a low γδ T cell percentage was characteristic of patients who develop cancer in the upcoming years. Previously, we had demonstrated that these γδ T cells were induced by a past CMV infection, and we observed that CMV seronegative recipients who did not develop postgraft CMV infection developed cancer after kidney transplantation more frequently than CMV-infected patients.
The main limitation of this study is the modest sample size. Obviously, large register studies yield more reliable findings, but even these types of studies can be limited by incomplete data collection. Our single-center study has the advantage of a long follow-up period, and the data set includes all cancers, including skin cancers.
Immunosurveillance is greatly impaired by immunosuppressive drugs as evidenced by the association of CD4+ T cell depletion with skin cancer in KTRs.28 However, the development of γδ T cells appears to be resistant to cyclosporin A in mice.18 Among their multiple functions, the antitumor role of γδ T cells has been widely documented. First, a high blood Vδ2neg γδ T cell percentage is associated with a lower melanoma occurrence.29 Second, γδ T cells are observed near many epithelial tumors.12,13 Third, even if some breast tumor-infiltrating Vδ2neg γδ T cells have been reported to suppress T cell and dendritic cell function in vitro,30 these cells are usually cytotoxic against tumor epithelial cell lines and thus behave as conventional T cells.12 Finally, γδ T cells in mice contribute to protection against epithelial malignancies.11 For all of these reasons, we hypothesize that the Vδ2neg γδ T cells observed in the epithelia function as sentinels aimed at killing emerging tumor cells.12
Reports have indicated that γδ T cells are involved in anti-infection and antitumor responses.10,31 This dual function of γδ T cells has been explained by the expression of common antigens in transformed and infected cells. Compelling evidence indicated that Vδ2pos γδ T cells are activated by phosphoantigens overexpressed by microorganisms and tumor cells.32 Although the study presented here was performed on a limited number of patients, the results are consistent and extend our previous in vitro data showing that Vδ2neg γδ T cells share killing activity against CMV-infected cells and tumor epithelial cells.25 In KTRs who experienced either pre- or postgraft CMV infections, cancer occurrence was associated with low Vδ2neg γδ T cell percentages (Figure 1). A decreased ability to have these cells amplified at the periphery could be associated with an increased risk of developing cancer. However, the factors necessary for a significant Vδ2neg γδ T cell expansion after CMV infection are still unknown. Furthermore, we demonstrated an inverse relationship between exposure to CMV and cancer occurrence (Figure 2). Interestingly, a recent multicenter retrospective analysis also observed that CMV seronegative patients with CMV seronegative donors had a higher risk of developing lymphoma than CMV seropositive recipients.33 This result was unexpected because the mechanisms of immune evasion induced by CMV may decrease immune response against tumor cells.34 In addition, this result is in apparent contrast to the previously reported presence of the CMV genome and antigens in diverse types of carcinomas. However, it is not yet clear whether CMV plays a direct role in carcinogenesis or if it represents an epiphenomenon. CMV has rather been proposed to mediate indirect oncomodulatory functions such as inhibition of apoptosis, tumor invasiveness through modulation of adhesion molecule expression and cell motility, increase of angiogenesis, and modulation of host immune system.34 However, one protein coded by the CMV genome (US28) induces tumor transformation in animal models.35 All of these studies may be consistent with our results if we assume that CMV-infected cells and tumor cells express the same stress-induced molecules, resulting in the selection of common immune effector cells among which Vδ2neg γδ T cells play an important role. Presence of CMV-infected cells in tumors from three patients of this cohort was searched through PCR but all were found to be CMV-negative (data not shown).
In conclusion, this study reveals a dual role for CMV-induced Vδ2neg γδ T cells in KTRs. This cell subset is not only involved in control of the virus but is also associated with a lower incidence of subsequent malignancy. In this respect, a simple measurement of Vδ2neg γδ T cells in the blood of KTRs may provide an interesting parameter for the evaluation of the cancer risk of this long-term immunosuppressed population.
