Abstract
Modest induction of endoplasmic reticulum (ER) stress confers resistance to inflammation in glomeruli. Recently, we found that ER stress leads to mesangial insensitivity to cytokine-induced activation of NF-κB, but the underlying mechanisms are incompletely understood. ER stress can trigger expression of CCAAT/enhancer-binding proteins (C/EBPs), which interact with transcription factors including NF-κB. Here, we investigated a role for C/EBPs in the ER stress–induced resistance to cytokines. Mesangial cells preferentially induced C/EBPβ after exposure to thapsigargin or tunicamycin; induction of C/EBPδ was modest and transient, and expression of C/EBPα was absent. The induction of C/EBPβ correlated with accumulation of C/EBPβ protein and enhanced transcriptional activity of C/EBP. Overexpression of C/EBPβ markedly suppressed TNF-α–induced activation of NF-κB, independent of its transacting potential. Knockdown of C/EBPβ by small interfering RNA reversed the suppressive effect of ER stress on NF-κB. In vivo, preconditioning of mice with ER stress induced renal C/EBPβ and suppressed NF-κB–dependent gene expression in response to LPS. Using dominant negative mutants and null mutants for individual branches of the unfolded protein response, we identified the RNA-dependent protein kinase–like ER kinase (PERK) and the inositol-requiring ER-to-nucleus signal kinase 1 (IRE1) pathways as the unfolded protein response responsible for ER stress–induced C/EBPβ. These results suggest that ER stress blunts cytokine-triggered activation of NF-κB, in part through PERK- and IRE1-mediated preferential induction of C/EBPβ.
Endoplasmic reticulum (ER) stress is defined as accumulation of unfolded or misfolded proteins in the ER, which triggers an adaptive program called the unfolded protein response (UPR).1,2 Three major transmembrane transducers for sensing ER stress have been identified in the ER. Those are RNA-dependent protein kinase–like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring ER-to-nucleus signal kinase 1 (IRE1). Activation of PERK leads to phosphorylation of eukaryotic translation initiation factor 2α (eIF2α), which causes general inhibition of protein synthesis and induction of a transcription factor ATF4 that binds to the amino acid response element. In response to ER stress, p90ATF6 transits to the Golgi, where it is cleaved by the proteases S1P and S2P, yielding a free cytoplasmic domain p50ATF6, which functions as an active transcription factor. Similarly, activated IRE1 catalyzes removal of a small intron from X-box–binding protein 1 (XBP1) mRNA. This splicing event creates a translational frameshift in XBP1 mRNA to produce an active transcription factor. p50ATF6 and XBP1 subsequently bind to the ER stress response element (ERSE) and the UPR element (UPRE), leading to expression of target genes including ER chaperones and genes involved in ER stress–associated degradation. Activation of IRE1 also allows for interaction of IRE1 with TNF receptor–associated factor 2 (TRAF2), leading to recruitment and activation of apoptosis signal–regulating kinase 1 (ASK1) and downstream c-Jun N-terminal kinase (JNK).1,2
ER stress is observed under various pathologic situations, including inflammation.3 Previous studies suggested that ER stress has the potential to activate NF-κB and thereby contributes to the development of inflammation, as reviewed by Zhang and Kaufman4; however, in the kidney, previous reports showed that UPR was induced in glomeruli of rats with passive Heymann nephritis and that ER stress preconditioning attenuated proteinuria in this experimental model.5,6 Inagi et al.7 also reported that, in the anti-Thy1 model of mesangioproliferative glomerulonephritis in rats, UPR was induced in nephritic glomeruli, especially in podocytes and mesangial cells. They also showed that preconditioning with subnephritogenic doses of ER stress inducers ameliorated the pathologic manifestations of the disease; however, mechanisms underlying the anti-inflammatory potential of ER stress and UPR have not been elucidated.
Recently, we reported that preceding ER stress causes insensitivity of mesangial cells to cytokine-induced activation of NF-κB via upregulation of A20 and downregulation of TRAF28; however, our data indicated that the roles of A20 and TRAF2 are partial and that other major targets of ER stress may also be present. To uncover additional mechanisms involved, we focused this investigation on a role of CCAAT/enhancer-binding protein (C/EBP) in the ER stress–induced cytokine insensitivity in mesangial cells. C/EBP is a family of transcription factors that are required for development, physiologic function, and responses to injury in various tissues.9 A previous report showed that some ER stress inducers triggered expression and production of C/EBP in human hepatoma cells.10 It is also known that C/EBP family members can interact with various transcription factors, including NF-κB,11 which may modulate function of NF-κB. We therefore investigated a role of C/EBP in the ER stress–induced suppression of NF-κB in mesangial cells. In this report, we demonstrate that ER stress depresses cytokine-induced activation of NF-κB through preferential induction of C/EBPβ. We also elucidate that particular UPRs, especially the PERK- and IRE1-initiated pathways, are involved in this process.
