Abstract
Earlier detection of antibody-mediated rejection of kidney allografts may improve graft outcomes. Profiling of gene expression holds promise for the diagnosis and prognosis of antibody-mediated rejection. Here, we identified 730 patients who received kidney transplants during 2002–2005, including 21 patients (2.9%) who experienced early acute antibody-mediated rejection. We also identified a matched group of 43 patients with early acute T cell-mediated rejection to serve as controls. Compared with patients with T cell-mediated rejection, those with antibody-mediated rejection had significantly higher intrarenal mRNA expression of the cytoprotective heme oxygenase-1 but had lower expression of the regulatory T cell marker forkhead box P3 (FoxP3), the B cell marker CD20, and the chemokine regulated upon activation, normal T cell expressed and secreted (RANTES). T cell infiltration was similar in both groups of patients. Compared with grafts that had a favorable course, those that failed as a result of antibody-mediated rejection had expression profiles suggesting a lack of regulation (less FoxP3, TGF-β1, RANTES, and CD20). Grafts that failed as a result of T cell-mediated rejection only revealed lower expression of CD20 mRNA. In summary, these data suggest that severe antibody-mediated rejection and T cell-mediated rejection result in graft loss by distinct mechanisms. Molecular phenotypes of early acute rejection might help to identify grafts with poor prognosis, allowing earlier application of additional therapies.
Despite improvements in the first year renal allograft outcome, in the long term both acute and chronic rejections have been implicated to reduce renal allograft survival. Early antibody-mediated rejection (AMR) occurring within first 3 wk after kidney transplantation represents just a small part of all acute rejection episodes.1–3 Although the fate of grafts developing early AMR likewise has been found to be superior to that of patients developing AMR later, the risk of graft loss is quite high despite aggressive immunosuppressive therapy.4,5 Besides the well established therapy of acute T cell-mediated rejection (TCMR), the treatment schemes in early AMR still vary from center to center. Plasmapheresis and intravenous immunoglobulins have been used frequently, whereas rituximab, an anti-CD20 monoclonal antibody, or splenectomy is used mostly in resistant cases only.6–8
Fitting with the diversity of therapeutical strategies, the AMR pathogenesis remains poorly understood. Whereas immunohistochemistry is well established,9 gene expression analyses of AMR just started. Surprisingly, AMR was shown to be associated with similar changes in the transcriptome like TCMR.10 Because microarray analyses are expensive, time-consuming, and often evaluated with improper statistics, the current update of the Banff classification recommends for future cytokine gene expression analysis using the reverse transcription (RT)-PCR method for the prediction of graft dysfunction.11
Recently, we found that TGF-β1 and monocyte chemoattractant protein 1 (MCP-1) gene expression in case biopsies with allograft nephropathy help to identify those patients at risk for premature graft failure.12,13 Early AMR is a rare complication of renal transplantation. Thus, there is limited information about gene expression profiles in early AMR in the literature. The aim of this study was thus to evaluate the patterns of intrarenal expression of several genes associated with B cell- and T cell-mediated inflammation, rejection, and tolerance14,15 in relation to the renal allograft outcome in patients with early AMR and TCMR.
Results
Early AMR was diagnosed in 21 (2.9%) of 730 patients. Eight patients had revealed combined early AMR and TCMR (Banff IIa, IIB, and III in four, two, and two patients, respectively). Early AMR was diagnosed in average on the ninth postoperative day. As a control group, patients with early TCMR occurring within the same time window were enrolled in the study. Ten patients with AMR were treated using only plasmapheresis, and 11 patients were treated by combination of plasmapheresis and intravenous immunoglobulins (IVIGs). Despite trends toward better graft survival in the later group, Kaplan–Maier analysis showed no significant difference in graft survival. Grafts that failed had higher both glomerulitis and peritubular capillaries scores in the biopsy (data not shown).
First, we evaluated the gene expression differences of the selected genes between patients with biopsy-proven early acute AMR, TCMR, and combined AMR/TCMR. Biopsies from patients with early AMR and early TCMR showed comparable expression of CD3 and cytotoxic T lymphocyte (CTL) markers, suggesting similar levels of T cell infiltration. Remarkably, CD20 B cell expression was approximately five times higher in TCMR versus AMR (P < 0.05). Similarly, early TCMR was associated with higher intrarenal expression of CCL5, regulated upon activation, normal T cell expressed and secreted (RANTES) (P < 0.05), and forkhead box P3 (FoxP3) (P < 0.01), whereas the stress marker heme oxygenase-1 (HMOX1) was enhanced in AMR versus TCMR (P < 0.05) (Figure 1). All other markers were comparably expressed in all groups. Expression levels of all studied transcripts did not differ between acute tubular necrosis-like AMR and combined AMR/TCMR.
Figure 1.
