Abstract
WNK kinase is a serine/threonine kinase that plays an important role in electrolyte homeostasis. WNK4 significantly inhibits the surface expression of the sodium chloride co-transporter (NCC) by enhancing the degradation of NCC through a lysosomal pathway, but the mechanisms underlying this trafficking are unknown. Here, we investigated the effect of the lysosomal targeting receptor sortilin on NCC expression and degradation. In Cos-7 cells, we observed that the presence of WNK4 reduced the steady-state amount of NCC by approximately half. Co-transfection with truncated sortilin (a dominant negative mutant) prevented this WNK4-induced reduction in NCC. NCC immunoprecipitated with both wild-type sortilin and, to a lesser extent, truncated sortilin. Immunostaining revealed that WNK4 increased the co-localization of NCC with the lysosomal marker cathepsin D, and NCC co-localized with wild-type sortilin, truncated sortilin, and WNK4 in the perinuclear region. These findings suggest that WNK4 promotes NCC targeting to the lysosome for degradation via a mechanism involving sortilin.
WNK (with no lysine [K]) kinase is a subfamily of serine/threonine kinases.1 Mutations in two members of this family, WNK1 and WNK4, result in pseudohypoaldosteronism type II,2 featuring hypertension, hyperkalemia, and metabolic acidosis. Previous studies showed that wild-type (WT) WNK4 inhibits the activity and surface expression of sodium chloride co-transporter (NCC) in Xenopus oocytes.3,4 Interestingly, one study showed that a NCC harboring five different Gitelman-type mutations exhibits low activity that is mainly due to a reduction of functional NCC inserting into the plasma membrane.5 Our previous study6 also indicated that NCC surface expression is regulated by altering its degradation through the lysosomal pathway. These combined studies suggest that alteration of NCC function can result from perturbing its protein synthesis,7,8 glycosylation and processing,9,10 and delivery to the plasma membrane.8,10 Golbang et al.11 showed that WNK4 also blocks the forward trafficking of NCC; however, the exact molecular basis of interference of WNK4 kinase with NCC forward trafficking and enhancing its degradation through a lysosomal pathway remains to be clarified.
There are two major sorting mechanisms involving targeting lysosomal proteins to lysosomes.12,13 One is the mannose-6-phosphate receptor (M6PR)-mediated mechanism,14–16 and the other is mediated by sortilin.17 The trans-Golgi network (TGN) is the major site of sorting for newly synthesized proteins that are targeted to lysosomes for degradation. These newly synthesized proteins first bind to either M6PR18–20 or sortilin21 in the TGN and subsequently recruit the Golgi-localized, γ-ear–containing, ADP-ribosylation factor–binding proteins (GGAs)22–24 clathrin and adaptor protein 1 (AP1) or AP325,26 to form clathrin-coated vesicles to initiate their trafficking to endosomes, following a secretory pathway, or to lysosomes for degradation.
Sortilin is a newly identified lysosomal targeting receptor that is involved in the alternative sorting of the lysosomal sphingolipid activator protein prosaposin27,28 and the GM2 activator protein.28,29 It is a homolog of the yeast vacuolar sorting receptor Vps10p and belongs to the type I Vps10p superfamily.17 The human sortilin gene encodes 833 amino acids. It consists of an N-terminal propeptide, a furin cleavage site, a large luminal domain, a single transmembrane region, and a short cytoplasmic tail.17 The major pool of sortilin accumulates in the TGN and vesicles, whereas 10% of sortilin is present in the plasma membrane. The truncation of the cytoplasmic tail of sortilin (sortilin TRU) leads to a disruption of the lysosomal sorting function, causing a majority of sortilin TRU to be retained in the TGN; however, a small portion of sortilin TRU may leak to the plasma membrane or vesicles. Sortilin TRU can serve as a dominant negative mutant.28 Sortilin seems to have multiple functions. Sortilin not only binds different ligands such as neurotensin, receptor associated protein, and prosaposin,17,30 but also is involved in intracellular sorting, endocytosis, and signal transduction.31 Studies have shown that sortilin binds the glucose transporter Glut4 and is translocated with Glut4 to the plasma membrane in response to insulin stimulation,32–34 suggesting that sortilin may be involved in the regulation of membrane transporters; therefore, we speculated that WNK4 might promote the degradation of NCC through the sortilin-mediated lysosomal pathway. Here, we report that WNK4 downregulates the steady-state protein levels of NCC in Cos-7 cells. Sortilin TRU reverses the degradation of NCC promoted by WNK4. The N-terminus of NCC binds sortilin WT as well as sortilin TRU, to a lesser extent. WNK4 co-localizes with NCC and sortilin and increases NCC co-localization with cathepsin D, a lysosomal marker. These data suggest that WNK4, NCC, and sortilin interact with one another to facilitate the WNK4-promoted degradation of NCC through a lysosomal pathway by a sortilin-mediated targeting mechanism.
Results
Effect of Sortilin on WNK4-Mediated Inhibition of NCC Protein Expression
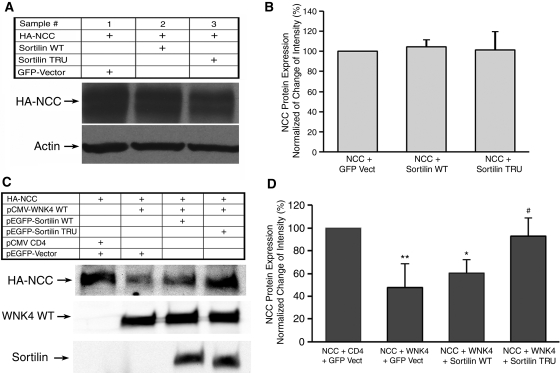
We have shown that WNK4 WT enhances NCC degradation through the lysosomal pathway.6 To determine whether a lysosomal sorting mechanism such as sortilin is involved in WNK4-promoted degradation, we co-transfected Cos-7 cells with hemagglutinin (HA)-NCC in combination with either green fluorescence protein (GFP)-sortilin WT or sortilin TRU (Figure 1) in the absence (Figure 2, A and B) or the presence of myc-WNK4 WT (Figure 2, C and D). There was no significant change in the steady-state amount of NCC in the absence of WNK4 in both NCC + sortilin WT and NCC + sortilin TRU groups as compared with NCC alone (control) group (P > 0.05; n = 4). In the presence of WNK4 WT, NCC protein level was significantly reduced by 52% (47.9 ± 8.7% in NCC + WNK4 [WNK4 WT group] versus 100% in the NCC alone [control group]; P < 0.001; n = 6). There was also a significant reduction in NCC expression in NCC + WNK4 + sortilin WT (sortilin WT group, 60.2 ± 5.0%) as compared with the control group (P < 0.01; n = 6); however, there was no significant difference in NCC expression between sortilin WT group and WNK4 WT group. In contrast, in NCC + WNK4 + sortilin TRU (sortilin TRU group), NCC protein expression (92.9 ± 6.8%) was significantly increased as compared with either WNK4 WT group (P < 0.001; n = 6) or sortilin WT group (P < 0.05; n = 6), indicating that the sortilin TRU likely reverses the inhibitory effect of WNK4 on NCC protein expression through its dominant negative effect on endogenous sortilin, because Cos-7 cells express endogenous sortilin protein. These data suggest that WNK4 enhances the degradation of NCC through a lysosomal pathway via sortilin.