Results
Preferential Induction of C/EBPβ by ER Stress
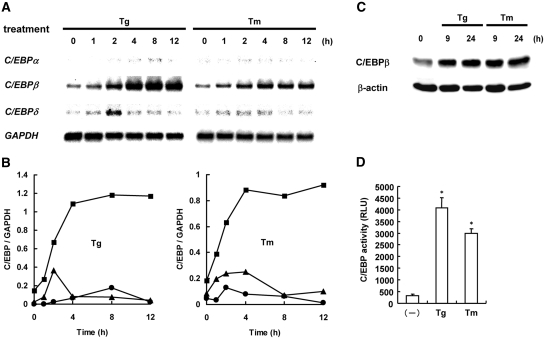
To examine whether ER stress has the potential to induce the C/EBP family members, we treated mesangial cells with thapsigargin or tunicamycin for up to 12 h and examined expression of C/EBPα, C/EBPβ, and C/EBPδ by Northern blot analysis (Figure 1, A and B). After stimulation with the ER stress inducers, we observed induction of C/EBP-homologous protein (CHOP) and 78-kD glucose-regulated protein (GRP78), indicators of ER stress, within a few hours (Supplemental Figure S1).8 In parallel with the induction of ER stress, C/EBPβ was also induced within a few hours, and the expression level increased in a time-dependent manner. In contrast, induction of C/EBPδ was only modest and transient with a peak at 2 h. Substantial induction of C/EBPα was not observed by the treatment with ER stress inducers. Of note, the preferential induction of C/EBPβ by ER stress is not specific to mesangial cells, because the similar phenomenon was observed in NRK-52E tubular epithelial cells (data not shown). To investigate whether ER stress increases C/EBPβ at a protein level, we performed Western blot analysis. As shown in Figure 1C, treatment with either thapsigargin or tunicamycin increased C/EBPβ protein at both 9 and 24 h. To examine further whether the induced C/EBPs, especially C/EBPβ, are functional, we transiently transfected mesangial cells with pC/EBP-Luc and treated them with ER stress inducers for 8 h. Consistent with the results of Northern and Western blot analyses, the reporter assay showed that ER stress inducers significantly increased the transacting potential of C/EBP (Figure 1D). The magnitude of induction was correlated with the induction level of C/EBPβ mRNA (Figure 1A). These results suggest that ER stress predominantly induces functional C/EBPβ.
Figure 1.
Preferential induction of C/EBPβ by ER stress is shown. (A) Mesangial cells were treated with thapsigargin (Tg; 100 nM) or tunicamycin (Tm; 10 μg/ml) for up to 12 h, and expression of C/EBPα, C/EBPβ, and C/EBPδ was examined by Northern blot analysis. Expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is shown at the bottom as a loading control. (B) Densitometric analysis of A. The levels of C/EBPs were normalized by the levels of GAPDH. ●, C/EBPα; ■, C/EBPβ; ▴, C/EBPδ. (C) Mesangial cells were treated with Tg or Tm for 9 or 24 h and subjected to Western blot analysis of C/EBPβ. The level of β-actin is shown at the bottom as a loading control. (D) Mesangial cells were transiently transfected with pC/EBP-Luc, stimulated with Tg or Tm for 8 h, and subjected to luciferase assay. Assays were performed in quadruplicate, and data are means ± SEM. *Statistically significant differences versus untreated controls (P < 0.05). RLU, relative light unit.
Suppression of Cytokine-Triggered NF-κB Activation by C/EBP
To examine whether C/EBP has the potential to suppress cytokine-triggered activation of NF-κB, we transiently transfected mesangial cells with pNFκB-Luc together with C/EBPβ or C/EBPδ and stimulated them with TNF-α. Reporter assays showed that overexpression of either C/EBPβ or C/EBPδ significantly inhibited TNF-α–induced NF-κB activation (Figure 2A). Consistent with this result, overexpression of C/EBPβ or C/EBPδ also significantly suppressed IL-1β–induced activation of NF-κB (Figure 2B). Of note, transfection with C/EBPα also blocked activation of NF-κB by TNF-α and IL-1β (Supplemental Figure S2), suggesting that all of C/EBPα, C/EBPβ, and C/EBPδ have the similar inhibitory potential.
Figure 2.
Suppression of cytokine-triggered NF-κB activation by C/EBP is shown. (A and B) Mesangial cells were transiently transfected with pNFκB-Luc together with empty vector, pCMV-C/EBPβ, or pCMV-C/EBPδ; treated with TNF-α (10 ng/ml; A) or IL-1β (1 ng/ml; B) for 8 h; and subjected to luciferase assay. (C and D) Cells were transfected with pNFκB-Luc together with empty vector, pCMV-C/EBPβ, or pCMV-A-C/EBP (C/EBP-DN); treated with TNF-α (C) or IL-1β (D) for 8 h; and subjected to luciferase assay. Assays were performed in quadruplicate, and data are means ± SEM. *Statistically significant differences versus vector controls (P < 0.05).