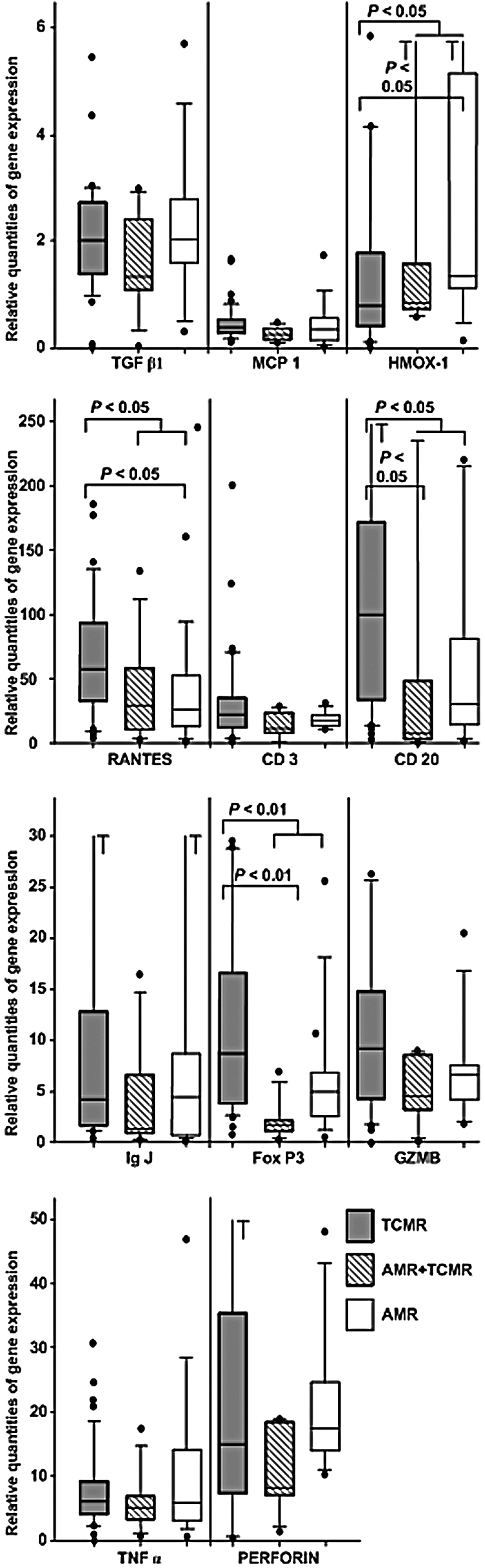
Quantitative intrarenal mRNA expression within grafts that suffered from early acute AMR or TCMR. Filled boxes represent TCMR, blank boxes AMR, and stripped boxes combined AMR/TCMR. The results are expressed as the ratio of the gene of interest to the housekeeping gene HPRT. The box plots show 25th and 75th (boundaries of boxes), 50th (median), 10th and 90th (error bars) percentile values. *P < 0.05; **P < 0.01. Samples for gene expression analysis were obtained before the antirejection therapy.
Next, we wondered whether the gene expression patterns were associated with the outcome of AMR/TCMR. Nine out of 20 patients with AMR that resulted in graft failure during follow-up experienced approximately tenfold lower infiltration by B cells (CD20, P < 0.01) and plasma cells (IgJ P < 0.01) as well as the fivefold lower expression of the regulatory T cell (Treg) master transcription factor FoxP3 (P = 0.02). With Bonferroni correction for multiple testing, however, a critical P value was set at 0.01, and thus the FoxP3 expression achieved just borderline significance. The T cell (CD3) and CTL [perforin (PRF) and granzyme B (GZMB)] infiltration and the stress marker HMOX1 were not predictable for the outcome of AMR (Table 1).
Table 1.
