Abstract
Left ventricular hypertrophy (LVH) associates with increased risk for cardiovascular disease. Hypertension leads to LVH in adults, but its role in the pathogenesis of LVH in children is not as well established. To examine left ventricular mass and evaluate factors associated with LVH in children with stages 2 through 4 chronic kidney disease (CKD), we analyzed cross-sectional data from children who had baseline echocardiography (n = 366) and underwent ambulatory BP monitoring (n = 226) as a part of the observational Chronic Kidney Disease in Children (CKiD) cohort study. At baseline, 17% of children had LVH (11% eccentric and 6% concentric) and 9% had concentric remodeling of the left ventricle. On the basis of a combination of ambulatory and casual BP assessment (n = 198), 38% of children had masked hypertension (normal casual but elevated ambulatory BP) and 18% had confirmed hypertension (both elevated casual and ambulatory BP). There was no significant association between LVH and kidney function. LVH was more common in children with either confirmed (34%) or masked (20%) hypertension compared with children with normal casual and ambulatory BP (8%). In multivariable analysis, masked (odds ratio 4.1) and confirmed (odds ratio 4.3) hypertension were the strongest independent predictors of LVH. In conclusion, casual BP measurements alone are insufficient to predict the presence of LVH in children with CKD. The high prevalence of masked hypertension and its association with LVH supports early echocardiography and ambulatory BP monitoring to evaluate cardiovascular risk in children with CKD.
Left ventricular hypertrophy (LVH), a frequent finding in adults with chronic kidney disease (CKD), poses an increased risk for cardiovascular disease.1 Similarly, LVH has been detected in approximately one third of children with stages 2 through 4 CKD.2–5 Whereas hypertension is directly linked to the development of LVH in adults with CKD,1 the relationship between elevated BP and the development of LVH in pediatric CKD remains unclear. In a multicenter study from Europe,4 no significant associations were found between casual or ambulatory BP and LVH in children with stages 2 through 4 CKD, suggesting only a minor role of hypertension in the pathogenesis of LVH in mild to moderate pediatric CKD.
The National Institutes of Health recently established the Chronic Kidney Disease in Children (CKiD) study, a prospective, observational study of children with mild to moderate CKD. The overall study design has been previously published.6 Identification of the prevalence and evolution of traditional and novel cardiovascular disease risk factors in progressive CKD is one of the primary aims of this study. As a part of the CKiD cardiovascular evaluation, echocardiography and 24-h ambulatory BP monitoring (ABPM) are performed biannually. In this report, baseline echocardiographic and ambulatory BP data from the CKiD cohort were analyzed to (1) describe LV structure in the CKiD cohort and (2) analyze the associations of casual and ambulatory BP, kidney function, and other demographic and clinical characteristics with LVH in this same group of children.
Results
Cohort Characteristics
Demographic and clinical characteristics of the 366 participants who had LVM index (LVMI) data available are summarized in Table 1. The majority were male and white, and most had nonglomerular forms of CKD. A significant number of participants were preterm at birth or were reported to have a low birth weight. As expected for children with CKD, participants tended to be short with preserved weight; 15% were obese. Two thirds of the participants were taking antihypertensive medications; the majority were taking angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs). There was no significant difference in demographic and clinical characteristics among children who had ABPM (n = 226) and those who did not have ABPM data available (n = 140; Supplemental Table 1).
Table 1.
Characteristics of study population (n = 366)
| Characteristic | Value |
|---|---|
| Age (yr; median [IQR]) | 12 (9 to 15) |
| Female gender (n [%]) | 146 (40) |
| Race/ethnicity (n [%]) | |
| white | 269 (74) |
| black | 59 (16) |
| other | 37 (10) |
| Hispanic | 52 (14) |
| Weight percentile (median [IQR]) | 43 (18 to 74) |
| Height percentile (median [IQR]) | 24 (7 to 50) |
| BMI percentile (median [IQR]) | 59 (33 to 84) |
| obese, BMI >95th percentile (n [%]) | 55 (15) |
| Low birth weight, <2500 g (n [%]) | 65 (19) |
| Preterm birth, <36 wk (n [%]) | 79 (22) |
| Iohexol-based GFR (ml/min per 1.73 m2; median [IQR]) | 43 (31 to 53) |
| Glomerular CKD (n [%]) | 72 (20) |
| Duration of CKD (yr; median [IQR]) | 8 (4 to 11) |
| Antihypertensive medications (n [%]) | 242 (67) |
| ACEIs/ARBs | 203 (56) |
| Ca blocker | 54 (15) |
| other | 12 (3) |
| ≥2 medications | 39 (11) |
BMI, body mass index; IQR, interquartile range.