C/EBP family members share substantial sequence identity in the basic region and the leucine zipper domain, form heterodimers, and bind to the C/EBP recognition sequence in the promoters of target genes.12 We tested whether the inhibition of NF-κB by C/EBP is ascribed to its transacting potential. We transfected mesangial cells with pNFκB-Luc together with C/EBPβ or C/EBP-DN encoding a dominant negative mutant of C/EBP lacking the transacting potential. The results showed that, like C/EBPβ, C/EBP-DN suppressed activation of NF-κB by TNF-α (Figure 2C) or IL-1β (Figure 2D). These results indicate that the inhibitory effect of C/EBPs on NF-κB is independent of their binding to the C/EBP recognition sequence.
Involvement of C/EBPβ in the Suppression of NF-κB by ER Stress
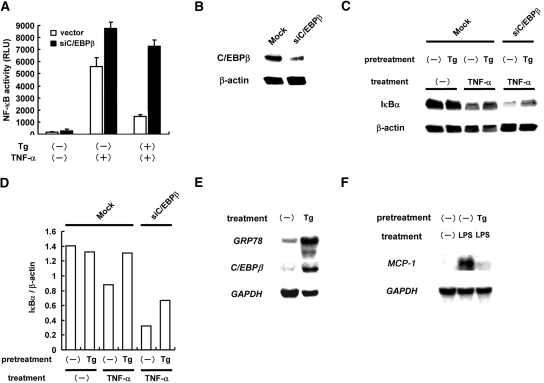
To examine further the involvement of C/EBPβ in the suppression of NF-κB by ER stress, we performed loss-of-function studies. For this purpose, pRNA-U6.1-siC/EBPβ was constructed to introduce small interfering RNA (siRNA) targeting rat C/EBPβ. We transiently co-transfected mesangial cells with pNFκB-Luc and pRNA-U6.1-Neo or pRNA-U6.1-siC/EBPβ. After the transfection, we treated the cells with or without thapsigargin for 6 h, exposed them to TNF-α for 18 h, and subjected them to luciferase assay to evaluate NF-κB activity. As shown in Figure 3A, knockdown of endogenous C/EBPβ significantly attenuated the suppressive effect of ER stress on NF-κB. Of note, TNF-α–induced activation of NF-κB was enhanced by overexpression of siC/EBPβ. The similar result was also obtained in the cells treated with IL-1β (data not shown).
Figure 3.
Involvement of C/EBPβ in the suppression of NF-κB by ER stress is shown. (A) Mesangial cells were transiently co-transfected with pNFκB-Luc and pRNA-U6.1-Neo (vector) or pRNA-U6.1-siC/EBPβ (siC/EBPβ). After the transfection, the cells were pretreated with or without Tg for 6 h, exposed to TNF-α for 18 h, and subjected to luciferase assay. Assays were performed in quadruplicate, and data are means ± SEM. (B) Mesangial cells were stably transfected with pRNA-U6.1-Neo or pRNA-U6.1-siC/EBPβ, and SM/U6.1-Neo cells (Mock) and SM/siC/EBPβ cells (siC/EBPβ) were established. The level of basal C/EBPβ protein was examined by Western blot analysis. (C) SM/U6.1-Neo cells and SM/siC/EBPβ cells were pretreated with Tg, treated with TNF-α for 30 min, and subjected to Western blot analysis of IκBα. (D) Densitometric analysis of C. The levels of IκBα were normalized by the levels of β-actin. (E) Mice were administered Tg (1 mg/kg) intraperitoneally, and after 16 h, kidneys were subjected to Northern blot analysis of GRP78 and C/EBPβ. (F) Mice were administered an intraperitoneal injection of LPS (200 μg/mouse) at 16 h after the Tg injection, and after 12 h, kidneys were subjected to Northern blot analysis of MCP-1.
To confirm these results, we established mesangial cells that stably express C/EBPβ siRNA. Overexpression of siC/EBPβ partially depressed the level of endogenous C/EBPβ in the established SM/siCEBPβ cells (Figure 3B). Using the established transfectants, we evaluated cytokine-induced degradation of IκBα, another indicator of NF-κB activation. Western blot analysis revealed that, in mock-transfected cells, degradation of IκBα was induced by the treatment with TNF-α. This degradation was reversed by the pretreatment with thapsigargin. In SM/siCEBPβ cells, however, degradation of IκBα by TNF-α was enhanced, and blockade of IκBα degradation by thapsigargin was reversed partially (Figure 3, C and D). These results suggest involvement of C/EBPβ in the suppression of cytokine-triggered NF-κB activation by ER stress.
To confirm further our conclusion in vivo, we preconditioned mice with ER stress by intraperitoneal injection of thapsigargin. After 16 h, kidneys were removed and subjected to Northern blot analysis. Consistent with the results in cultured mesangial cells, administration with thapsigargin upregulated expression of C/EBPβ, as well as expression of GRP78 (Figure 3E). Under this experimental setting, we examined induction of monocyte chemoattractant protein 1 (MCP-1), a NF-κB–dependent chemokine, by LPS. As shown in Figure 3F, expression of MCP-1 was induced in the kidney by LPS, and it was markedly suppressed by ER stress preconditioning. This result showed that expression of C/EBPβ is induced by ER stress in vivo, which may be causative of ER stress–mediated suppression of NF-κB.