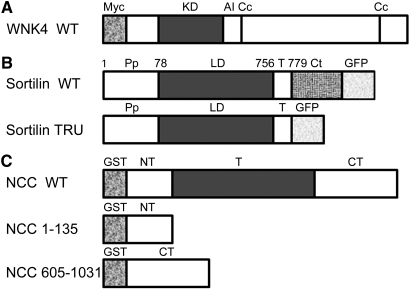
Figure 1.
Human WNK4, sortilin, and NCC are shown with predicted domains used in the experiment. (A) Myc-tagged WNK4 WT with predicted domains. KD, kinase domain; AI, autoinhibitory domain; Cc, coiled coil domain. (B) GFP-tagged sortilin WT and its truncated mutant sortilin TRU lacking its cytoplasmic tail. Pp, propeptide; LD, luminal domain; T, transmembrane region; Ct, cytoplasmic tail. (C) GST-tagged NCC WT and its amino terminus of NCC (1 through 135) and carboxyl terminus of NCC (605 through 1031). NT, amino terminus; T, transmembrane domain, CT, carboxyl terminus.
Figure 2.
The effect of sortilin on NCC protein expression is shown. Two days after transfection of Cos-7 cells with HA-NCC WT and either GFP-sortilin WT or truncated sortilin TRU in the absence or presence of myc-WNK4 WT as indicated, cells were lysed. Lysates were subjected to 5% SDS-PAGE followed by immunoblotting with anti-HA, anti-GFP, and anti-Myc antibodies. (A and C) Representative Western blot results. The top blot shows the steady-state protein level of NCC in both A and C. The bottom blot in A shows actin for protein loading control. The middle and bottom blots in C indicate WNK4 WT and sortilin protein expression, respectively. (B and D) Summary of the results of steady-state NCC protein expression for A (n = 4) and C (n = 6), respectively. Data are presented as the ratio of change from control groups (normalized to 100%) in which NCC+GFP Vect is for A and NCC+CD4+ GFP Vect is for C. There is no statistical difference in NCC protein expression in the absence of WNK4 in either NCC+sortilin WT or NCC+sortilin TRU groups as compared with control group in B. In the presence of WNK4 WT, NCC protein expression is significantly reduced in NCC+WNK4+GFP Vect group as compared with either control group or NCC+WNK4+sortilin TRU group in D (**P < 0.001). NCC protein expression in NCC+WNK4+sortilin WT is also significantly reduced as compared with the control group (*P < 0.01). The level of NCC protein expression in NCC+WNK4+sortilin TRU group was significantly higher as compared with the NCC+WNK4+sortilin WT group (#P < 0.05).
NCC Interacts with Sortilin WT and Its Truncated Sortilin
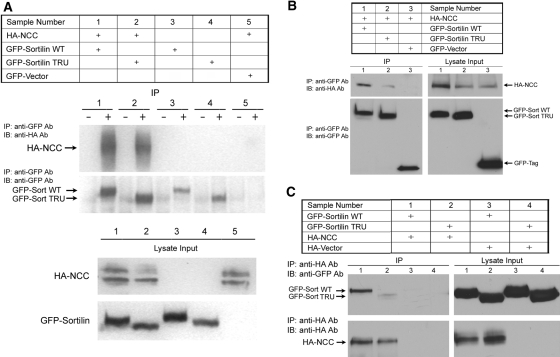
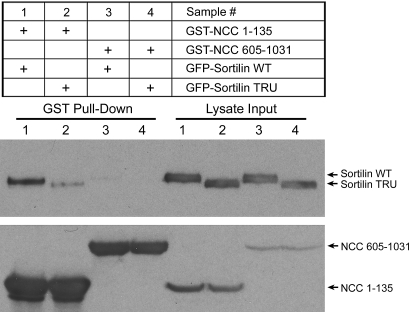
Because sortilin TRU reverses the inhibitory effect of WNK4 on NCC protein expression, we next tested whether NCC interacts with sortilin using co-immunoprecipitation (co-IP) experiments in Cos-7 cells. As shown in Figure 3C, A and B, in the cells co-transfected with HA-NCC WT in combination with either GFP-tagged sortilin WT or TRU, the anti-GFP antibody immunoprecipitated both sortilin WT and TRU. In addition, both sortilin WT and TRU co-immunoprecipitated with NCC (samples 1 and 2 in Figure 3, A and B), although sortilin TRU bound to NCC with much less avidity. In contrast, in the cells co-transfected with a GFP vector and HA-NCC WT without sortilin, the anti-GFP antibody did not immunoprecipitate NCC (sample 5 in Figure 3A and sample 3 in Figure 3B). The reciprocal co-IP also showed that NCC co-precipitated sortilin WT as well as sortilin TRU to a lesser extent (samples 1 and 2 in Figure 3C). These data suggest that NCC interacts with both sortilin WT and TRU. Because sortilin TRU without its cytoplasmic tail still binds NCC, albeit with a lesser affinity, the luminal domain of sortilin could be the binding site. We also investigated which region of NCC contains the binding site for sortilin. As shown in Figure 4, in Cos-7 cells transiently transfected with either GST-NCC (1 through 135) or GST-NCC (605 through 1031) truncation mutants in combination with either GFP-sortilin WT or TRU, NCC (1 through 135) was able to pull down sortilin WT (sample 1) as well as sortilin TRU (sample 2) but to a lesser extent, whereas NCC (605 through 1031) did not pull down either sortilin WT (sample 3) or sortilin TRU (sample 4). These results suggest that the amino terminus of NCC is the site of interaction with sortilin.
Figure 3.