Quantitative intrarenal mRNA expression in early acute AMR
| Graft outcome | Failed grafts (n = 9) | Surviving grafts (n = 11) | P |
|---|---|---|---|
| TGF-β1 | 1.26 [0.03 to 6.95] | 2.44 [1.03 to 3.60] | 0.0367 |
| TNF-α | 5.12 [0.19 to 46.94] | 4.91 [2.31 to 17.40] | 0.4060 |
| MCP-1 | 0.23 [0.02 to 1.73] | 0.33 [0.09 to 0.61] | 0.4938 |
| RANTES | 15.02 [0.83 to 66.49] | 47.00 [8.96 to 159.65] | 0.0118 |
| HMOX1 | 1.18 [0.70 to 25.06] | 1.35 [0.14 to 9.07] | 0.2650 |
| CD3 | 13.65 [0.57 to 17.93] | 18.55 [7.65 to 28.54] | 0.1091 |
| CD20 | 4.17 [1.00 to 32.63] | 53.65 [7.72 to 278.29] | 0.0035 |
| IgJ | 0.85 [0.18 to 6.19] | 7.27 [0.73 to 212.78] | 0.0086 |
| FoxP3 | 1.20 [0.29 to 5.00] | 5.34 [1.73 to 25.51] | 0.0200 |
| GZMB | 4.01 [0.15 to 6.74] | 6.64 [1.72 to 20.31] | 0.0955 |
| PRF | 10.04 [1.28 to 17.82] | 17.65 [6.98 to 48.17] | 0.0763 |
| FoxP3/CD3 | 0.16 [0.11 to 2.13] | 0.22 [0.09 to 1.82] | NS |
| GZMB/CD3 | 0.34 [0.20 to 0.60] | 0.31 [0.11 to 1.10] | NS |
| PRF/CD3 | 1.08 [0.75 to 2.26] | 0.82 [0.35 to 4.27] | NS |
Relative quantities of specific gene expression relative to thatof the housekeeping gene HPRT and the calibrator sample are expressed asmedians [range]. Results reflect the gene expression patterns at thetime of diagnosis of early AMR and prior to therapy. One patient who died withfunctioning graft was not included in the analysis. The critical P fromBonferroni correction for multiple testing in Sankoh's modification is 0.01.Statistically significant values are represented in boldface type.
In contrast, grafts that failed after early TCMR during the follow-up showed before rejection therapy lower intrarenal mRNA expression of B cells (CD20, P < 0.01) and plasma cells (IgJ, P < 0.01) compared with grafts making a favorable course (Table 2). The CTL markers (PRF and GZMB) were comparable in both groups, and the GZMB-to-CD3 ratio was higher in the failing grafts; however, the low number of subjects who failed did not enable us to make a proper conclusion.
Table 2.
Quantitative intrarenal mRNA expression within grafts that suffered from early TCMR
| Graft outcome | Failed grafts (n = 4) | Surviving grafts (n = 37) | P |
|---|---|---|---|
| TGF-β1 | 2.69 [0.04 to 5.46] | 1.92 [0.01 to 7.03] | 0.5762 |
| TNF-α | 3.70 [0.99 to 24.55] | 6.07 [0.11 to 30.90] | 0.5657 |
| MCP-1 | 0.42 [0.22 to 1.62] | 0.39 [0.12 to 1.66] | 0.4394 |
| RANTES | 30.72 [9.39 to 180.12] | 63.82 [4.13 to 184.98] | 0.5730 |
| HMOX1 | 0.82 [0.12 to 16.21] | 0.79 [0.01 to 13.80] | 0.5831 |
| CD3 | 13.09 [2.00 to 17.00] | 26.28 [0.29 to 199.68] | 0.0809 |
| CD20 | 20.63 [2.90 to 31.13] | 108.46 [7.09 to 1519.93] | 0.0033 |
| IgJ | 1.62 [1.18 to 2.73] | 6.76 [0.37 to 220.48] | 0.0003 |
| FoxP3 | 4.38 [0.83 to 6.30] | 9.99 [1.56 to 33.25] | 0.0503 |
| GZMB | 9.26 [8.92 to 9.61] | 8.16 [0.0005 to 69.20] | 0.2732 |
| PRF | 22.24 [2.16 to 42.33] | 14.96 [0.007 to 89.60] | 0.9183 |
| FoxP3/CD3 | 0.41 [0.26 to 0.48] | 0.48 [0.12 to 1.19] | NS |
| GZMB/CD3 | 2.51 [0.57 to 4.46] | 0.26 [0.00001 to 2.56] | NS |
| PRF/CD3 | 1.79 [1.08 to 2.49] | 0.47 [0.0002 to 6.54] | NS |
Relative quantities of specific gene expression relative to that of the housekeeping gene HPRT and the calibrator sample are expressed as median [range]. Samples for gene expression analyses were obtained prior to the antirejection therapy. The critical P from Bonferroni correction for multiple testing in Sankoh's modification is 0.01. Statistically significant values are represented in boldface type. Two patients who died with functioning graft were not included in the analysis.
Next, we analyzed the correlations between the expression of various genes. With Spearman rank analysis, the correlation of all measured genes with FoxP3 mRNA expression was performed. There were strong correlations of intrarenal expression of TGF-β1 (rs = 0.709, P = 0.006), RANTES (rs = 0.684, P = 0.004), and CD20 (rs = 0.764, P = 0.004) with the intrarenal expression of FoxP3 mRNA during early AMR.