Hypertension Status
The frequency of hypertension was higher on the basis of the mean 24-h wake or sleep BP as compared with casual BP. When BP load ≥25% was used to define elevated ambulatory BP, the prevalence reached 36% (systolic) and 31% (diastolic) during the wake period and 38% (systolic) and 42% (diastolic) during sleep. On the basis of a combination of casual and ambulatory BP, 12% of patients were classified as having confirmed systolic hypertension and 11% as having confirmed diastolic hypertension. Masked hypertension was found in 33% for systolic and 34% for diastolic BP (DBP). Overall, 18% of the CKiD participants had confirmed and 38% had masked systolic or diastolic hypertension. Among children with masked and confirmed hypertension, 29% and 15%, respectively, were not taking antihypertensive medications (Table 2).
Table 2.
Casual (n = 347) and ambulatory BP (n = 226) status of study population
| Variable | SBP | DBP |
|---|---|---|
| Casual BP | ||
| mmHg (median [IQR]) | 107 (100 to 117) | 65 (59 to 74) |
| ≥95th percentile (n [%]) | 43 (12) | 37 (11) |
| 24-h BP | ||
| mmHg (median [IQR]) | 110 (101 to 117) | 66 (60 to 71) |
| ≥95th percentile (n [%]) | 37 (16) | 38 (17) |
| load (%; median [IQR]) | 15 (4 to 37) | 13 (4 to 34) |
| load ≥25% (n [%]) | 89 (39) | 80 (35) |
| Wake BP | ||
| mmHg (median [IQR]) | 116 (108 to 122) | 70 (66 to 76) |
| ≥95th percentile (n [%]) | 39 (17) | 35 (15) |
| load (%; median [IQR]) | 13 (2 to 35) | 8 (2 to 30) |
| load ≥25% (n [%]) | 81 (36) | 69 (31) |
| Sleep BP | ||
| mmHg (median [IQR]) | 102 (94 to 109) | 59 (53 to 64) |
| ≥95th percentile (n [%]) | 49 (22) | 50 (22) |
| load (%; median [IQR]) | 14 (0 to 42) | 16 (4 to 41) |
| load ≥25% (n [%]) | 86 (38) | 95 (42) |
| Dipping | ||
| % (mean [IQR]) | 11 (7 to 14) | 17 (12 to 21) |
| abnormal (<10%) | 88 (39) | 36 (16) |
| BP statusa | ||
| normotension | 106 (54) | 108 (55) |
| white-coat hypertension | 3 (1.5) | 2 (1) |
| masked hypertension | 66 (33) | 67 (34) |
| confirmed hypertension | 23 (12) | 21 (11) |
Casual hypertension defined as BP ≥95th percentile according to the National High Blood Pressure Education Program (NHBPEP) Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents.25 Ambulatory hypertension defined as (1) mean wake and/or sleep SBP or DBP ≥95th percentile for ABPM and/or (2) SBP or DBP load ≥25%. BP status is based on combination of casual and ambulatory BP: (1) Normotensive: Normal casual and ambulatory BP; (2) confirmed hypertension: Presence of both casual and ambulatory hypertension; (3) white-coat hypertension: Casual hypertension in the presence of normal ambulatory BP; or (4) masked hypertension: Ambulatory hypertension in the presence of normal casual BP.
an = 198 for casual + ABPM.
Characteristics Associated with LVH
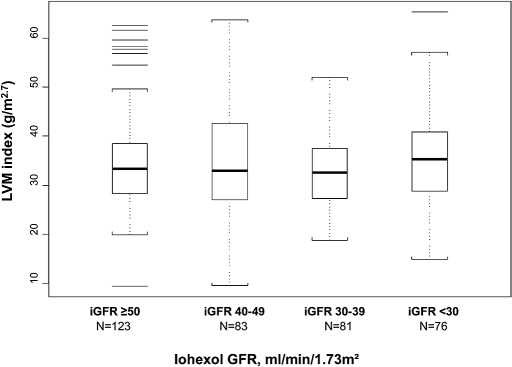
The median (interquartile range) LVMI was 33 g/m2.7 (28 to 39) and relative wall thickness (RWT) was 0.33 cm (0.30 to 0.37). The majority (74%) of patients had normal LV geometry, 17% (61) had LVH (11% eccentric and 6% concentric), and 9% (33) had concentric LV remodeling. There was no significant difference in LVMI on the basis of iohexol GFR status (Figure 1).
Figure 1.
Distribution of LVMI by iohexol GFR (n = 363) is shown. Overall P = 0.449.