Sortilin interacts with NCC. Cos-7 cells were co-transfected with either GFP-sortilin WT or truncated GFP-sortilin TRU alone or HA-NCC in combination with GFP-sortilin WT or GFP-sortilin TRU as indicated. Forty-eight hours after transfection, cells were lysed, IP and co-IP were performed, and cells were subjected to Western blot analysis. (A and B) Representative IP and co-IP results. The top blots in the IP section indicate the co-IP results using a polyclonal anti-GFP antibody for IP and probing with a monoclonal anti-HA antibody. The bottom blots in the IP section indicate IP results using an anti-GFP antibody for IP and probing with anti-GFP antibody. The blots in the lysate input section indicate the lysates expressing NCC and sortilin, respectively. In both samples 1 and 2 in A and B, anti-GFP antibody precipitates both sortilin WT and TRU, and both sortilin WT and TRU are able to pull down HA-NCC. In sample 5 in A and sample 3 in B, anti-GFP antibody cannot pull down NCC as the negative control. Samples 3 and 4 in A serve as negative controls as well, because cells were not transfected with HA-NCC. (C) Reciprocal co-IP results. Again, the top blot in the IP section indicates co-IP results using monoclonal anti-HA antibody for IP and probing with a polyclonal anti-GFP antibody. The bottom blot in the IP section indicates IP results using an anti-HA antibody and probing with anti-HA antibody. The blots in the lysate input section indicate the lysates expressing sortilin and NCC, respectively. Again, in samples 1 and 2, anti-HA antibody precipitates HA-NCC, and NCC is able to pull down sortilin WT as well as sortilin TRU, although to a lesser extent. Samples 3 and 4 serve as negative control. −, Lysates incubated with preimmune rabbit serum; +, lysates incubated with rabbit anti-GFP antibody.
Figure 4.
The N-terminal cytoplasmic region of human NCC is responsible for its association with sortilin. Forty-eight hours after transfection, Cos-7 cells transfected with indicated plasmids were lysed, and the cell lysates were subjected to GST pull-down assay. (Left) GST pull-down results. (Right) Lysate input. (Left, top blot) GST-NCC (1 through 135) is able to pull down GFP-sortilin WT and GFP-sortilin TRU as indicated in lanes 1 and 2, whereas GST-NCC (605 through 1031) is not able to pull down either GFP-sortilin WT or GFP-sortilin TRU as indicated in lanes 3 and 4. The ability of glutathione to pull down both NCC (1 through 135) and NCC (605 through 1031) is shown in lanes 1 to 4 in the bottom blot. (Right) The lysate inputs for sortilin and NCC are shown in the top and bottom blots, respectively. These findings indicate that the amino terminus of NCC is responsible for its interaction with both sortilin WT and sortilin TRU.
Distribution of Sortilin and Truncated Sortilin
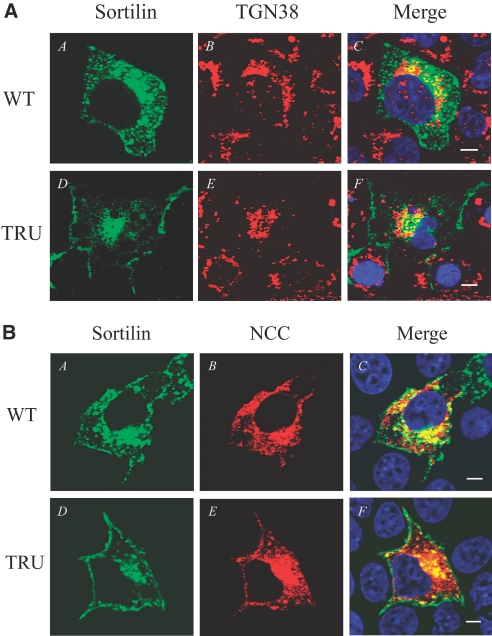
We then examined the distribution of sortilin WT and TRU in Cos-7 cells. Sortilin WT is seen in the perinuclear region, peripheral granular structures, and plasma membrane (Figure 5AA), whereas sortilin TRU is mainly seen in the perinuclear region with some in plasma membrane (Figure 5AD). Sortilin WT co-localizes with TGN 38, a TGN marker, in the perinuclear region (Figure 5AC). Sortilin TRU also co-localizes with TGN 38 in the perinuclear region (Figure 5AF), but, unlike sortilin WT, the expression in the peripheral granular structure is lost. These data indicate that sortilin WT is distributed in the TGN in addition to vesicles and the plasma membrane, and sortilin TRU is mainly retained in the TGN.
Figure 5.
Distribution of sortilin and its co-localization with NCC. Two days after Cos-7 cells were transfected with indicated plasmids, immunostaining and confocal microscopy were performed. (A) In A, sortilin WT in green seems to be distributed in the perinuclear region and peripheral granular structure as well as plasma membrane. In B, TGN 38 is shown in red. C shows the merged picture, indicating the co-localization of sortilin WT with TGN 38 in the perinuclear region in yellow. In D, the truncated sortilin TRU in green is mainly retained in the perinuclear region and some in the plasma membrane, but its expression pattern seems to be different from sortilin WT's, because the peripheral granular structure is lost. In E, TGN 38 is shown in red. F shows the merged picture, indicating the co-localization of sortilin TRU with TGN 38 mainly in the perinuclear region in yellow. (B) In A and D, sortilin WT and sortilin TRU seem to be distributed in the similar patterns as described in A, respectively. In B, NCC in red is distributed in the perinuclear, cytoplasmic, and plasma membrane regions. C shows the merged picture, indicating the co-localization of NCC with sortilin WT in the perinuclear and cytoplasmic regions in yellow. In E, NCC in red is mainly distributed in the perinuclear region with some in the plasma membrane. F shows the merged picture, indicating that the co-localization of NCC with sortilin TRU in yellow is mainly in the perinuclear region, and its peripheral co-localization with NCC seems be less. These findings suggest that the truncated sortilin TRU may prevent NCC from WNK4-promoted degradation through the lysosomal pathway by retaining NCC in the perinuclear region (TGN) or diverting NCC to other vesicular compartments other than lysosomes. Bar = 10 μM.
NCC Co-localizes with Sortilin WT and Its Mutant Sortilin TRU
We further investigated the cellular location where NCC interacts with sortilin in Cos-7 cells transiently transfected with HA-NCC WT in combination with either GFP-sortilin or TRU. NCC in red (Figure 5BB) is co-localized with sortilin WT in green (Figure 5BA) in the perinuclear region and peripheral granular structure (Figure 5BC), whereas a majority of NCC in red (Figure 5BE) is co-localized with sortilin TRU (Figure 5BD) in the perinuclear region and only a small amount of NCC is co-localized in the peripheral region (Figure 5BF), suggesting that sortilin TRU complexed with NCC is mainly retained in the TGN. These findings suggest that the truncated sortilin TRU may bind the endogenous sortilin via a dominant negative effect and interfere with its interaction with NCC and therefore prevent NCC from WNK4-mediated lysosomal degradation.