Induction of C/EBPβ by the PERK and IRE1 Branches of UPR
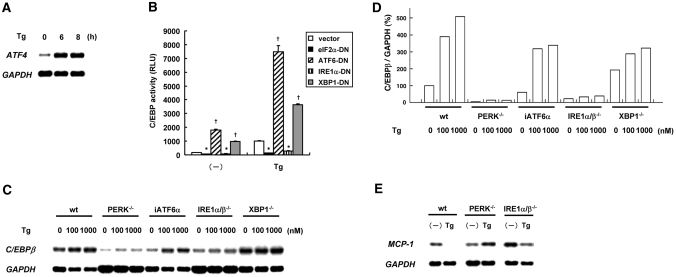
ER stress generally triggers three major branches of UPR that cause downstream transcriptional events. Those are the PERK-eIF2α, ATF6, and IRE1 pathways. We previously reported that treatment of SM43 mesangial cells with thapsigargin caused activation of the ATF6 and the IRE1 pathways.13 To confirm that the PERK-eIF2α pathway is also activated by ER stress, we treated mesangial cells with thapsigargin and tested induction of ATF4, the indicator of UPR downstream of PERK and eIF2α. Northern blot analysis revealed that ATF4 was substantially induced by the treatment with thapsigargin (Figure 4A).
Figure 4.
Induction of C/EBPβ by the PERK and IRE1 branches of UPR is shown. (A) Mesangial cells were treated with Tg for up to 8 h, and induction of ATF4 was examined by Northern blot analysis. (B) Mesangial cells were transiently transfected with pC/EBP-Luc together with empty vector, pcDNA3-heIF2αS51A (eIF2α-DN), pcDNA3.1-ATF6α(171 to 373)ΔAD (ATF6-DN), pCAG-hIRE1αK699A (IRE1α-DN), or pcDNA3.1-dnXBP1 (XBP1-DN); pretreated with Tg for 8 h; and subjected to luciferase assay. *Significant suppression, †significant upregulation versus vector controls. (C) Wild-type MEF (wt), PERK−/− MEF, siATF6α-expressing MEF (iATF6α), IRE1α/β−/− MEF, and XBP1−/− MEF were treated with Tg (100 or 1000 nM) for 6 h, and Northern blot analysis of C/EBPβ was performed. (D) Densitometric analysis of C. The levels of C/EBPβ were normalized by the levels of GAPDH, and relative percentages are shown. (E) Wild-type MEF, PERK−/− MEF, and IRE1α/β−/− MEF were treated with 100 nM Tg for 24 h and subjected to Northern blot analysis of MCP-1.
To identify UPR branches responsible for the ER stress–triggered induction of C/EBPβ, we performed experiments using dominant negative mutants of eIF2α, ATF6, IRE1α, and XBP1. We transiently co-transfected mesangial cells with pC/EBP-Luc together with eIF2α-DN, ATF6-DN, IRE1α-DN, or XBP1-DN; treated them with thapsigargin; and subjected them to luciferase assay. As shown in Figure 4B, treatment with thapsigargin significantly induced activation of C/EBP in mock-transfected cells. This activation was suppressed by transfection with eIF2α-DN or IRE1α-DN. In contrast, transfection with ATF6-DN or XBP1-DN increased basal activity of C/EBP significantly and rather enhanced ER stress–induced C/EBP activation. These results indicate a possibility that the PERK and IRE1 pathways are involved in the induction of C/EBPβ by ER stress.
To confirm this possibility, we used PERK−/− mouse embryonic fibroblasts (MEF), IRE1α/β−/− MEF, XBP1−/− MEF, and iATF6 MEF stably expressing ATF6 siRNA. We treated wild-type MEF and mutant MEF with thapsigargin for 6 h and subjected them to Northern blot analysis of C/EBPβ (Figure 4, C and D). In wild-type MEF, iATF6 MEF, and XBP1−/− MEF, we observed substantial induction of C/EBPβ after the treatment with thapsigargin. Consistent with the results in mesangial cells, however, MEF deficient for PERK or IRE1 exhibited blunted induction of C/EBPβ in response to thapsigargin. Furthermore, treatment with thapsigargin caused downregulation of MCP-1, an indicator of NF-κB activity, in wild-type MEF, whereas the suppression was blunted in both PERK−/− MEF and IRE1α/β−/− MEF (Figure 4E). These results further suggest that the PERK and IRE1 pathways are involved and responsible for the induction of C/EBPβ by ER stress.
In general, the IRE1 pathway transduces signals via XBP1 or TRAF2. Our finding that IRE1 but not XBP1 was involved in the induction of C/EBPβ indicates a possibility that IRE1 induces C/EBPβ independent of the IRE1-XBP1 pathway. To confirm this result, we used mesangial cells stably expressing a dominant negative mutant of XBP1.13 We treated mock-transfected SM/Neo cells and SM/XBP1-DN cells with thapsigargin and subjected them to Northern blot analysis. The result showed that dominant negative inhibition of XBP1 did not attenuate ER stress–triggered induction of C/EBPβ (data not shown), confirming the lack of involvement of the XBP1 pathway.