Concise Methods
Patients
Case-Control Study.
Between 1996 and 2000, 313 kidneys from deceased donors were transplanted in our department. We retrospectively identified 18 KTRs who had developed cancer and for whom blood γδ T cells had been enumerated at M-18, M-12, and M-6 as well as at 6 and 12 mo after cancer diagnosis. Comparisons were made with a case-control group of 45 cancer-free patients that were matched for sex, year of transplantation, CMV infection status, and ATG regimen.
Cohort Study.
Retrospectively, we included 131 consecutive KTRs who received transplants in our department between 1997 and 1999. Only patients older than 38 yr of age were included because no younger patient developed cancer after transplantation. Eight patients failed to complete the follow-up. Eighteen graft losses occurred during the follow-up until January 2007: 2 deaths, 13 chronic allograft dysfunctions, 1 vascular thrombosis, 1 toxic nephropathy, and 1 relapse of the primary kidney disease. None of these patients developed cancer until their graft loss. Finally, 105 KTRs with a functional graft were followed for 8.33 ± 1 yr.
All patients received an immunosuppressive regimen comprised of cyclosporin or tacrolimus, azathioprine or mycophenolate mofetil, and corticosteroids. Some patients also received induction treatment with ATG or anti-IL-2 receptor antibody. Delayed graft function was defined by the need for dialysis for the first week. All acute rejection episodes were proven by biopsy.
Pretransplant CMV infection was defined by positive CMV serology on the day of the graft (R+). Posttransplant CMV infection was diagnosed using CMV pp65 antigen positivity in peripheral blood leukocytes. For all CMV seronegative patients, CMV DNA analyses of three historical sera (month 3, 6, and 12) from the first year of posttransplantation were retrospectively performed and confirmed that patients were truly CMV negative (data not shown). From 1999, CMV seronegative patients who received CMV seropositive allograft (D+R−) and CMV seropositive recipients (R+) treated with ATG received oral ganciclovir for the first 3 mo posttransplantation. Patients suffering from postgraft CMV infection also received intravenous ganciclovir.
The diagnosis of cancer was based on histologic examination. This study was carried out on samples harvested for medical care. This study did not require agreement from any ethics committees.
Flow Cytometric Analysis of γδ T Lymphocytes
Whole blood was incubated with different monoclonal antibodies (anti-CD45 and anti-CD3 from BD Bioscience, anti-panδ and anti-Vδ2 from Beckman Coulter). After red cell lysis and fixation, at least 5000 lymphocytes were processed by the FACScalibur flow cytometer, and the percentages of cell populations were obtained using the CELLQUEST software (BD Bioscience). Absolute counts of lymphocytes were obtained using the single platform lyse/no wash Trucount (BD Biosciences).
In Vitro Analysis
The conservation of frozen PBMCs was approved by our relevant local institutional review board (CHU Bordeaux), and the patients gave their written consent. γδ T cells were sorted from PBMCs by a flow cytometer cell sorter (ARIA, BD Bioscience). γδ T cells were then expanded in culture RPMI medium supplemented with 10% human serum, 1000 U/ml rIL-2, 15 ng/ml rIL-15, and irradiated autologous PBMCs. After 1 mo, 30,000 γδ T cells were incubated with subconfluent A431 (epidermoid carcinoma) and the Daudi (Burkitt's lymphoma) cell line layers in flat-bottomed 96-well plates for 24 h at 37°C in the presence of rIL-12 and rInterferon-α (two cytokines that enhance IFN-γ production by Vδ2neg γδ T cells). IFN-γ released into the supernatant was quantified by ELISA (Bender Medsystems, Austria) to evaluate the response of Vδ2neg γδ T cell lines against tumor antigens
Statistical Analysis
Case-Control Study.