Sortilin Expresses and Co-localizes with NCC in the Renal Distal Convoluted Tubule
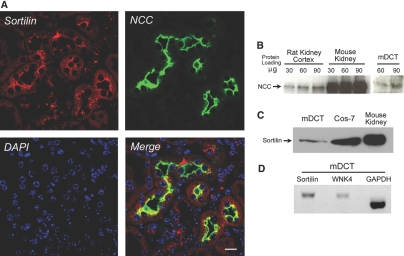
To confirm sortilin expression in native kidney tissue, particularly in the distal convoluted tubule (DCT), where NCC is exclusively expressed, we performed immunohistochemistry, Western blot analysis, and reverse transcriptase–PCR. As shown in Figure 6A, endogenous sortilin was expressed in the cytoplasm and subapical region of the mouse DCT (mDCT) and cortical collecting duct and co-localized with NCC mainly in the subapical region of mDCT. Sortilin mRNA and protein were also expressed in Cos-7, mouse kidney and mDCT cell lines (Figure 6, C and D). In addition, NCC and WNK4 were expressed in the kidney and mDCT cells (Figure 6, B and D). These data suggest that sortilin, NCC, and WNK4 are coexpressed in native DCT.
Figure 6.
Sortilin is expressed and co-localized with NCC in the renal DCT. (A) Mouse kidney cortex was fixed and sectioned, and immunohistochemistry was performed. Monoclonal anti-sortilin antibody and polyclonal rabbit anti-NCC antibody (gift of Dr. Mark Knepper, National Institutes of Health) were used to stain the endogenous sortilin (in red) and NCC (in green), respectively. DAPI was used to stain the nucleus. The merged picture shows that sortilin is co-localized with NCC in the subapical region of DCT cells (in yellow). (B) A Western blot shows the endogenous NCC protein expression in rat kidney cortex, mouse whole kidney, and mDCT cell lines. (C) A Western blot shows that endogenous sortilin is expressed in mDCT and Cos-7 cells as well as mouse kidney. (D) Reverse transcriptase–PCR shows endogenous sortilin and WNK4 expressions in mDCT cells. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was used as a positive control. These results demonstrate that sortilin is expressed in the kidney, especially in DCT cells, and co-localize with NCC, indicating that sortilin and NCC may interact each other in vivo. Bar = 20 μM.
WNK4 Promotes NCC Targeting to Lysosome for Degradation
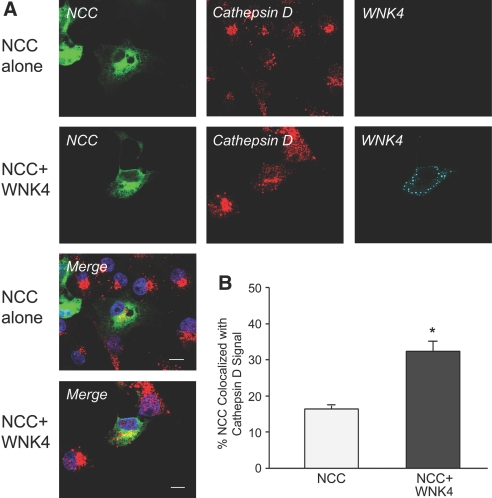
We further investigated whether WNK4 increases NCC targeting to the lysosomal compartment by immunofluorescence confocal microscopy. Cos-7 cells were transiently transfected with GFP-NCC with or without myc-WNK4. The cells were pretreated with lysosomal inhibitors leupeptin (30 μM) and E64 (50 μM) for 14 h before immunostaining; therefore, the pool of NCC that would normally be degraded in lysosomes could be visualized. As shown in Figure 7A, in the NCC alone group, NCC is distributed in plasma membrane, cytoplasm, and perinuclear region and has some degree of co-localization with lysosomal marker cathepsin D. In contrast, in the NCC plus WNK4 group, NCC seems to be mainly distributed in cytoplasm and the perinuclear region, and the degree of co-localization of NCC with cathepsin D seems to be increased. To verify these findings, we quantified the overlapping signals between NCC and cathepsin D in both groups. As shown in Figure 7B, the percentage of NCC co-localization with cathepsin D in NCC plus WNK4 group significantly increased as compared with NCC alone group (16.4 ± 1.3 versus 32.3 ± 3.2%; P < 0.001; n = 19). These data suggest that WNK4 promotes NCC accumulation in lysosomal compartment for degradation.
Figure 7.
WNK4 promotes NCC targeting to the lysosomal compartment. Two days after Cos-7 cells transiently co-transfected with GFP-NCC alone or with myc-WNK4 WT, immunostaining and confocal microscopy were performed. Sixteen hours before fixation, Cos-7 cells were pretreated with lysosomal inhibitors leupeptin (30 μM) and E64 (50 μM); therefore, the pool of NCC that would normally be degraded in lysosomes could be visualized and the percentage of NCC co-localized with lysosomal marker cathepsin D was quantified for individual cells. (A) GFP-NCC is seen in green. Cathepsin D is detected by a polyclonal rabbit anti-cathepsin D followed by Cy3-conjugated secondary antibody in red. WNK4 is shown in cyan. DAPI in blue shows the nucleus staining. The merged pictures show the co-localization of NCC with cathepsin D in yellow. In the absence of WNK4, NCC is distributed in plasma membrane, cytoplasmic, and perinuclear regions, and it shows some extent of co-localization with cathepsin D, whereas in the presence of WNK4, NCC is seen mainly in cytoplasmic and perinuclear regions as well as less in plasma membrane, and the degree of its co-localization with cathepsin D seems to be increased. (B) To quantify the percentage of NCC co-localization with cathepsin D in these two groups, the images of 19 cells from three separate experiments in each group were obtained by z axis scanning under confocal microscopy. The percentage of the co-localization of NCC with cathepsin D in yellow was quantified using the manufacturer's software (Zen; Zeiss). In the presence of WNK4, the percentage of co-localization of NCC with lysosomal marker cathepsin D is significantly higher than that in the absence of WNK4, indicating that WNK4 promotes NCC targeting to lysosomal compartments for degradation.