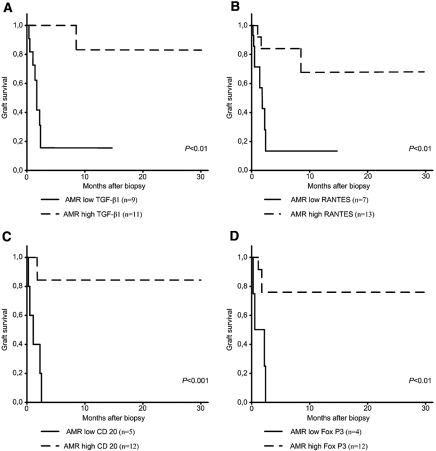
The Kaplan–Meier analysis of renal allograft survival showed patients with early AMR and low intrarenal expression of TGF-β1, RANTES, CD20, and FoxP3 at the time of biopsy [cutoff relative quantities (RQs) assessed from receiver operating characteristic analysis were 2.0, 16.7, 5.7, and 1.3, respectively] to have significantly shorter graft survival as compared with other patients (Figure 2). Similarly, patients with enhanced intrarenal CD20 mRNA expression had longer graft survival in TCMR (P < 0.01). Low mRNA expression of TGF-β1, RANTES, CD20, and FoxP3 in the biopsy revealing early AMR implied an increased risk for renal graft failure within next 12 mo [hazard ratio (HR) 11.96, P < 0.05; HR 5.61, P < 0.05; HR 11.46, P < 0.01; and HR 6.38, P < 0.05, respectively], and a similar effect was found in TCMR only for CD20 (HR 10.47, P < 0.05; Table 3). The analysis of multiway frequency tables confirmed the influence of low TGF-β1 intragraft mRNA expression during early AMR on renal graft failure (P < 0.01), independently of the treatment modality.
Figure 2.
Kaplan–Meier analysis of variables on renal graft outcome. (A) Intragraft TGF-β1 mRNA in AMR (log rank statistic 10.34; P < 0.01). (B) Intragraft RANTES mRNA in AMR (log rank statistic 7.32; P < 0.01). (C) Intragraft CD20 mRNA in AMR (log rank statistic 12.74; P < 0.001). (D) Intragraft FoxP3 mRNA in AMR (log rank statistic 7.56; P < 0.01).
Table 3.
Intrarenal gene expression during the early acute rejection and the risk of graft failure
| Variable | Hazard ratio | 95% Confidence interval | P | |
|---|---|---|---|---|
| AMR | TGF-β1 ≤ 2 (n = 11) | 11.96 | 1.41 to 101.29 | 0.023 |
| RANTES ≤ 16.71 (n = 7) | 5.61 | 1.38 to 22.73 | 0.016 | |
| CD20 ≤ 5.7 (n = 5) | 11.46 | 2.17 to 60.49 | 0.004 | |
| FoxP3 ≤ 1.3 (n = 4) | 6.38 | 1.41 to 28.89 | 0.016 | |
| TCMR | CD20 ≤ 31 (n = 8) | 10.47 | 1.09 to 101.01 | 0.042 |
Low intragraft TGF-β1 (RQ ≤ 2), RANTES (RQ ≤ 16.71), CD20 (RQ ≤ 5.7), and FoxP3 (RQ ≤ 1.3) gene expressions in the biopsy with proved early AMR prior to the therapy and low intragraft CD20 (RQ ≤ 31) gene expression in the biopsy with proved early TCMR prior to the therapy were related with an increased risk of renal graft failure within the first 12 mo after kidney transplantation (univariate Cox regression model).
Discussion
The aim of this study was to evaluate the expression patterns of selected genes during early acute AMR and early acute TCMR. In contrast to some reports,10 we found significant differences between AMR and TCMR. Interestingly, T cell and CTL infiltrates were only slightly higher in TCMR, suggesting similar levels of T cell infiltration (total and CTL) in TCMR and AMR. The higher levels of FoxP3 in TCMR suggest a stronger counter-regulation by Tregs in TCMR compared with AMR. Because CCL5 (RANTES) seems to be involved in Treg recruitment, the higher RANTES expression in TCMR might explain the FoxP3 enrichment in cellular rejection. Remarkably, B cell infiltration (CD20) was also higher in TCMR versus AMR. Very recent data suggest an association of B cell expansion and allograft tolerance (RISET [Reprogramming the Immune System for Establishing of Tolerance]/Tolerance Indices Network, Hernandez et al., submitted). In summary, our data suggest a higher level of regulation in cellular versus humoral rejection that might explain the better long-term outcome of antirejection therapy in the former.
Therefore, we asked then whether a relation between gene expression and outcome is seen in early AMR and TCMR.
As suggested above, AMR shows fewer events of regulation (Tregs and B cells) compared with TCMR that have a better outcome in general, but looking at the failed grafts following AMR, the lack of regulation (low FoxP3, RANTES, TGF-β1, and CD20) was even more prominent. However, effector markers (T cells, CTL, macrophage inflammatory protein, etc.) do not predict the outcome of early AMR. The strongest prediction of graft failure within the next 12 mo is defined by low TGF-β1 and CD20 gene expression levels in the biopsy, revealing early AMR (HR 11.96, P = 0.023 and HR 11.46, P = 0.004, respectively). In fact, multiway frequency tables confirmed the key association of low TGF-β1 intragraft mRNA expression levels and renal graft failure after AMR, independently of the treatment modality. These data suggest that a lack of adequate counter-regulation might be a key element in the pathogenesis of severe AMR, resulting in graft loss.