Discussion
Preconditioning by ER stress confers resistance on glomerular cells to inflammatory stimuli in vitro and in vivo.5–8 In this investigation, we describe a novel role of C/EBPβ in the ER stress–induced insensitivity of glomerular cells to cytokine-triggered activation of NF-κB. We found that C/EBPβ was preferentially induced in mesangial cells in response to ER stress. The predominant induction of C/EBPβ was not specific to the particular cell type but was also observed in other cells. Overexpression of C/EBPβ markedly suppressed TNF-α–induced activation of NF-κB independent of its transacting potential, and knockdown of C/EBPβ reversed the suppressive effect of ER stress. We also disclosed that the PERK and the IRE1 pathways were the UPRs responsible for the induction of C/EBPβ by ER stress. Our results suggest that ER stress blunts cytokine-triggered activation of NF-κB, in part through UPR-mediated preferential induction of C/EBPβ.
It is widely known that ER stress induces expression of CHOP, also called C/EBPζ, via activation of ERSE and the amino acid response element.14 In contrast, information is limited regarding regulation of other C/EBP family members by ER stress. In this report, we demonstrated that, among other members of the C/EBP family, C/EBPβ was predominantly induced by ER stress. Molecular mechanisms underlying this event are not well understood, but a previous report suggested that a UPRE was located at the 3′ end of the human C/EBPβ gene and that it could be responsible for induction of C/EBPβ by ER stress10; however, it is not the case in the regulation of rat and mouse cells. We found that dominant negative inhibition of XBP1 did not attenuate but rather enhanced activation of C/EBP by ER stress in rat mesangial cells. Consistent with this result, XBP1-null mutant MEF exhibited a higher level of basal C/EBPβ. Because UPRE is activated by XBP1 under ER stress conditions, our results exclude a possibility that C/EBPβ is upregulated by ER stress via activation of UPRE.
ER stress has the potential to activate NF-κB and mitogen-activated protein kinases (MAPKs) including JNK.15,16 A previous report indicated that expression of some C/EBP family member may be regulated by MAPKs and NF-κB.17 To examine possible involvement of MAPKs or NF-κB in the induction of C/EBPβ by ER stress, we pretreated mesangial cells with selective inhibitors of extracellular signal–regulated kinase (PD098059), JNK (SP600125), p38 MAPK (SB203580), or NF-κB (SC-514); stimulated them by thapsigargin; and subjected them to Northern blot analysis. Our preliminary results showed that SP600125 but not other inhibitors attenuated induction of C/EBPβ by ER stress (Supplemental Figure S3), indicating possible involvement of JNK. Of note, ER stress causes interaction of IRE1 with TRAF2, which allows for recruitment and activation of ASK1 and downstream JNK.2 A previous report showed that SP600125 inhibited expression of C/EBPβ in LPS-stimulated microglia.18 ER stress possibly induces expression of C/EBPβ via activation of the IRE1-JNK pathway.
In this study, we elucidated that the PERK-eIF2α and the IRE1 pathways are responsible for the induction of C/EBPβ by ER stress. Conversely, our data showed that the ATF6 and the IRE1-XBP1 pathway are not involved in the induction, indicating that neither ERSE nor UPRE is involved in the induction of C/EBPβ. In general, the IRE1 pathway transduces signals via XBP1 or TRAF2. Our finding that IRE1 but not XBP1 is involved in the induction of C/EBPβ indicates that IRE1 induces C/EBPβ not via the IRE1-XBP1 pathway but by other IRE1-dependent signaling cascade such as the IRE1-TRAF2 pathway. Interestingly, previous reports suggested that ER stress triggered activation of NF-κB through the IRE1-TRAF2 pathway19,20 and/or the PERK-eIF2α pathway.21,22 Both pathways may be involved in the biphasic, bidirectional regulation of NF-κB by ER stress, as we recently reviewed.23 That is, although ER stress may induce proinflammatory molecules via transient activation of NF-κB in the early phase,15 consequent UPR may suppress NF-κB in the later phase. Indeed, as we reported recently, ER stress triggered transient activation of NF-κB with a peak at 6 to 12 h in renal tubular cells24; however, this activation was transient and subsided within 24 h. Thereafter, activity of NF-κB was further depressed below its initial, basal level.24 Furthermore, like mesangial cells, ER stress preconditioning for 24 h markedly suppressed activation of NF-κB by TNF-α in tubular cells (our unpublished data). Similarly, in podocytes, short-term preexposure to ER stress (1 h) enhanced induction of MCP-1 by TNF-α, whereas longer term preexposure (6 h) to ER stress attenuated TNF-α–triggered MCP-1 expression.25 ER stress is possibly involved not only in the initiation of renal inflammation but also in its resolution.26 That ER stress preconditioning attenuated LPS-induced inflammatory responses in the kidney (Figure 3, E and F) and other organs27 supports our current hypothesis.
In this investigation, we found that dominant negative inhibition of ATF6 or XBP1 significantly increased basal activity of C/EBP in mesangial cells. One possible explanation is that inhibition of ATF6 or XBP1 attenuated basal activity of ERSE and thereby reduced basal expression of downstream genes, especially ER chaperones, including GRP78. This molecular event may lead to ER stress and consequent induction of C/EBPs.