NCC Co-localizes with Sortilin and WNK4
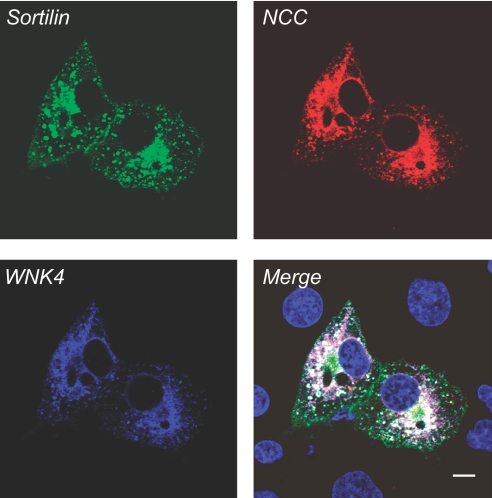
We previously showed that WNK4 interacts with NCC.6 We now showed that NCC interacts with sortilin. To examine whether NCC, sortilin, and WNK4 coexpress in the same subcellular compartment, we evaluated the co-distribution of these proteins in Cos-7 cells co-transfected with HA-NCC, GFP-sortilin WT, and myc-WNK4 WT. As shown in Figure 8, NCC, sortilin, and WNK4 are co-localized in the perinuclear region and peripheral granular structures. These combined data suggest that NCC, sortilin, and WNK4 might be associated together and NCC might sequentially bind sortilin and WNK4 that ultimately facilitates the WNK4-promoted NCC targeting to the lysosome for degradation via a sortilin-mediated mechanism.
Figure 8.
NCC co-localizes with sortilin and WNK4. Cos-7 cells were co-transfected with myc-WNK4 WT, GFP-sortilin WT, and HA-NCC WT. Forty-eight hours after transfection, immunostaining and confocal microscopy were performed. The distribution pattern for sortilin WT in green seems to be similar to sortilin WT as described in Figure 5A, and NCC expression pattern in red seems to be similar to the NCC pattern as described in Figure 5B. WNK4 in blue is expressed in the perinuclear and cytoplasmic regions. The merged picture shows the co-localization of WNK4, sortilin, and NCC in the perinuclear region in white, indicating that WNK4, sortilin, and NCC might be associated together.
Discussion
Emerging evidence has shown WNK kinase plays an important role in maintaining homeostasis of ion channels and transporters.35–37 Our previous study showed that WNK4 enhances NCC degradation through a lysosomal pathway.6 In this study, we showed that sortilin TRU reverses the inhibitory effect of WNK4 on NCC. We also showed that NCC interacts with sortilin WT as well as TRU, albeit to a lesser extent, via its N-terminus. Sortilin WT is co-localized with NCC throughout the cytoplasm, whereas the sortilin TRU is co-localized with NCC mainly in the perinuclear region. Sortilin is endogenously expressed in the kidney, especially in DCT cells, and co-localized with NCC in vivo. WNK4 increases NCC targeting to lysosomal compartment. In addition, WNK4, sortilin, and NCC seem to be co-localized in the perinuclear region. These data suggest that WNK4, NCC, and sortilin interact with one another and facilitate NCC targeting to the lysosome for degradation.
Previous studies showed that sortilin TRU is mainly retained in the perinuclear region (Golgi and TGN).28 We showed that sortilin TRU bind NCC even though its affinity to NCC is less than sortilin WT; therefore, the reversal of the inhibitory effect of WNK4 on NCC could be due to sortilin TRU's dominant negative effect on endogenous sortilin, which interferes with its interaction with NCC and therefore prevents NCC from moving to the lysosome. We also showed that WNK4 significantly increases NCC accumulation in the lysosomal compartment. These data suggest that WNK4 downregulates NCC by promoting NCC to the lysosome via a sortilin-mediated mechanism, which represents a novel mechanism for the regulation of NCC trafficking by WNK4 kinase.
Protein trafficking in the eukaryotic systems involves a series of protein–protein interactions between the cargo and protein transport carriers. Cargo proteins are selected for sorting to specific destinations by coat proteins or by receptors that interact with coat proteins.38 For a cargo protein to exit a sorting compartment, a receptor needs to interact with cytoplasmic coat proteins, such as adaptor proteins and clathrin; then small-coated vesicles are formed from donor membranes in the TGN and traffic the cargo to acceptor membranes such as endosomes and lysosomes. Clathrin is a structural protein that is the main constituent in clathrin-coated vesicles (CCV). These vesicles also include the GGAs and either of two heterotetrameric AP complexes, AP1 or AP3.25,26 The GGAs are newly identified monomeric clathrin APs,22–24 which have similar function as the AP complexes. The VHS domain of the GGA protein functions as a recognition module for sorting signals.39 The cytoplasmic tail of M6PR or sortilin binds GGA via its DXXLL motif and subsequently recruits clathrin to form CCVs designated to endosomes and lysosomes. In this study, we showed that NCC interacts with sortilin, most likely in the TGN. It is probable that NCC binds sortilin, subsequently recruiting GGA and clathrin to form CCV.24,38,39 The CCV containing NCC may also associate with AP3 to target to the lysosome for degradation. A recent study also showed that WNK4 increases the association of NCC with AP3 to promote NCC delivery to the lysosomal compartment for degradation,40 which is consistent with our conclusion. Our findings further support the notion that NCC is regulated through affecting its forward trafficking in addition to other mechanisms such as altering its abundance,41–43 glycosylation,10 and phosphorylation.44,45
In the kidney, NCC is exclusively expressed in DCT and responsible for 5 to 7% of sodium reabsorption.46 It is likely that, under normal physiologic conditions, NCC constantly traffics to both the plasma membrane via a secretory pathway and the lysosome for degradation to maintain its basal steady protein level, which is subject to regulation by many mediators.3,6,10,41–45 Renal DCT cells contain endogenous WNK4 kinase.2 In the presence of WNK4, the rate of NCC targeting to the lysosomal compartment is much higher than that of NCC without WNK4. We also showed that WNK4, NCC, and sortilin co-localize together in the perinuclear region, indicating that they might be associated together and/or interact in the same vesicles, traffic together, and facilitate the WNK4-promoted NCC targeting to the lysosome for degradation through a sortilin receptor–mediated mechanism; however, because we used overexpression system, overall protein degradation will inevitably be increased to cope with the excess protein, which is a potential limitation in our study. We believe that, overall, increased protein degradation would not necessarily alter the effect of WNK4 on the regulation of NCC. Taken together, our data suggest that WNK4 kinase could play a role in redirecting NCC's destination. The exact mechanism of how WNK4 alters NCC trafficking needs to be further explored.