Comparisons between cases and controls were performed using conventional statistics for matched data, including McNemar χ2 test for qualitative variables, t test, or Wilcoxon rank-test. To identify a γδ T cell threshold predicting malignancy occurrence, conditional logistic regression models were used at M-18, M-12, and M-6 with the γδ T cell value as the explanatory variable. Results were expressed as AIC, Wald test P value, and OR.
Cohort Study.
The variables potentially associated with the occurrence of cancer were subjected to univariate analysis. Risk factors associated with cancer occurrence with P < 0.25 in univariable analysis were included in a multivariable model. Then, a backward selection procedure was used to select a final multivariate model including all significant variables with P < 0.05. Kaplan–Meier analysis was used to construct patient survival curves without cancer occurrence after kidney transplantation. Comparison was made using the log-rank test. Analyses were performed with SAS Software (version 9.1, Cary, NC).
Disclosures
None.
Acknowledgments
We acknowledge Catherine Rio for her help and BioScience Writers for manuscript editing. We are indebted to J.C. Carron, M. Garcie, and F. Saussais, who skillfully carried out the flow cytometric analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Protection from Cancer in Kidney Transplant Patients by γδ T Cells: Role of CMV Infection?” on pages 11–13.
References
- 1.Webster AC, Craig JC, Simpson JM, Jones MP, Chapman JR: Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: A cohort study of 15,183 recipients. Am J Transplant 7: 2140–2151, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE: Cancer incidence before and after kidney transplantation. JAMA 296: 2823–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C: Cancer after kidney transplantation in the United States. Am J Transplant 4: 905–913, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Ramsay HM, Fryer AA, Hawley CM, Smith AG, Harden PN: Non-melanoma skin cancer risk in the Queensland renal transplant population. Br J Dermatol 147: 950–956, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD: IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410: 1107–1111, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K: Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 356: 1795–1799, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J: Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353: 2654–2666, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD: Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A 99: 16168–16173, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C: Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med 358: 2698–2703, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrarini M, Ferrero E, Dagna L, Poggi A, Zocchi MR: Human gammadelta T cells: A nonredundant system in the immune-surveillance against cancer. Trends Immunol 23: 14–18, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC: Regulation of cutaneous malignancy by gammadelta T cells. Science 294: 605–609, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T: Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci U S A 96: 6879–6884, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeurer MJ, Martin D, Walter W, Liu K, Zitvogel L, Halusczcak K, Rabinowich H, Duquesnoy R, Storkus W, Lotze MT: Human intestinal Vdelta1+ lymphocytes recognize tumor cells of epithelial origin. J Exp Med 183: 1681–1696, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barcy S, De Rosa SC, Vieira J, Diem K, Ikoma M, Casper C, Corey L: Gamma delta+ T cells involvement in viral immune control of chronic human herpes virus 8 infection. J Immunol 180: 3417–3425, 2008 [DOI] [PubMed] [Google Scholar]
- 15.De Maria A, Ferrazin A, Ferrini S, Ciccone E, Terragna A, Moretta L: Selective increase of a subset of T cell receptor gamma delta T lymphocytes in the peripheral blood of patients with human immunodeficiency virus type 1 infection. J Infect Dis 165: 917–919, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Giacomelli R, Matucci-Cerinic M, Cipriani P, Ghersetich I, Lattanzio R, Pavan A, Pignone A, Cagnoni ML, Lotti T, Tonietti G: Circulating Vdelta1+ T cells are activated and accumulate in the skin of systemic sclerosis patients. Arthritis Rheum 41: 327–334, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Giacomelli R, Parzanese I, Frieri G, Passacantando A, Pizzuto F, Pimpo T, Cipriani P, Viscido A, Caprilli R, Tonietti G: Increase of circulating gamma/delta T lymphocytes in the peripheral blood of patients affected by active inflammatory bowel disease. Clin Exp Immunol 98: 83–88, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin T, Matsuzaki G, Umesue M, Omoto K, Yoshida H, Harada M, Singaram C, Hiromatsu K, Nomoto K: Development of TCR-gamma delta CD4-CD8+ alpha alpha but not TCR-alpha beta CD4-CD8+ alpha alpha i-IEL is resistant to cyclosporin A. J Immunol 155: 4224–4230, 1995 [PubMed] [Google Scholar]