It is known that C/EBP family members interact with NF-κB subunits11 and may inhibit activation of NF-κB. A previous report indicated that the inhibitory effect of C/EBP on NF-κB may be mediated by protein–protein interactions distinct from the role of C/EBP in gene expression.28 For example, C/EBPα/β may interact with p65 NF-κB subunit and thereby suppress cytokine-induced NF-κB activation even in the absence of direct binding of p65-C/EBP to the κB site.29 Consistent with this report, we demonstrated that overexpression of C/EBPβ, as well as C/EBPα and C/EBPδ, markedly suppressed TNF-α–induced activation of NF-κB independent of its function as a transcription factor. It is because a C/EBP mutant lacking the transacting potential similarly inhibited activation of NF-κB. The C/EBP family members have the highly conserved, basic-leucine zipper domain that is required for protein–protein interactions.12 Interaction of C/EBP with NF-κB subunits, especially p65, may be a possible mechanism underlying the inhibition of NF-κB by C/EBPβ under ER stress conditions.
Taken together, we elucidated that, in addition to upregulation of A20 and downregulation of TRAF2,8 induction of C/EBPβ is another mechanism involved in the suppression of NF-κB by ER stress. A recent report showed that ER stress preconditioning ameliorated glomerular injury and proteinuria in mesangioproliferative glomerulonephritis.7 Our current data suggest that PERK- and IRE1-mediated preferential induction of C/EBPβ may explain the anti-inflammatory potential of ER stress via suppression of NF-κB in glomerulonephritis.
Concise Methods
Cells
The rat mesangial cell line SM43 was established as described previously.30 Wild-type MEF, XBP1−/− MEF, and iATF6 MEF that stably express ATF6α siRNA were provided by Dr. Laurie Glimcher (Harvard School of Public Health).31 PERK−/− MEF and IRE1α/β−/− MEF were provided by Dr. David Ron (New York University School of Medicine). Medium containing 1% FBS was generally used for studies.
Reagents
Thapsigargin and tunicamycin were purchased from Sigma-Aldrich Japan (Tokyo, Japan). Human recombinant TNF-α and human recombinant IL-1β were obtained from R&D Systems (Minneapolis, MN). PD098059 and SB203580 were purchased from Calbiochem (San Diego, CA), and SP600125 was obtained from Sigma-Aldrich Japan. SC-514 was purchased from BIOMOL Int. (Plymouth Meeting, PA).
Stable Transfectants
SM/siCEBPβ cells were established by stable transfection of SM43 cells with pRNA-U6.1-siC/EBPβ32 that introduces rat C/EBPβ siRNA. Effective knockdown of C/EBPβ was confirmed by Western blot analysis. Mock-transfected cells were also established by stable transfection with pRNA-U6.1-Neo (GenScript, Piscataway, NJ). SM/XBP1-DN cells were established by stable transfection of SM43 cells with pcDNA3.1-dnXBP1 (provided by Dr. Laurie H. Glimcher)33 encoding a dominant negative mutant of XBP1, as described previously.13
Transient Transfection
Using GeneJuice (Novagen, Madison, WI), SM43 cells were transiently co-transfected with pNFκB-Luc (Panomics, Fremont, CA) together with pcDNA3.1 (Invitrogen, Carlsbad, CA), pCMV-C/EBPα, pCMV-C/EBPβ, pCMV-C/EBPδ (provided by Dr. Ez-Zoubir Amri, CNRS, France),34 pCMV-A-C/EBP (encoding a dominant negative mutant of C/EBP, provided by Dr. Charles Vinson, National Institutes of Health),35 pRNA-U6.1-Neo, or pRNA-U6.1-siC/EBPβ32 at 1:4 ratio. After 12 to 24 h, cells were treated with or without thapsigargin for 6 h, exposed to cytokines for 8 to 24 h, and subjected to luciferase assay, as described later. SM43 cells were also transiently co-transfected with pC/EBP-Luc (provided by Dr. Yoshihiko Nishio, Shiga University of Medical Science, Japan)36 together with pCAG-hIRE1αK699A (provided by Dr. Masayuki Miura, University of Tokyo, Japan),37 pcDNA3.1-dnXBP1, pcDNA3.1-ATF6α(171 to 373)ΔAD (provided by Dr. Kazutoshi Mori, Kyoto University, Japan),38 or pcDNA3-heIF2αS51A (provided by Dr. David Ron) encoding a dominant negative mutant of IRE1α, XBP1, ATF6, or eIF2α, respectively (1:2 ratio). After 12 to 24 h, cells were treated with or without thapsigargin or tunicamycin for 8 h and subjected to luciferase assay. Assays were performed in quadruplicate.
Northern Blot Analysis
Total RNA was extracted by a single-step method, and Northern blot analysis was performed as described previously.39 cDNAs for C/EBPs (α, β and δ) (provided by Dr. Ez-Zoubir Amri),34 MCP-1,40 GRP78 (provided by Dr. Kazunori Imaizumi, University of Miyazaki, Miyazaki, Japan),36 CHOP,41 and ATF4 (provided by Dr. David Ron) were used to prepare radiolabeled probes. Expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Densitometric analysis was performed using Scion Image (Scion Corp., Frederick, MO).