Sortilin not only is involved in sorting of lysosomal hydrolases13 but also is engaged in intracellular sorting of Glut4,32,34,47 endocytosis,48 and signal transduction.49,50 In this study, we showed that NCC interacts with sortilin WT as well as TRU, to a lesser extent. Sortilin TRU, which lacks the cytoplasmic tail, is still able to bind NCC, indicating that NCC interacts with the luminal domain of sortilin. Because sortilin TRU has low binding affinity to NCC, other potential binding sites in the cytoplasmic tail of sortilin cannot be excluded. The exact reason that sortilin TRU has a low affinity for NCC needs to be clarified. We also found that the N-terminus of NCC is responsible for its interaction with sortilin, but we do not know which sequence or motif in the N-terminus is involved in this interaction. Previous studies demonstrated that WNK4 inhibits NCC surface expression and its function in Xenopus oocytes3,4 and mammalian cells6,11 by a kinase-dependent mechanism. Because the N-terminus of NCC is responsible for interaction with sortilin, it is speculated that WNK4 might phosphorylate the N-terminus of NCC directly or indirectly and alter its interaction with sortilin. Although the mechanism remains to be clarified, it is clear that WNK4 affects NCC sorting direction and targeting. Further experiments will be needed to examine whether the phosphorylation status at the N-terminus of NCC affects its trafficking or final sorting destination. Unlike WNK4, WNK3 stimulates NCC activity in Xenopus oocytes.51,52 WNK3 and WNK4 share a high degree of amino acid identity within the kinase domain but a much lower degree of amino acid identity in the amino and carboxyl terminal domains. San-Cristobal et al.53 reported that amino terminus of WNK3 or WNK4 plays an important role in regulation of NCC activity. How the amino terminal domain of WNK4 affects NCC function or trafficking, affects the interaction with sortilin and NCC, and ultimately changes the direction of NCC sorting remains unknown. Elucidating these molecular mechanisms will definitely provide a novel view on NCC regulation by WNK kinase.
Sortilin has been shown to play an important role in participating in sorting membrane proteins or transporters such as NCC. This study showed that WNK4 promotes NCC degradation through a lysosomal pathway via a sortilin-mediated mechanism, which provides a novel mechanism underlying NCC regulation by WNK kinase.
Concise Methods
Plasmids and Constructs
Human WNK4 WT and human NCC WT were amplified by PCR from a human kidney cDNA library and then subcloned into pCMV-taq 3B vector (Stratagene, La Jolla, CA) and pCMV-HA vector (Clontech, Palo Alto, CA), respectively, as described previously.6 The GST-tagged full-length NCC, N-terminal intracellular NCC (1 through 135), and C-terminal intracellular NCC (605 through 1031) constructs were generated by PCR amplification using pCMV-HA-NCC as template and by insertion of respective cDNA into modified PRK5-GST vectors. The pEGFP-sortilin WT and its truncated sortilin lacking cytoplasmic tail (pEGFP-sortilin TRU), a dominant negative mutant, were provided by Dr. Carlos R. Morales (McGill University, Hamilton, Ontario, Canada).27 All constructs, shown in Figure 1, were confirmed by sequence analysis. These constructs have been successfully expressed in African green monkey kidney cells (Cos-7) and confirmed by Western blot analysis.
Cell Culture, Transfection, and Animals
Cos-7 cells obtained from American Type Culture Collection (Manassas, VA) were maintained in DMEM supplemented with 10% FCS, l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). mDCT cells were a gift of Peter Friedman (University of Pittsburgh, Pittsburgh, PA).43,54 mDCT cells were maintained in DMEM/F-12 medium containing 5% FCS, 1% penicillin, 1% streptomycin, and 1% neomycin. All other media and components were purchased from Invitrogen (Carlsbad, CA). Cells were transiently transfected using LipofectAMINE 2000 (Invitrogen) according to the manufacturer's instructions. Forty-eight hours after transfection, cells were used for Western blot analysis, IP, or immunostaining. All animal protocols were approved by the Emory University Institutional Animal Care and Use Committee. The kidneys from adult male Sprague-Dawley rats (Charles River Laboratory, Wilmington, MA) and C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME), were harvested for Western blot analysis and immunohistochemistry.
Western Blotting Analysis
Cos-7 cells were harvested and processed as described previously.6 Briefly, cells transiently transfected with various DNA constructs as indicated were lysed in lysis buffer containing 20 mM HEPES (pH 7.5), 120 mM NaCl, 5.0 mM EDTA, 1.0% Triton X-100, 0.5 mM dithiothreitol, 1.0 mM PMSF, and complete protease inhibitor (Roche Diagnostics, Mannheim, Germany; 1 tablet per 50 ml of solution). The lysates were spun at 6000 × g for 5 min to pellet the insoluble material, and the proteins from supernatant were quantified using a Pierce BCA Protein Assay kit (Pierce, Rockford, IL). After mixing in Laemmli buffer (Bio-Rad, Hercules, CA) and incubating at 37°C for 30 min, the protein sample was separated by SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (Amersham Biosciences, Piscataway, NJ) for Western blot. The probing with specific antibodies and subsequent detection with ECL plus system (Amersham Biosciences) or Super signal (Pierce) were performed according to standard procedures as described previously.6,55
IP and Co-IP
IP and co-IP were preformed as described previously.55 In brief, Cos-7 cells were transfected with plasmids as indicated. Forty-eight hours after transfection, the cells were lysed. The cell lysates were incubated with primary antibodies for 2 h, then Protein G/A Sepharose beads were added, and the lysates were further mixed and incubated overnight at 4°C. After washing with lysis buffer twice and PBS once, beads were then eluted with Laemmli buffer (Bio-Rad). The eluted proteins were separated by SDS-PAGE, transferred onto PVDF membrane, and probed with appropriate antibodies. For co-IP experiments, blots were first probed with an antibody to the first binding partner. The PVDF membranes were then stripped and reprobed with an antibody to the second binding partner. Reciprocal IP was also performed with respective antibodies.
GST Pull-Down Assay
GST pull-down assay was modified and carried out as described previously.56 In brief, Cos-7 cells were co-transfected with GST-tagged N-terminus or C-terminus of NCC in combination with either sortilin WT or sortilin TRU. Forty-eight hours after transfection, cells were lysed in lysis buffer, and cell lysates were prepared as described previously.6 The resultant supernatants were incubated with Glutathione-Sepharose 4B (Amersham Biosciences) overnight at 4°C. The Glutathione-Sepharose 4B beads were then washed five times with lysis buffer. The bound protein complexes were resolved on a 7% SDS-PAGE and transferred to a PVDF membrane. Subsequently, the blots were probed with the indicated antibodies.