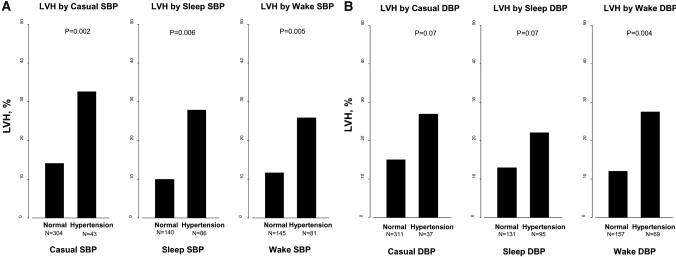
No significant difference was found in any demographic or clinical characteristics between children with eccentric and concentric LVH; therefore, we performed further analysis of LVH by evaluating those with eccentric and concentric LVH combined. We compared children who had LVH with those who did not have LVH (Table 3). There were more black, female, and younger children with LVH. Those with LVH were shorter, had a shorter duration of CKD, had more nephrotic-range proteinuria and anemia, and were receiving ACEIs or ARBs significantly less often than children without LVH. No significant difference in the family history of hypertension, preterm birth, or low birth weight was seen between children with or without LVH. LVH was more frequent in children with systolic casual, wake, and sleep ambulatory hypertension than in children without systolic casual or ambulatory hypertension (Figure 2A). For DBP, no statistically significant difference in the frequency of LVH was seen in children with a diagnosis of casual hypertension versus those without casual hypertension (Figure 2B). In contrast to casual DBP, LVH was more frequent in children with wake diastolic hypertension versus those without diastolic hypertension.
Table 3.
Characteristics of study population by LVH status (n = 366)
| Characteristic | No LVH (n = 305) | LVH (n = 61) | P |
|---|---|---|---|
| Black race (n [%]) | 44 (15) | 15 (25) | 0.051 |
| Female gender (n [%]) | 114 (37) | 32 (53) | 0.028 |
| Age (yr) | |||
| <6 (n [%]) | 38 (13) | 13 (21) | 0.068 |
| median (IQR) | 12 (9 to 16) | 11 (6 to 15) | 0.067 |
| Height (cm) | |||
| median (IQR) | 146 (126 to 162) | 135 (111 to 153) | 0.006 |
| percentile (median [IQR]) | 27.0 (10.0 to 51.0) | 7.7 (2.0 to 29.0) | <0.001 |
| Weight (kg) | |||
| median (IQR) | 41 (27 to 57) | 34 (20 to 53) | 0.066 |
| percentile (median [IQR]) | 43 (19 to 75) | 41 (12 to 65) | 0.261 |
| BMI percentile (median [IQR]) | 57 (32 to 84) | 69 (45 to 90) | 0.12 |
| Duration of CKD (yr; median [IQR]) | 8.1 (4.5 to 11.7) | 5.8 (3.0 to 9.4) | 0.002 |
| Glomerular CKD (n [%]) | 58 (19) | 14 (23) | 0.46 |
| Iohexol GFR (ml/min per 1.73 m2; median [IQR]) | 43 (32 to 55) | 41 (27 to 49) | 0.11 |
| Hypoalbuminemia <4 g/dl (n [%]) | 33 (11) | 12 (21) | 0.046 |
| Protein/creatinine ratio (median [IQR]) | 0.43 (0.17 to 1.11) | 0.64 (0.28 to 2.10) | 0.014 |
| Nephrotic proteinuria, >2 protein/creatinine ratio (n [%]) | 30 (10) | 14 (26) | 0.002 |
| Hemoglobin (g/dl; median [IQR]) | 13 (12 to 14) | 12 (11 to 13) | 0.003 |
| Anemia (n [%]) | 114 (39) | 32 (53) | 0.048 |
| wide range C-reactive protein (wrCRP) >0.3 mg/dl (n [%]) | 105 (43) | 21 (40) | 0.76 |
| Albumin-corrected calcium (mg/dl; median [IQR]) | 9.0 (8.8 to 9.2) | 9.0 (8.7 to 9.3) | 0.94 |
| Phosphate (mg/dl; median [IQR]) | 4.7 (4.2 to 5.2) | 4.7 (4.2 to 5.4) | 0.42 |
| Calcium × phosphate (mg2/dl2; median [IQR]) | 42 (37 to 47) | 43 (37 to 48) | 0.32 |
| ACEI/ARB use (n [%]) | 179 (59) | 24 (39) | 0.005 |
Figure 2.
(A and B) LVH by casual, wake, and sleep systolic (SBP; A) and diastolic BP (DBP; B) status is shown.