Western Blot Analysis
Western blot analysis was performed by the enhanced chemiluminescence system (Amersham Biosciences, Buckinghamshire, UK), as described previously.39,42 Primary antibodies used were: Anti-C/EBPβ antibody (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) and anti-IκBα antibody (1:200 dilution; Santa Cruz Biotechnology). As a loading control, identical filters were reprobed for β-actin using anti–β-actin antibody (1:30,000 dilution; Sigma-Aldrich Japan).
Luciferase Assay
Activity of luciferase was evaluated by Luciferase Assay System (Promega, Madison, WI) according to the manufacturer's protocol.8 Assays were performed in quadruplicate.
Animal Experiment
ER stress was induced in C57BL/6J mice (25 g body wt) by intraperitoneal injection of thapsigargin (1 mg/kg). After 16 h, kidneys were removed and subjected to Northern blot analysis. In some experiments, mice were administered LPS (200 μg/mouse, Escherichia coli 0111; B4; Sigma-Aldrich Japan) intraperitoneally at 16 h after the thapsigargin injection, and after 12 h, kidneys were subjected to analysis.
Statistical Analysis
Data were expressed as means ± SEM. Statistical analysis was performed using the nonparametric Mann-Whitney U test to compare data in different groups. P < 0.05 was considered to be a statistically significant difference.
Disclosures
None.
Acknowledgments
This work was supported, in part, by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (16390243, 19651024, and 20390235) to M.K. K.H. was a Research Fellow of the Japan Society of the Promotion Science.
We thank Dr. Laurie Glimcher (Harvard School of Public Health), Dr. David Ron (New York University School of Medicine), Dr. Kazunori Imaizumi (University of Miyazaki), Dr. Ez-Zoubir Amri (CNRS), Dr. Yoshihiko Nishio (Shiga University of Medical Science), Dr. Masayuki Miura (University of Tokyo), and Dr. Kazutoshi Mori (Kyoto University) for providing us with plasmids.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Rutkowski DT, Kaufman RJ: A trip to the ER: Coping with stress. Trends Cell Biol 14: 20–28, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Kim R, Emi M, Tanabe K, Murakami S: Role of the unfolded protein response in cell death. Apoptosis 11: 5–13, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Kitamura M: Endoplasmic reticulum stress and unfolded protein response in renal pathophysiology: Janus faces. Am J Physiol Renal Physiol 295: F323–F342, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Zhang K, Kaufman RJ: From endoplasmic-reticulum stress to the inflammatory response. Nature 454: 455–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cybulsky AV, Takano T, Papillon J, Khadir A, Liu J, Peng H: Complement C5b-9 membrane attack complex increases expression of endoplasmic reticulum stress proteins in glomerular epithelial cells. J Biol Chem 277: 41342–41351, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Cybulsky AV, Takano T, Papillon J, Bijian K: Role of the endoplasmic reticulum unfolded protein response in glomerular epithelial cell injury. J Biol Chem 280: 24396–24403, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Inagi R, Kumagai T, Nishi H, Kawakami T, Miyata T, Fujita T, Nangaku M: Preconditioning with endoplasmic reticulum stress ameliorates mesangioproliferative glomerulonephritis. J Am Soc Nephrol 19: 915–922, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayakawa K, Hiramatsu N, Okamura M, Yamazaki H, Nakajima S, Yao J, Paton AW, Paton JC, Kitamura M: Acquisition of anergy to proinflammatory cytokines in non-immune cells through endoplasmic reticulum stress response: A mechanism for subsidence of inflammation. J Immunol 182: 1182–1191, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Lekstrom-Himes J, Xanthopoulos KG: Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem 273: 28545–28548, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Dudenhausen EE, Pan YX, Zhong C, Kilberg MS: Human CCAAT/enhancer-binding protein β gene expression is activated by endoplasmic reticulum stress through an unfolded protein response element downstream of the protein coding sequence. J Biol Chem 279: 27948–27956, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Stein B, Cogswell PC, Baldwin AS, Jr: Functional and physical associations between NF-kappaB and C/EBP family members: A Rel domain-bZIP interaction. Mol Cell Biol 13: 3964–3974, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramji DP, Foka P: CCAAT/enhancer-binding proteins: Structure, function and regulation. Biochem J 365: 561–575, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endo S, Hiramatsu N, Hayakawa K, Okamura M, Kasai A, Tagawa Y, Sawada N, Yao J, Kitamura M: Geranylgeranylacetone, an inducer of HSP70, elicits unfolded protein response and coordinates cellular fate independently of HSP70. Mol Pharmacol 72: 1337–1348, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Brewer JW, Diehl JA, Hendershot LM: Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 318: 1351–1365, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Pahl HL, Baeuerle PA: A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappaB. EMBO J 14: 2580–2588, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D: Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287: 664–666, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Liu YW, Chen CC, Wang JM, Chang WC, Huang YC, Chung SY, Chen BK, Hung JJ: Role of transcriptional factors Sp1, c-Rel, and c-Jun in LPS-induced C/EBPδ gene expression of mouse macrophages. Cell Mol Life Sci 64: 