In contrast, graft failure after early TCMR was associated with a higher effector T cell ratio (CTL/CD3). In contrast to AMR, markers of regulation (FoxP3 and TGF-β1) were not significantly different between the TCMR responders and nonresponders, suggesting a dominance of uncontrolled CTL in the pathogenesis of severe early TCMR, resulting in graft loss. Like in AMR, a lack of B cells is associated with graft failure, making a protective (“tolerogenic”) role for B cells possible.
TGF-β1 has been suggested as having both immunosuppressive and profibrogenic properties. Although in the long term the higher TGF-β1 expression within the renal allograft is associated with the poor kidney graft outcome,13 early after transplantation its higher expression might be related with improved tissue remodeling and repair after ischemic injury16 along with a more profound suppression of host immune response and thus be associated with better graft outcome.17 TGF-β1 has been shown to be critical in the generation of CD4+CD25+Foxp3+ Tregs.18 The increased frequencies of Tregs were described as an additional mechanism that induces alloimmune tolerance.19 The quantification of Tregs in allograft rejection is thus of considerable clinical interest. The presence of intragraft FoxP3-positive cells was not confined to tolerated grafts but was suggested to be a part of the normal immune response during rejection.20 TGF-β1-driven Tregs may prevent an aberrant chronic T cell hyperactivation and were shown to inhibit inflammatory response to transplanted grafts and enhance its survival.21 Veronese et al.,9 using immunohistochemistry, analyzed 80 human renal transplant biopsies for the Treg transcription factor FoxP3. They found FoxP3-positive cells to be present within the interstitium in acute cellular rejection at a greater density than those in AMR. Similarly to the lower FoxP3 protein expression levels observed by Veronese et al., we find a lower level of FoxP3 mRNA expression in early AMR as compared with that in early TCMR. A recently published study by Bunnag et al. showed an association between FoxP3 mRNA expression in case biopsies and rejection.22 In this study, the authors observed no differences in FoxP3 mRNA expression between AMR and TCMR. They, however, evaluated just three early AMR cases; the other 11 AMRs were chronic AMR cases that occurred after the first year posttransplant. In multivariate analysis, higher FoxP3 mRNA levels did not correlate with favorable 6-mo graft outcomes, even when the analysis was restricted to biopsies with rejection. Contrary to this study22 and in agreement with the results of our study, Bestard et al.23 suggest that immunostaining of FoxP3+ Tregs in protocol biopsies seems to be useful for the prediction of long-term kidney transplant outcome. Similarly, in the mouse renal transplant model, the presence of FoxP3 Tregs decreased during ongoing rejection.24
Additionally, TGF-β1 might act as a potent inhibitor of complement C3 secretion under inflammatory conditions.25 The locally synthesized complement component C3 was shown to contribute to both TCMR and AMR, with the highest expression levels found in C4d-positive indication biopsies with signs of cellular rejection.26
In our study, there were also trends toward higher expression of markers related to B cell infiltration, such as CD20 within grafts with better prognosis. The B-lymphocyte infiltrate during acute rejection was shown to be associated with poor prognosis in some reports27,28 although not in others.29 B cells may be functioning as professional antigen-presenting cells in the graft and thus related to more severe rejection outcome. Recently, CD20 infiltrates during acute cellular rejection have been shown to possibly represent a heterogeneous population that might not help to identify a graft at risk for failure.30 Moreover, very recent data suggest an association between enhanced B cell levels and operational tolerance in kidney transplant patients (unpublished observations).
In conclusion, we documented that gene expression profiles differ between early AMR and early TCMR of renal allografts. The differences are even more pronounced in grafts with poor outcome after rejection, supporting the view of different pathogenesis for AMR and TCMR. Aggressive therapeutical maneuvers in acute rejection might be associated with late complications, such as tumors, diabetes, and cardiovascular diseases. Phenotypical molecular traits of both AMR and TCMR identified in our study, in particular the lower distribution of CD20-positive cells associated with poor graft prognosis, are thus of potential clinical relevance. Rare occurrence of early AMR warrants larger prospective multicenter and biomarker-driven clinical trials to validate our results to include molecular phenotypes of rejection in management decisions.