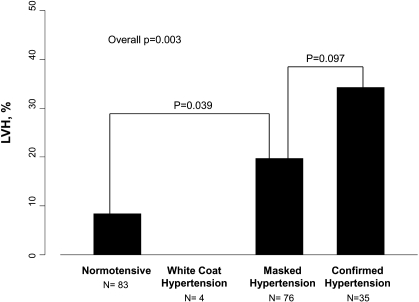
We then compared the frequency of LVH using a combination of casual and ambulatory BP. LVH was more frequent in children with confirmed (both casual and ambulatory; 34%; P < 0.003) and masked (20%; P = 0.039) systolic or diastolic hypertension than in children with normal casual and ambulatory BP (8%; Figure 3). In the multivariable analysis, confirmed or masked hypertension status was an independent predictor of LVH in CKiD children (Table 4). Female gender and lower hemoglobin were also associated with LVH.
Figure 3.
LVH by casual and ambulatory BP status (n = 198) is shown. Overall P = 0.003.
Table 4.
Multivariable analysis of LVH predictors in CKiD children
| Characteristics | OR (95% CI) |
|---|---|
| Female | 4.46 (1.60 to 12.39) |
| Hemoglobin, per 1 g/dl | 0.64 (0.45 to 0.91) |
| Masked hypertension | 4.13 (1.26 to 13.56) |
| Confirmed hypertension | 4.30 (1.01 to 18.26) |
Adjusted for age, height, race, CKD diagnosis, iohexol GFR, use of antihypertensive medications, protein/creatinine ratio, hypoalbuminemia, and years since diagnosis. CI, confidence interval; OR, odds ratio.
Discussion
This cross-sectional analysis of BP and cardiac structure in a large population of children with CKD demonstrates that LVH is strongly associated with hypertension. Our study shows that casual BP measurements alone are insufficient to detect the presence of LVH-inducing hypertension and indicates that ABPM should be performed routinely in this patient population.
Most previous studies of LV structure in children with CKD came from single centers and were limited by small sample sizes.2,3 The only multicenter study on the subject that has been published to date is the Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CKD in Pediatric Patients (ESCAPE) trial.4 In that study, echocardiographic data from 156 children who were aged 3 to 18 yr and had stages 2 through 4 CKD were analyzed, and 33% were found to have LVH. In this study (n = 366), LVH was found in 17% of patients. The difference in the prevalence of LVH might be explained by different cut points to define LVH in children. The ESCAPE trial defined LVH on the basis of a single cut point of LVMI ≥38 g/m2.7, the 95th percentile value in healthy children.7 Use of this LVMI cut point would yield a prevalence of LVH in our cohort of 30%; however, the use of this single cut point overestimates the prevalence of LVH, especially in young children. In the CKiD study, we used the normative values for LVMI developed at Cincinnati Children's Hospital Medical Center,8 the central site for reading and data analyses of echocardiographic data in the CKiD study. Pediatric reference values for LVMI (g/m2.7) were based on the analysis of 2273 (0 to 18 yr) nonobese, healthy children (1267 boys and 1006 girls). The data indicate that indexing of LVM to a power of height2.7 is age and gender specific. For example, the 95th percentile for LVMI varies from 40 to 45 g/m2.7 in adolescent girls and boys to approximately 70 g/m2.7 in 1-yr-old children; boys have a slightly larger LVM for a given height throughout childhood and adolescence. One of the potential pitfalls in using age-dependent LVM indexing in children with CKD is related to short stature. Recently, Foster et al.9 demonstrated that LVMI (g/m2.7) varies not only according to age but also according to absolute height with higher values in children with shorter height, especially in those with height <110 cm. Given that the study participants had significantly reduced height (median height percentile 24) relative to age, poor growth may have resulted in some misclassification of diagnosis of LVH in very young children. Indeed, children with LVH in our study were shorter than children without LVH; however, we found no significant independent relationship between height and LVH in the multivariable analysis.
Most studies of patients with CKD indicate that LVH becomes worse with progression of CKD. In contrast, we found no significant associations between LVMI or LVH and CKD stage. A potential explanation for this disparity is a difference in method used to determine kidney function. The primary prerequisite for identification and staging of CKD is an exact measurement of GFR. Most previous adult and pediatric studies have estimated GFR using serum creatinine or creatinine-based equations such as the Modification of Diet in Renal Disease (MDRD) Study equation, the Cockcroft-Gault formula, or the Schwartz formula; however, these methods are imprecise, especially for children.10,11 One of the major advantages and strengths of the CKiD study is the measurement of GFR by iohexol clearance, a method proved to measure kidney function precisely.12
In this study, children who were receiving ACEIs or ARBs were less likely to have LVH than children who were receiving other classes of antihypertensive medication. Both ACEIs and ARBs are known to decrease myocardial fibrosis in hypertensive heart disease.13,14 Interestingly, this effect could occur independent of any regression of LVH.13,14 The likely cause of the significant association between LVH and ACEIs/ARBs use is better BP control in the children on ACEIs or ARBs in our cohort, as recently demonstrated by Flynn et al.15 Once adjustment for hypertensive status was made in the multivariate model, no independent effect of ACEIs/ARBs on LVH was evident.