3282–3294, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ejarque-Ortiz A, Medina MG, Tusell JM, Pérez-González AP, Serratosa J, Saura J: Upregulation of CCAAT/enhancer binding protein beta in activated astrocytes and microglia. Glia 55: 178–188, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Kaneko M, Niinuma Y, Nomura Y: Activation signal of nuclear factor-kappaB in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull 26: 931–935, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH: Autocrine tumor necrosis factor-alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol 26: 3071–3084, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC: Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol 23: 5651–5663, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D: Translational repression mediates activation of nuclear factor-kappaB by phosphorylated translation initiation factor 2. Mol Cell Biol 24: 10161–10168, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitamura M: Biphasic, bidirectional regulation of NF-kappaB by endoplasmic reticulum stress. Antioxid Redox Signal 11: 2353–2364, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki H, Hiramatsu N, Hayakawa K, Tagawa Y, Okamura M, Ogata R, Huang T, Nakajima S, Yao J, Paton AW, Paton JC, Kitamura M: Activation of the Akt–NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol 183: 1480–1487, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamura M, Takano Y, Hiramatsu N, Hayakawa K, Yao J, Paton AW, Paton JC, Kitamura M: Suppression of cytokine responses by indomethacin in podocytes: A mechanism through induction of unfolded protein response. Am J Physiol Renal Physiol 295: F1495–F1503, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Kitamura M: Endoplasmic reticulum stress in glomerulonephritis: The bad guy turns good? J Am Soc Nephrol 20: 1871–1873, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Harama D, Koyama K, Mukai M, Shimokawa N, Miyata M, Nakamura Y, Ohnuma Y, Ogawa H, Matsuoka S, Paton AW, Paton JC, Kitamura M, Nakao A: A sub-cytotoxic dose of subtilase cytotoxin prevents LPS-induced inflammatory responses, depending on its capacity to induce the unfolded protein response. J Immunol 183: 1368–1374, 2009 [DOI] [PubMed] [Google Scholar]
- 28.McKnight SL: McBindall: A better name for CCAAT/enhancer binding proteins? Cell 107: 259–261, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Prösch S, Heine AK, Volk HD, Krüger DH: CCAAT/enhancer-binding proteins alpha and beta negatively influence the capacity of tumor necrosis factor alpha to up-regulate the human cytomegalovirus IE1/2 enhancer/promoter by NF-kappaB during monocyte differentiation. J Biol Chem 276: 40712–40720, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Kitamura M, Taylor S, Unwin R, Burton S, Shimizu F, Fine LG: Gene transfer into the rat renal glomerulus via a mesangial cell vector: Site-specific delivery, in situ amplification, and sustained expression of an exogenous gene in vivo. J Clin Invest 94: 497–505, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee AH, Iwakoshi NN, Glimcher LH: XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23: 7448–7459, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du S, Hiramatsu N, Hayakawa K, Kasai A, Okamura M, Huang T, Yao J, Takeda M, Araki I, Sawada N, Paton AW, Paton JC, Kitamura M: Suppression of NF-kappaB by cyclosporine A and tacrolimus (FK506) via induction of the C/EBP family: Implication for unfolded protein response. J Immunol 182: 7201–7211, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH: Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci U S A 100: 9946–9951, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bezy O, Elabd C, Cochet O, Petersen RK, Kristiansen K, Dani C, Ailhaud G, Amri EZ: Delta-interacting protein A, a new inhibitory partner of CCAAT/enhancer-binding protein beta, implicated in adipocyte differentiation. J Biol Chem 280: 11432–11438, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Ahn S, Olive M, Aggarwal S, Krylov D, Ginty DD, Vinson C: A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol 18: 967–977, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katayama T, Imaizumi K, Honda A, Yoneda T, Kudo T, Takeda M, Mori K, Rozmahel R, Fraser P, George-Hyslop PS, Tohyama M: Disturbed activation of endoplasmic reticulum stress transducers by familial Alzheimer's disease-linked presenilin-1 mutations. J Biol Chem 276: 43446–43454, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Iwawaki T, Akai R, Kohno K, Miura M: A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat Med 10: 98–102, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K: ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol 20: 6755–6767, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayakawa K, Meng Y, Hiramatsu N, Kasai A, Yamauchi K, Yao J, Kitamura M: Priming of glomerular mesangial cells by activated macrophages causes blunted responses to proinflammatory stimuli. J Immunol 176: 2529–2537, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Rollins BJ, Morrison ED, Stiles CD: Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci U S A 85: 3738–3742, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D: Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J 17: 5708–5717, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokouchi M, Hiramatsu N, Hayakawa K, Okamura M, Du S, Kasai A, Takano Y, Shitamura A, Shimada T, Yao J, Kitamura M: Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response. J Biol Chem 283: 4252–4260, 2008 [DOI] [PubMed] [Google Scholar]