- 19.Albonico HU, Braker HU, Husler J: Febrile infectious childhood diseases in the history of cancer patients and matched controls. Med Hypotheses 51: 315–320, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Krone B, Kolmel KF, Grange JM, Mastrangelo G, Henz BM, Botev IN, Niin M, Seebacher C, Lambert D, Shafir R, Kokoschka EM, Kleeberg UR, Gefeller O, Pfahlberg A: Impact of vaccinations and infectious diseases on the risk of melanoma—Evaluation of an EORTC case-control study. Eur J Cancer 39: 2372–2378, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Harkins L, Volk AL, Samanta M, Mikolaenko I, Britt WJ, Bland KI, Cobbs CS: Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 360: 1557–1563, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, Nabors LB, Cobbs CG, Britt WJ: Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 62: 3347–3350, 2002 [PubMed] [Google Scholar]
- 23.Dechanet J, Merville P, Lim A, Retiere C, Pitard V, Lafarge X, Michelson S, Meric C, Hallet MM, Kourilsky P, Potaux L, Bonneville M, Moreau JF: Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest 103: 1437–1449, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lafarge X, Merville P, Cazin MC, Berge F, Potaux L, Moreau JF, Dechanet-Merville J: Cytomegalovirus infection in transplant recipients resolves when circulating gammadelta T lymphocytes expand, suggesting a protective antiviral role. J Infect Dis 184: 533–541, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P, Dromer C, Emilie D, Moreau JF, Dechanet-Merville J: Shared reactivity of Vdelta2-gammadelta T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med 201: 1567–1578, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devaud C, Bilhere E, Loizon S, Pitard V, Behr C, Moreau JF, Dechanet-Merville J, Capone M: Antitumor activity of gammadelta T cells reactive against cytomegalovirus-infected cells in a mouse xenograft tumor model. Cancer Res 69: 3971–3978, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon ME, Merville P, Moreau JF, Dechanet-Merville J: Long term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood 112: 1317–1324, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ducloux D, Carron PL, Rebibou JM, Aubin F, Fournier V, Bresson-Vautrin C, Blanc D, Humbert P, Chalopin JM: CD4 lymphocytopenia as a risk factor for skin cancers in renal transplant recipients. Transplantation 65: 1270–1272, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Argentati K, Re F, Serresi S, Tucci MG, Bartozzi B, Bernardini G, Provinciali M: Reduced number and impaired function of circulating gamma delta T cells in patients with cutaneous primary melanoma. J Invest Dermatol 120: 829–834, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF: Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 27: 334–348, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Morita CT, Jin C, Sarikonda G, Wang H: Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: Discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev 215: 59–76, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Thedrez A, Sabourin C, Gertner J, Devilder MC, Allain-Maillet S, Fournie JJ, Scotet E, Bonneville M: Self/non-self discrimination by human gammadelta T cells: Simple solutions for a complex issue? Immunol Rev 215: 123–135, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Opelz G, Daniel V, Naujokat C, Fickenscher H, Dohler B: Effect of cytomegalovirus prophylaxis with immunoglobulin or with antiviral drugs on post-transplant non-Hodgkin lymphoma: A multicentre retrospective analysis. Lancet Oncol 8: 212–218, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Soderberg-Naucler C: Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med 259: 219–246, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Maussang D, Verzijl D, van Walsum M, Leurs R, Holl J, Pleskoff O, Michel D, van Dongen GA, Smit MJ: Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc Natl Acad Sci U S A 103: 13068–13073, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]