The key finding in our study is a strong association between BP parameters and LVH in CKiD participants. These results are in contrast to the ESCAPE trial,4 in which no significant relationships between casual or ambulatory BP and LVH were found. In our study, children with casual, wake, sleep, and 24-h ambulatory hypertension had LVH more frequently than children without casual or ambulatory hypertension. More important, the likelihood of having LVH was four times higher in children who were identified as having masked hypertension compared with children with normal clinic and ambulatory BP.
The association of masked hypertension with LVH was first described almost a decade ago by Liu et al.16 and confirmed in an Italian, population-based study (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA]).17 In both studies, adults with masked hypertension had a higher LVM than individuals with true normotension and LVMI values similar to those of individuals with true hypertension. A similar phenomenon was reported in a pediatric population18; however, the frequency of masked hypertension in our study (38%) was four to five times higher that what has been reported in unselected pediatric samples or pediatric samples referred to hypertension clinics (7 to 11%).18–22 A recent meta-analysis of masked hypertension in adult patients with CKD found the prevalence ranged from 4.7 to 31.3% in individual studies,23 with the overall prevalence of masked hypertension at approximately 8%. The authors explained the heterogeneity in prevalence rates by different cutoffs used to define normal and abnormal BP. In this study, we defined ambulatory hypertension on the basis of recent consensus recommendations from the American Heart Association (AHA) Atherosclerosis, Hypertension, and Obesity in Youth Committee.24 Its proposed BP classification scheme allows the capture of more patients with elevated BP, including patients with a BP load as low as 25%. Whereas the authors of the recommendations pointed out that this BP classification had never been validated in outcome studies, the data presented here have filled that void. Our study, using LVH as an intermediate cardiovascular outcome, suggests that the current ambulatory BP classification scheme might better identify children who have CKD and are at risk for end-organ damage than casual BP alone. The significance of our findings can be also underscored by the fact that nearly one third of the CKiD children with masked hypertension were not receiving antihypertensive medications. Equally important is that masked hypertension was found in treated children who had hypertension and in whom BP was thought to be well controlled on the basis of office measurements. These data add further strength to our previous finding that hypertension in pediatric CKD may be frequently under- or even untreated.15
The CKiD study has important strengths, including a large sample size; precise measurement of GFR by iohexol clearance11; and standardized demographic, clinical, laboratory, ABPM, and echocardiographic measures. Conversely, this report is limited by its cross-sectional analysis and the lack of information on the duration of hypertension, so we are unable to evaluate whether a cause-and-effect relationship exists between BP and LVH. This shortcoming will be overcome as the participants in the CKiD Study continue longitudinal follow-up with repeated measurements of iohexol GFR, ABPM, and echocardiography. Given that masked hypertension has been linked to a greater risk for ESRD in adults,25 long-term follow-up will also allow us to evaluate the association of masked hypertension with progression of CKD in our cohort.
There is no consensus on which children with CKD should have an echocardiographic evaluation and/or ABPM or on when these studies should be obtained. The current Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients1 recommend that “children commencing dialysis should be evaluated for the presence of cardiac disease (cardiomyopathy and valvular disease) using echocardiography once the patient has achieved dry weight (ideally within 3 mo of the initiation of dialysis therapy).” Unfortunately, these guidelines do not provide recommendations for echocardiographic evaluation of children before dialysis. The fourth report on BP control in children26 recommends echocardiographic evaluation for the presence of LVH in children with comorbid conditions (diabetes and kidney disease) and casual BP between the 90th and 95th percentiles and in all children with casual BP >95th percentile. The recently published AHA statement on standard assessment of ABPM in children and adolescents recommends the use of ABPM in pediatric renal transplant recipients only; it does not discuss the use of ABPM or echocardiography in children before or during maintenance dialysis.24 KDOQI, the AHA statement on ABPM, and the fourth report on BP in children do not recommend the use of ABPM to identify children who are at risk for LVH.