Immunostaining, Immunohistochemistry, and Confocal Microscopy
Immunostaining and confocal microscopy were performed as described previously.6 In brief, for immunostaining experiments, the transfected Cos-7 cells were fixed and blocked with 5% nonfat dry milk in PBS for 1 h, then incubated with primary antibodies for 30 min at 37°C. Cells were then washed with PBS three times followed by 30 min of incubation at 37°C with appropriate secondary antibodies conjugated to either fluorescein (FITC) or isothiocyanate Cy3 fluorescent dye (Jackson ImmunoResearch Laboratory, West Grove, PA). After staining, the coverslips were washed thoroughly with PBS three times, mounted with antiquenching medium (Vector Laboratories, Burlingame, CA), and sealed. For immunohistochemistry, in situ fixation of mouse kidneys was performed as described previously.57 For paraffin embedding, tissues were dehydrated in a series of graded ethyl alcohol followed by xylene and embedded in paraffin and then cut into 2-μm sections. Sections were deparaffinized and rehydrated. Endogenous peroxidase was blocked by 0.5% H2O2 in absolute methanol for 30 min at room temperature. For revealing antigens, sections were incubated in 1 mM Tris solution (pH 9.0) supplemented with 0.5 mM EGTA and heated in a microwave oven for 10 min. Nonspecific binding of IgG was prevented by incubating the sections in 50 mM NH4Cl-PBS for 30 min, followed by blocking in PBS supplemented with 1% BSA, 0.05% saponin, and 0.2% gelatin. Sections were incubated for 30 min at 37°C with primary antibodies (monoclonal anti-sortilin antibody [1:200] or rabbit anti-NCC antibody [1:200] from Dr. Mark Knepper, National Institutes of Health) diluted in PBS supplemented with 0.1% BSA and 0.3% Triton X-100. After rinsing with PBS, sections were incubated with either Cy-3 or FITC-conjugated secondary antibody (1:200) for 30 min at 37°C. After washing with PBS, sections were mounted with antiquenching medium (Vector Laboratories) and sealed. The slides were examined using a confocal laser scanning microscope (model LSM510; Zeiss). The images were acquired using the manufacturer's software and prepared for publication with Adobe Photoshop. The co-localization analyses were performed using the manufacturer's software (Zen; Zeiss).
Statistical Analysis
The data are presented as means ± SE. Statistical significance was determined by t test when two groups were compared or by one-way ANOVA, followed by Bonferroni post hoc tests when multiple groups were compared. We assigned significance at P < 0.05.
Disclosures
None.
Acknowledgments
This work is supported by National Institutes of Health grants DK068226 (H.C.) and DK R01 32753 (W.B.G.).
Part of this work was presented annual meeting of the American Society of Nephrology; San Francisco, CA; November 2, 2007.
We thank Dr. Carlos R. Morales (McGill University, Hamilton, Ontario, Canada) for kindly providing sortilin plasmid. We thank Drs. Janet D. Klein and Misti A. Blount for helpful suggestions and critical reading of the manuscript. We thank Dr. Young-Hee Kim for help in immunohistochemistry. We also thank Dr. William McClellan for help with the statistical analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Sorting out Lysosomal Trafficking of the Thiazide-Sensitive Na-Cl Co-transporter,” on pages 7–9.
References
- 1.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH: WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275: 16795–16801, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP: Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP: Molecular pathogenesis of inherited hypertension with hyperkalemia: The Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci U S A 100: 680–684, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang CL, Angell J, Mitchell R, Ellison DH: WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabath E, Meade P, Berkman J, de los Heros P, Moreno E, Bobadilla NA, Vazquez N, Ellison DH, Gamba G: Pathophysiology of functional mutations of the thiazide-sensitive Na-Cl cotransporter in Gitelman disease. Am J Physiol Renal Physiol 287: F195–F203, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Cai H, Cebotaru V, Wang YH, Zhang XM, Cebotaru L, Guggino SE, Guggino WB: WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int 69: 2162–2170, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP: Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Syren ML, Tedeschi S, Cesareo L, Bellantuono R, Colussi G, Procaccio M, Ali A, Domenici R, Malberti F, Sprocati M, Sacco M, Miglietti N, Edefonti A, Sereni F, Casari G, Coviello DA, Bettinelli A: Identification of fifteen novel mutations in the SLC12A3 gene encoding the Na-Cl co-transporter in Italian patients with Gitelman syndrome. Hum Mutat 20: 78, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Kunchaparty S, Palcso M, Berkman J, Velazquez H, Desir GV, Bernstein P, Reilly RF, Ellison DH: Defective processing and expression of thiazide-sensitive Na-Cl cotransporter as a cause of Gitelman's syndrome. Am J Physiol 277: F643–F649, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Hoover RS, Poch E, Monroy A, Vazquez N, Nishio T, Gamba G, Hebert SC: N-Glycosylation at two sites critically alters thiazide binding and activity of the rat thiazide-sensitive Na(+):Cl(−) cotransporter. J Am Soc Nephrol 14: 271–282, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Golbang AP, Cope G, Hamad A, Murthy M, Liu CH, Cuthbert AW, O'shaughnessy KM: Regulation of the expression of the Na/Cl cotransporter by WNK4 and WNK1: Evidence that accelerated dynamin-dependent endocytosis is not involved. Am J Physiol Renal Physiol 291: F1369–F1376, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Ni X, Morales CR: The lysosomal trafficking of acid sphingomyelinase is mediated by sortilin and mannose 6-phosphate receptor. Traffic 7: 889–902, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Ni X, Canuel M, Morales CR: The sorting and trafficking of lysosomal proteins. Histol Histopathol 21: 899–913, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Lobel P, Fujimoto K, Ye RD, Griffiths G, Kornfeld S: Mutations in the cytoplasmic domain of the 275 kd mannose 6-phosphate receptor differentially alter lysosomal enzyme sorting and endocytosis. Cell 57: 787–796, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Kornfeld S: Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem 61: 307–330, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Ghosh P, Dahms NM, Kornfeld S: Mannose 6-phosphate receptors: New twists in the tale. Nat Rev Mol Cell Biol 4: 202–212, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, Roigaard H, Gliemann J, Madsen P, Moestrup SK: Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem 272: 3599–3605, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Takatsu H, Katoh Y, Shiba Y, Nakayama K: Golgi-localizing, gamma-adaptin ear homology domain, ADP-ribosylation factor-binding (GGA) proteins interact with acidic dileucine sequences within the cytoplasmic domains of sorting receptors through their Vps27p/Hrs/STAM (VHS) domains. J Biol Chem 276: 28541–28545, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Puertollano R, Aguilar RC, Gorshkova I, Crouch RJ, Bonifacino JS: Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science 292: 1712–1716, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Doray B, Poussu A, Lehto VP, Kornfeld S: Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science 