Concise Methods
Patient Population
A total of 730 kidney transplant procedures were performed in our center in Prague from January 2002 through October 2005. Early acute AMR (within the first 3 wk posttransplant) was diagnosed in 21 patients (2.9%). A control group of 43 patients with TCMR out of these 730 patients were matched for posttransplant time, age, gender, and immunosuppression. Their basic demographic parameters are shown in Table 4. All patients were treated with maintenance immunosuppression using either tacrolimus or cyclosporin A (CsA) along with mycophenolate mofetil (MMF) and corticosteroids. In cases of increased panel reactive antibody (PRA) frequencies (>50%) before transplantation, patients received induction with antithymocyte globulin (ATG, Thymoglobuline, Genzyme) or anti-CD3 monoclonal antibody (OKT3-Muromonab, Janssen-Cillag). A survey of maintenance immunosuppression used is shown in Table 4. All patients were on follow-up in our outpatient clinic. Graft failure was defined as a return to dialysis treatment.
Table 4.
Basic patient characteristics
| AMR | TCMR | |
|---|---|---|
| N | 21 | 43 |
| Age (yr) | 47.0 ± 12.4 | 47 ± 10.7 |
| First/second/third transplants | 13/8/1 | 40/3/0 |
| Donor gender (male %) | 16/(76.2%) | 22/(51.2%) |
| Number of A mismatches | 1.1 ± 0.6 | 1.3 ± 0.6 |
| Number of B mismatches | 1.4 ± 0.6 | 1.3 ± 0.7 |
| Number of DR mismatches | 0.9 ± 0.7 | 1.0 ± 0.7 |
| PRA (%) | 44 ± 36 | 9 ± 16a |
| Hemodialysis duration (mo) | 35.1 ± 26.5 | 27.2 ± 26.5 |
| Immunosuppression: ATG or OKT3-Muromonab induction | 10 (47.6%) | 11 (25.6%) |
| Tacrolimus | 19 (90.5%) | 36 (83.7%) |
| CsA | 2 (9.5%) | 7 (16.3%) |
| MMF | 20 (95.2%) | 39 (90.7%) |
| SIR | 1 (4.8%) | 4 (9.3%) |
| Early AMR/TCMR: | ||
| Diagnosis (day) | 9.0 ± 2.8 | 8.6 ± 4.0 |
| Biopsy Cr (mg/dl) | 5.1 ± 2.8 | 4.4 ± 3.0 |
| Biopsy CCr (ml/min) | 27.7 ± 16.4 | 33.5 ± 19.0 |
| C4d+ (%) | 100 | 0 |
| CDCXM+ (%) | 90.5 | Not done |
| FXCM+ (%) | 90.5 | Not done |
| 12 months Cr (mg/dl) | 2.0 ± 0.8 | 1.7 ± 0.7 |
| 12 months CCr (ml/min) | 43.5 ± 17.8 | 61.9 ± 20.1b |
CCr, creatinine clearance; Cr, creatinine; SIR, sirolimus.
aP < 0.001.
bP < 0.05.
Histology
Biopsies were indicated either in the case of missing graft function within the first 5 to 7 postoperative days (delayed graft function) or stagnation of decreasing serum creatinine at pathologic levels or of rising serum creatinine. Biopsy procedures using 14-gauge biopsy needles were performed under ultrasound control. Diagnosis was established on the basis of histologic verification using the Banff 05 classification.31 Patients signed their informed consent before each biopsy to participate in the gene expression profile study, and the protocol was approved by the Ethical Committee of the Institute for Clinical and Experimental Medicine, Prague, Czech Republic.
The diagnosis of early AMR was characterized by C4d-positive immunohistology within the first 2 wk posttransplant along with evidence of donor-specific antibodies. C4d was documented using anti-C4d monoclonal antibody (Quidel Corp.) on frozen sections by indirect immunofluorescence method or immunoperoxidase methods on paraffin sections with a polyclonal anti-C4d antibody (Biomedika).
Small portions of renal tissue from the cortex were placed into tissue storage reagent RNAlater (Sigma) immediately after biopsy and stored at −20°C for further gene expression analysis, although most of the renal tissue taken by core biopsy was used for routine histology.
Evidence of Anti-HLA Antibody
Anti-donor-HLA antibody was demonstrated by either cytotoxic cross-match (CDCXM) or flow cytometry cross-match (FCXM). These tests were performed invariably if histology suspected early AMR. Non-HLA endothelial antibodies were not determined.
Treatment of Early Rejection
Ten patients with early AMR were treated using repeated sessions of plasmapheresis, and 11 patients were treated by a combination of repeated plasmapheresis followed by IVIGs (0.5 g/kg, Endobulin, Kiovig, Baxter-Immuno).
Acute TCMR BANFF I and IIa were treated with a methylprednisolone (MP) bolus, whereas ATG was administered in patients with rejections of BANFF IIb and III or in a case of steroid resistance. In eight cases that showed histologic signs of early AMR in combination with TCMR, a combined therapy of plasmapheresis/IVIG and MP/ATG was used as appropriate.