The high prevalence of masked hypertension and associated LVH in our study supports the case for the early echocardiographic evaluation and ABPM as a part of standard care to screen for the discussed cardiovascular risks in children with mild to moderate CKD. The study also suggests that the oscillometric technique of BP assessment outside the health care environment is beneficial in the detection of cardiac end-organ damage in children with CKD. The use of home BP monitoring could be considered as an intermediate step and a lower cost alternative to ABPM.27,28 Results of this study also raise the question of whether the same strategy should be applied to other pediatric populations at high risk for cardiovascular disease (e.g., obesity, diabetes).
Concise Methods
Study Population and Design
The CKiD study is an observational cohort study of CKD in children being conducted at 46 pediatric nephrology centers (Supplemental Table 1) in North America in response to National Institutes of Health RFA DK-03-012 entitled “Prospective Study of Chronic Kidney Disease in Children.”6,29 The CKiD study protocol has been reviewed and approved by the institutional review boards of each participating center. Eligibility criteria for enrollment in CKiD include age 1 to 16 yr and estimated GFR as calculated by the Schwartz formula11 of 30 to 90 ml/min per 1.73 m2.
This study is a cross-sectional analysis of baseline echocardiographic and ABPM data (year 2 of the CKiD study) from the first 389 children who had echocardiography as of January 2009. A total of 366 participants had a good-quality echocardiographic study with LVMI calculated, and 226 had an ABPM evaluation of good quality. Combined, echocardiographic, casual BP, and ABPM data were available for analysis on 198 children; those who had a >30-d interval between casual and ambulatory BP measurements were excluded from the analysis.
Casual BP Measurements
CKiD participants have casual BP measurements obtained from the right arm at study entry (baseline), then at annual intervals after enrollment. Casual BP in CKiD is obtained by auscultation using an aneroid sphygmomanometer (Mabis MedicKit 5; Mabis Healthcare, Waukegan, IL); all participating sites have been provided the same equipment by the CKiD Clinical Coordinating Centers. The Clinical Coordinating Centers also provide standardized training and certification in the auscultatory BP measurement protocol described next to all study personnel responsible for casual BP measurement. Recertification in auscultatory BP measurement technique and central equipment calibration take place at annual intervals. The specific procedure for BP determination in the CKiD study has been described elsewhere.15
The average of three BP measurements is recorded as the participants' casual BP for the study visit. The participants' BP obtained on the day of echocardiographic evaluation is included in this study. Participants' casual BP in CKiD are classified according to the National High Blood Pressure Education Program (NHBPEP) Fourth Report on the Diagnosis, Evaluation, and Treatment of High BP in Children and Adolescents26: Normotensive (<90th percentile), prehypertensive (≥90th and <95th percentiles), and hypertensive (≥95th percentile).
Ambulatory BP Monitoring
Ambulatory BP is measured by protocol using a SpaceLabs 90217 oscillometric device (SpaceLabs Healthcare, Issaquah, WA). Monitors are programmed centrally at the ABPM center located at the University of Texas at Houston and then shipped to the clinical sites, where they are placed on the individual's nondominant arm. Arm circumference is measured at the local sites, and a cuff is selected according to the fourth report recommendations.26 All participating clinical sites receive annual standardized training in monitor placement from the ABPM center.
The monitoring is performed for 24 h, and then the monitor is sent back to the ABPM center for downloading and entry into the database. For each 24-h recording, measurements are obtained every 20 min through the day and night at a bleed step of 8 mmHg. A diary is kept during the monitoring to record the time of sleep, time of waking, time of any napping, and time of medication administration, particularly antihypertensive medication. ABPM studies are considered successful only when at least one successful BP measurement is obtained during each hour of monitoring and the overall success rate is >75%. At least one repeat monitoring was attempted for participants whose first study was unsuccessful.
The means of systolic (SBP) and DBP are determined for 24 h and wake and sleep periods. BP load is defined as the percentage of BP readings that exceeded the ambulatory BP 95th percentiles.30 Ambulatory hypertension is defined as (1) mean wake and/or sleep SBP or DBP ≥95th percentile for ambulatory BP and/or (2) SBP or DBP load ≥25%. Normative data for ABPM and the definition of ambulatory hypertension were based on AHA recommendations on ambulatory BP monitoring for children and adolescents.24
On the basis of the combination of casual and ambulatory BP, hypertensive status was classified as (1) confirmed hypertension: Presence of both casual and ambulatory hypertension; (2) white-coat hypertension: Casual hypertension in the presence of normal ambulatory BP (mean BP <95th percentile and BP load <25%); (3) masked hypertension: Ambulatory hypertension in the presence of normal casual BP; or (4) normotension: Normal casual BP (<95th percentile) in the presence of normal ambulatory BP (mean BP <95th percentile and BP load <25%).24 Hypertensive status was defined regardless of whether the participant was or was not receiving treatment for high BP or was on antihypertensive medications for other reasons (e.g., proteinuria). Dipping status was defined as a percentage drop in the mean BP from wake to sleep periods. Abnormal dipping was defined as a decline of <10%.