292: 1716–1718, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, Pohlmann R, Petersen CM: The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J 20: 2180–2190, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boman AL, Zhang C, Zhu X, Kahn RA: A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol Biol Cell 11: 1241–1255, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirst J, Lui WW, Bright NA, Totty N, Seaman MN, Robinson MS: A family of proteins with gamma-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J Cell Biol 149: 67–80, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dell'Angelica EC, Puertollano R, Mullins C, Aguilar RC, Vargas JD, Hartnell LM, Bonifacino JS: GGAs: A family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J Cell Biol 149: 81–94, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapuy B, Tikkanen R, Muhlhausen C, Wenzel D, von FK, Honing S: AP-1 and AP-3 mediate sorting of melanosomal and lysosomal membrane proteins into distinct post-Golgi trafficking pathways. Traffic 9: 1157–1172, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Bonifacino JS: Insights into the biogenesis of lysosome-related organelles from the study of the Hermansky-Pudlak syndrome. Ann N Y Acad Sci 1038: 103–114, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Lefrancois S, Zeng J, Hassan AJ, Canuel M, Morales CR: The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin. EMBO J 22: 6430–6437, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefrancois S, Canuel M, Zeng J, Morales CR: Inactivation of sortilin (a novel lysosomal sorting receptor) by dominant negative competition and RNA interference. Biol Proced Online 7: 17–25, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan AJ, Zeng J, Ni X, Morales CR: The trafficking of prosaposin (SGP-1) and GM2AP to the lysosomes of TM4 Sertoli cells is mediated by sortilin and monomeric adaptor proteins. Mol Reprod Dev 68: 476–483, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Munck PC, Nielsen MS, Jacobsen C, Tauris J, Jacobsen L, Gliemann J, Moestrup SK, Madsen P: Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. EMBO J 18: 595–604, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lintzel J, Franke I, Riedel IB, Schaller HC, Hampe W: Characterization of the VPS10 domain of SorLA/LR11 as binding site for the neuropeptide HA. Biol Chem 383: 1727–1733, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Shi J, Kandror KV: Sortilin is essential and sufficient for the formation of Glut4 storage vesicles in 3T3–L1 adipocytes. Dev Cell 9: 99–108, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Shi J, Kandror KV: The luminal Vps10p domain of sortilin plays the predominant role in targeting to insulin-responsive Glut4-containing vesicles. J Biol Chem 282: 9008–9016, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Lin BZ, Pilch PF, Kandror KV: Sortilin is a major protein component of Glut4-containing vesicles. J Biol Chem 272: 24145–24147, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Kahle KT, Rinehart J, Giebisch G, Gamba G, Hebert SC, Lifton RP: A novel protein kinase signaling pathway essential for blood pressure regulation in humans. Trends Endocrinol Metab 19: 91–95, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Kahle KT, Ring AM, Lifton RP: Molecular physiology of the WNK kinases. Annu Rev Physiol 70: 329–355, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Huang CL, Yang SS, Lin SH: Mechanism of regulation of renal ion transport by WNK kinases. Curr Opin Nephrol Hypertens 17: 519–525, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Bonifacino JS, Lippincott-Schwartz J: Coat proteins: Shaping membrane transport. Nat Rev Mol Cell Biol 4: 409–414, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Bonifacino JS: The GGA proteins: Adaptors on the move. Nat Rev Mol Cell Biol 5: 23–32, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Subramanya AR, Liu J, Ellison DH, Wade JB, Welling PA: WNK4 diverts the thiazide-sensitive NaCl cotransporter to the lysosome and stimulates AP-3 interaction. J Biol Chem 284: 18471–18480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA: The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci U S A 95: 14552–14557, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S: Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 74: 1403–1409, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Ko B, Joshi LM, Cooke LL, Vazquez N, Musch MW, Hebert SC, Gamba G, Hoover RS: Phorbol ester stimulation of RasGRP1 regulates the sodium-chloride cotransporter by a PKC-independent pathway. Proc Natl Acad Sci U S A 104: 20120–20125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR: Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci 121: 675–684, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Pacheco-Alvarez D, Cristobal PS, Meade P, Moreno E, Vazquez N, Munoz E, Diaz A, Juarez ME, Gimenez I, Gamba G: The Na+:Cl− cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem 281: 28755–28763, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Gamba G: Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Morris NJ, Ross SA, Lane WS, Moestrup SK, Petersen CM, Keller SR, Lienhard GE: Sortilin is the major 110-kDa protein in GLUT4 vesicles from adipocytes. J Biol Chem 273: 3582–3587, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Nielsen MS, Jacobsen C, Olivecrona G, Gliemann J, Petersen CM: Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase. J Biol Chem 274: 8832–8836, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL: ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci 25: 5455–5463, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin S, Navarro V, Vincent JP, Mazella J: Neurotensin receptor-1 and -3 complex modulates the cellular signaling of neurotensin in the HT29 cell line. Gastroenterology 123: 1135–1143, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Yang CL, Zhu X, Ellison DH: The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Invest 117: 3403–3411, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rinehart J, Kahle KT, de los Heros P, Vazquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP: WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl- cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci U S A 102: 16777–16782, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.San-Cristobal P, Ponce-Coria J, Vazquez N, Bobadilla NA, Gamba G: WNK3 and WNK4 amino terminal domain defines their effect on the renal Na+:Cl− cotransporter. Am J Physiol Renal Physiol 295: F1199–F1206, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gesek FA, Friedman PA: Sodium entry mechanisms in distal convoluted tubule cells. Am J Physiol 268: F89–F98, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Cheng J, Moyer BD, Milewski M, Loffing J, Ikeda M, Mickle JE, Cutting GR, Li M, Stanton BA, Guggino WB: A Golgi-associated PDZ domain protein modulates cystic fibrosis transmembrane regulator plasma membrane expression. J Biol Chem 277: 3520–3529, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Mistry AC, Mallick R, Frohlich O, Klein JD, Rehm A, Chen G, Sands JM: The UT-A1 urea transporter interacts with snapin, a SNARE-associated protein. J Biol Chem 282: 30097–30106, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, Verlander JW: Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol 284: F229–F241, 2003 [DOI] [PubMed] [Google Scholar]