RNA Isolation and Real-Time Quantitative RT-PCR
The renal tissue was homogenized; total RNA was extracted using RNA Blue (Top-Bio) and reversely transcribed into cDNA, using the SuperScript II Reverse Transcriptase (Invitrogen). Complementary DNA was amplified by real-time quantitative PCR (TaqMan, 7300 Real Time PCR Systems, Applied Biosystems) using commercial primers and fluorogenic probes. Samples were tested for genomic DNA contamination and, if tested positive, were excluded from the study. On the basis of our preliminary data, we selected the following markers for gene expression analysis: cytokines such as TGF-ββ1, TNF-α, MCP-1, and RANTES (CCL5), for quantifying T, B, or plasma cell infiltration the markers CD3, CD20, and IgJ, respectively, FoxP3 as master transcription factor of Tregs, the cytotoxic markers GZMB and PRF for quantifying cytotoxic T cells/NK cells, and HMOX1 as a cytoprotective stress protein. Specific gene expression was calculated relative to that of the housekeeping gene hypoxanthine guanine phosphoribosyl transferase (HPRT), and the calibrator sample [comparative threshold cycle method (2−ΔΔ[supi]cT). RQ software, Applied Biosystems) and was expressed as RQ.
Statistical Analysis
Basic statistical parameter data are given as absolute and relative frequency, average and SD, or median and range. Survival curves were estimated using the Kaplan–Meier method. Agreement between groups was tested using the log-rank test. The intergroup difference was analyzed by the chi-square test for discrete variables and t test for continuous variables. The differences in gene expression patterns were analyzed after logarithmic transformation, and their correlations were analyzed using the Spearman rank correlation test. Due to a strong correlation between the transcripts, Bonferroni correction modified by Sankoh was applied to correct for multiple testing.32The univariate Cox regression model was used for analyzing the risk for renal graft failure. The influence of intrarenal gene expression in combination with the different treatment of AMR on graft survival was analyzed using the log-linear model in multiway frequency tables. The receiver operating characteristic curve analysis was used to calculate the cutoff values for low and high intrarenal gene expression. Unless stated otherwise, the P value <0.05 was considered to be statistically significant. Calculations were performed using the StatsDirect 2.6.6 program (StatsDirect).
Disclosures
None.
Acknowledgments
Authors are indebted to Katerina Hyklova, Irena Brabcova, Vladena Homolkova, and Jelena Skibova for their technical assistance as well as the patients and nurses for cooperation and help. This work was supported Grants MZO 00023001 and NR/9388-3/2007 from Internal Grant Agency of the Ministry of Health of the Czech Republic and RISET (Reprogramming of the Immune System for Establishing of Tolerance) of the European Union.
A part of the study was presented at The World Transplant Congress, August 10 through 14, 2008, Sydney, Australia.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Davis CL, Hricik DE: Transplant: Immunology and treatment of rejection. Am J Kidney Dis 43: 1116–1137, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Feucht HE: Complement C4d in graft capillaries—The missing link in the recognition of humoral alloreactivity. Am J Transplant 3: 646–652, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Feucht HE, Schneeberger H, Hillebrand G, Burkhardt K, Weiss M, Riethmuller G, Land W, Albert E: Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int 43: 1333–1338, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Halloran PF, Schlaut J, Solez K, Srinivasa NS: The significance of the anti-class I response. II. Clinical and pathologic features of renal transplants with anti-class I-like antibody. Transplantation 53: 550–555, 1992 [PubMed] [Google Scholar]
- 5.Halloran PF, Wadgymar A, Ritchie S, Falk J, Solez K, Srinivasa NS: The significance of the anti-class I antibody response. I. Clinical and pathologic features of anti-class I-mediated rejection. Transplantation 49: 85–91, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Gloor J, Cosio F, Lager DJ, Stegall MD: The spectrum of antibody-mediated renal allograft injury: Implications for treatment. Am J Transplant 8: 1367–1373, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Kaplan B, Gangemi A, Thielke J, Oberholzer J, Sankary H, Benedetti E: Successful rescue of refractory, severe antibody mediated rejection with splenectomy. Transplantation 83: 99–100, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Sun Q, Liu ZH, Ji S, Chen J, Tang Z, Zeng C, Zheng C, Li LS: Late and early C4d-positive acute rejection: Different clinico-histopathological subentities in renal transplantation. Kidney Int 70: 377–383, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Veronese F, Rotman S, Smith RN, Pelle TD, Farrell ML, Kawai T, Benedict Cosimi A, Colvin RB: Pathological and clinical correlates of FOXP3+ cells in renal allografts during acute rejection. Am J Transplant 7: 914–922, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Mueller