Echocardiography
M-mode and Doppler echocardiography are performed at individual participating centers, but reading and analyses of echocardiographic data are performed by the Cardiovascular Core Imaging Research Laboratory at Cincinnati Children's Hospital Medical Center. For achievement of standardization and uniformity of echocardiographic images across the centers, qualifying recordings were sent to each center and then certified at the Cardiovascular Core Imaging Research Laboratory.
LVM was measured by two-dimensional directed M-mode echocardiography at rest according to the American Society of Echocardiography criteria.31 This method applies measurements of LV end-diastolic cavity (LVED) and septal (IVS) and posterior wall (PW) thicknesses and accurately predicts LVM through the following equation: 0.8{1.04[(LVED + PW + IVS)3 − LVED3]} + 0.6. LVM was indexed (mass divided by height raised to a power of 2.7 [g/m2.7]) to account for body size.32 LVH was defined as LVMI ≥the 95th percentile for normal children and adolescents.8 RWT was calculated [RWT = (PW + IVS)/LVED] to assess the LV geometry.
Patients with increased LVMI (≥95th percentile) and elevated RWT (≥0.41) had concentric LVH; those with increased LVMI and normal RWT (<0.41) had eccentric LVH. Concentric remodeling was defined by the presence of an elevated RWT but with a normal LVMI.
Other Variables
Demographic and medical history information was collected as per protocol. Weight, height, GFR, and blood and urine collection were obtained on the day of the echocardiographic evaluation. GFR was determined by plasma iohexol disappearance curves with four time points at 10, 30, 120, and 300 min after infusion of 5 ml of iohexol; details of the GFR assessment methods have been previously published.12 Collected blood and urine samples were analyzed at the central biochemistry laboratory (University of Rochester, Rochester, NY).
Statistical Analysis
Continuous variables in this report are described as medians and interquartile ranges; categorical variables are described as frequencies and percentages. Children with LVH were compared with those with normal LVMI in a univariate analysis. Adjusted odds ratios for LVH were then calculated using a logistic model. Only variables with P < 0.1 were included in the multivariable analysis. Results were summarized as odd ratios and 95% confidence intervals. All analyses were performed using SAS 9.1 statistical software (SAS Institute, Cary, NC).
Disclosures
None.
Acknowledgments
Data in this article were collected by the Chronic Kidney Disease in Children (CKiD) prospective cohort study with clinical coordinating centers (principal investigators) at Children's Mercy Hospital and the University of Missouri–Kansas City (Bradley Warady) and Johns Hopkins School of Medicine (Susan Furth) and the data coordinating center (principal investigator) at the Johns Hopkins Bloomberg School of Public Health (Alvaro Munoz). The CKiD is funded by the National Institute of Diabetes and Digestive and Kidney Diseases with additional funding from the National Institute of Neurological Disorders and Stroke; the National Institute of Child Health and Human Development; and the National Heart, Lung, and Blood Institute (UO1-DK-66143, UO1-DK-66174, and UO1-DK-66116). The CKiD website is located at http://www.statepi.jhsph.edu/ckid.