TF, Einecke G, Reeve J, Sis B, Mengel M, Jhangri GS, Bunnag S, Cruz J, Wishart D, Meng C, Broderick G, Kaplan B, Halloran PF: Microarray analysis of rejection in human kidney transplants using pathogenesis-based transcript sets. Am J Transplant 7: 2712–2722, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Hribova P, Lacha J, Kotsch K, Volk HD, Brabcova I, Skibova J, Vitko S, Viklicky O: Intrarenal cytokine and chemokine gene expression and kidney graft outcome. Kidney Blood Press Res 30: 273–282, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Pribylova-Hribova P, Kotsch K, Lodererova A, Viklicky O, Vitko S, Volk HD, Lacha J: TGF-β1 mRNA upregulation influences chronic renal allograft dysfunction. Kidney Int 69: 1872–1879, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Melk A, Mansfield ES, Hsieh SC, Hernandez-Boussard T, Grimm P, Rayner DC, Halloran PF, Sarwal MM: Transcriptional analysis of the molecular basis of human kidney aging using cDNA microarray profiling. Kidney Int 68: 2667–2679, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Weintraub LA, Sarwal MM: Microarrays: A monitoring tool for transplant patients? Transpl Int 19: 775–788, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Lario S, Mendes D, Bescos M, Inigo P, Campos B, Alvarez R, Alcaraz A, Rivera-Fillat F, Campistol JM: Expression of transforming growth factor-β1 and hypoxia-inducible factor-1α in an experimental model of kidney transplantation. Transplantation 75: 1647–1654, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Ozdemir BH, Ozdemir FN, Demirhan B, Haberal M: TGF-β1 expression in renal allograft rejection and cyclosporine A toxicity. Transplantation 80: 1681–1685, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM: Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med 198: 1875–1886, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi S: Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 6: 345–352, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Haanstra KG, Wubben JA, Korevaar SS, Kondova I, Baan CC, Jonker M: Expression patterns of regulatory T-cell markers in accepted and rejected nonhuman primate kidney allografts. Am J Transplant 7: 2236–2246, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Fu S, Zhang N, Yopp AC, Chen D, Mao M, Chen D, Zhang H, Ding Y, Bromberg JS: TGF-β induces Foxp3 + T-regulatory cells from CD4 + CD25 − precursors. Am J Transplant 4: 1614–1627, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Bunnag S, Allanach K, Jhangri GS, Sis B, Einecke G, Mengel M, Mueller TF, Halloran PF: FOXP3 expression in human kidney transplant biopsies is associated with rejection and time post transplant but not with favorable outcomes. Am J Transplant 8: 1423–1433, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Bestard O, Cruzado JM, Rama I, Torras J, Goma M, Seron D, Moreso F, Gil-Vernet S, Grinyo JM: Presence of FoxP3+ regulatory T cells predicts outcome of subclinical rejection of renal allografts. J Am Soc Nephrol 19: 2020–2026, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Jiang J, Guan Q, Lan Z, Wang H, Nguan CY, Jevnikar AM, Du C: Reduction of Foxp3-expressing regulatory T cell infiltrates during the progression of renal allograft rejection in a mouse model. Transpl Immunol 19: 93–102, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Andoh A, Shimada M, Takaya H, Hata K, Fujiyama Y, Bamba T: Transforming growth factor-β1 acts as a potent inhibitor of complement C3 biosynthesis in human pancreatic cancer cell lines. Pancreas 20: 138–145, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Serinsoz E, Bock O, Gwinner W, Schwarz A, Haller H, Kreipe H, Mengel M: Local complement C3 expression is upregulated in humoral and cellular rejection of renal allografts. Am J Transplant 5: 1490–1494, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M, Salvatierra O, Jr: Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med 349: 125–138, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Tsai EW, Rianthavorn P, Gjertson DW, Wallace WD, Reed EF, Ettenger RB: CD20+ lymphocytes in renal allografts are associated with poor graft survival in pediatric patients. Transplantation 82: 1769–1773, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Bagnasco SM, Tsai W, Rahman MH, Kraus ES, Barisoni L, Vega R, Racusen LC, Haas M, Mohammed BS, Zachary AA, Montgomery RA: CD20-positive infiltrates in renal allograft biopsies with acute cellular rejection are not associated with worse graft survival. Am J Transplant 7: 1968–1973, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Kayler LK, Lakkis FG, Morgan C, Basu A, Blisard D, Tan HP, McCauley J, Wu C, Shapiro R, Randhawa PS: Acute cellular rejection with CD20-positive lymphoid clusters in kidney transplant patients following lymphocyte depletion. Am J Transplant 7: 949–954, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ: Banff '05 Meeting Report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant 7: 518–526, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Sankoh AJ, Huque MF, Dubey SD: Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med 16: 2529–2542, 1997 [DOI] [PubMed] [Google Scholar]