We thank Chrissy Schulte and Vicky Moore, sonographers at Cardiovascular Core Imaging Research Laboratory at Cincinnati Children's Hospital Medical Center, and Tim Poffenbarger, data analyst at the ABPM center at the University of Texas, Houston.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45[Suppl 3]: S1–S153, 2005 [PubMed] [Google Scholar]
- 2.Johnstone LM, Jones CL, Grigg LE, Wilkinson JL, Walker RG, Powell HR: Left ventricular abnormalities in children, adolescents and young adults with renal disease. Kidney Int 50: 998–1006, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Mitsnefes MM, Kimball TR, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Left ventricular mass and systolic performance in pediatric patients with chronic renal failure. Circulation 107: 864–868, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Matteucci MC, Wuhl E, Picca S, Mastrostefano A, Rinelli G, Romano C, Rizzoni G, Mehls O, de Simone G, Schaefer FESCAPE Trial Group: Left ventricular geometry in children with mild to moderate chronic renal insufficiency. J Am Soc Nephrol 17: 218–226, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Progression of left ventricular hypertrophy in children with early chronic kidney disease: 2-Year follow-up study. J Pediatr 149: 671–675, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Simone G, Daniels SR, Kimball TR, Roman MJ, Romano C, Chinali M, Galdeirsi M, Devereux RB: Evaluation of concentric left ventricular geometry in humans: Evidence for age-related systematic underestimation. Hypertension 45: 64–68, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Khoury PR, Mitsnefes MM, Daniels SR, Kimball TR: Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr 22: 709–714, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Foster BJ, Mackie AS, Mitsnefes MM, Ali H, Mamber S, Colan SD: A novel method of expressing left ventricular mass relative to body size in children. Circulation 117: 2769–2775, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, de Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Brion LP, Spitzer A: The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34: 571–590, 1987 [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GJ, Furth S, Cole SR, Warady B, Muñoz A: Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Brilla CG, Reinhard C: Funck RC, Rupp H: Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 102: 1388–1393, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Díez J, Querejeta R, López B, González A, Larman M, Martínez Ubago JL: Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 105: 2512–2517, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BAChronic Kidney Disease in Children Study Group: Blood pressure in children with chronic kidney disease: A report from the Chronic Kidney Disease in Children study. Hypertension 52: 631–637, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu JE, Roman MJ, Pini R, Schwartz JE, Pickering TG, Devereux RB: Cardiac and arterial target organ damage in adults with elevated ambulatory and normal office blood pressure. Ann Intern Med 131: 564–572, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Sega R, Trocino G, Lanzarotti A, Carugo S, Cesana G, Schiavina R, Valagussa F, Bombelli M, Giannattasio C, Zanchetti A, Mancia G: Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study). Circulation 104: 1385–1392, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Lurbe E, Torro I, Alvarez V, Nawrot T, Paya R, Redon J, Staessen JA: Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension 45: 493–498, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka S, Awazu M: Masked hypertension in children and young adults. Pediatr Nephrol 19: 651–654, 2004 [DOI] [PubMed] [Google Scholar]
- 20.McNiece KL, Gupta-Malhotra M, Samuels J, Bell C, Garcia K, Poffenbarger T, Sorof JM, Portman RJNational High Blood Pressure Education Program Working Group: Left ventricular hypertrophy in hypertensive adolescents: Analysis of risk by 2004 National High Blood Pressure Education Program Working Group staging criteria. Hypertension 50: 392–395, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stabouli S, Kotsis V, Toumanidis S, Papamichael C, Constantopoulos A, Zakopoulos N: White-coat and masked hypertension in children: Association with target-organ damage. Pediatr Nephrol 20: 1151–1155, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Verberk WJ, Kessels AG, de Leeuw PW: Prevalence, causes, and consequences of masked hypertension: A meta-analysis. Am J Hypertens 21: 969–975, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Bangash F, Agarwal R: Masked hypertension and white-coat hypertension in chronic kidney disease: A meta-analysis. Clin J Am Soc Nephrol 4: 656–664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels SAmerican Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee: Ambulatory blood pressure monitoring in children and adolescents: Recommendations for standard assessment–A scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young and the Council for High Blood Pressure Research. Hypertension 52: 433–451, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Agarwal R, Andersen MJ: Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int 69: 406–411, 2006 [DOI] [PubMed] [Google Scholar]
- 26.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents: The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114: 555–576, 2004 [PubMed] [Google Scholar]
- 27.Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff DAmerican Heart Association, American Society of Hypertension; Preventive Cardiovascular Nurses Association: Call to action on use and reimbursement for home blood pressure monitoring: Executive summary—A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension 52: 1–9, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Andersen MJ, Khawandi W, Agarwal R: Home blood pressure monitoring in CKD. Am J Kidney Dis 45: 994–1001, 2005 [DOI] [PubMed] [Google Scholar]
- 29.National Institute of Diabetes and Digestive and Kidney Diseases: RFA DK-03-012. Prospective Study of Chronic Kidney Disease in Children, November 26, 2002. Available at: http://grants.nih.gov/grants/guide/rfa-files/RFA-DK-03-012.html Accessed January 15, 2009
- 30.Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, Krull F, Reichert H, Reusz GS, Rascher W: Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: A multicenter trial including 1141 subjects. J Pediatr 130: 178–184, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Devereux RB, Reichek N: Echocardiographic determination of left ventricular mass in man: Anatomic validation of the method. Circulation 55: 613–618, 1977 [DOI] [PubMed] [Google Scholar]
- 32.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH: Left ventricular mass and body size in normotensive children and adults: Assessment of allometric relations and impact of overweight. J Am Coll Cardiol 20: 1251–1260, 1992 [DOI] [PubMed] [Google Scholar]