Abstract
Anabolic steroid abuse adversely affects the endocrine system, blood lipids, and the liver, but renal injury has not been described. We identified an association of focal segmental glomerulosclerosis (FSGS) and proteinuria in a cohort of 10 bodybuilders (six white and four Hispanic; mean body mass index 34.7) after long-term abuse of anabolic steroids. The clinical presentation included proteinuria (mean 10.1 g/d; range 1.3 to 26.3 g/d) and renal insufficiency (mean serum creatinine 3.0 mg/dl; range 1.3 to 7.8 mg/dl); three (30%) patients presented with nephrotic syndrome. Renal biopsy revealed FSGS in nine patients, four of whom also had glomerulomegaly, and glomerulomegaly alone in one patient. Three biopsies revealed collapsing lesions of FSGS, four had perihilar lesions, and seven showed ≥40% tubular atrophy and interstitial fibrosis. Among eight patients with mean follow-up of 2.2 yr, one progressed to ESRD, the other seven received renin-angiotensin system blockade, and one also received corticosteroids. All seven patients discontinued anabolic steroids, leading to weight loss, stabilization or improvement in serum creatinine, and a reduction in proteinuria. One patient resumed anabolic steroid abuse and suffered relapse of proteinuria and renal insufficiency. We hypothesize that secondary FSGS results from a combination of postadaptive glomerular changes driven by increased lean body mass and potential direct nephrotoxic effects of anabolic steroids. Because of the expected rise in serum creatinine as a result of increased muscle mass in bodybuilders, this complication is likely underrecognized.
Index Case
The index case (patient 1) is a 30-yr-old white male professional bodybuilder who had no significant medical history and presented to a local hospital with lower extremity edema. The patient was on no prescription medications, but as part of his bodybuilding regimen, he regularly consumed a high-protein diet (>550 g/d) and dietary supplements including 10 g/d creatine monohydrate, 1000 mg/d branched-chain amino acids, 10 g/d glutamine, and multivitamins. For more than a decade, he regularly used anabolic androgenic steroids (AASs), including injectable testosterone, methyl-1-testosterone (taken orally), growth hormone, and insulin to augment his bodybuilding. At the time of biopsy, his steroid regimen consisted of growth hormone 4 IU 5 d/wk and testosterone 500 mg intramuscularly twice weekly. In addition, he took 75 mg of ephedrine and 600 mg of caffeine before each workout session.
Physical examination revealed a height of 71 inches (180 cm), a weight of 295 pounds (134 kg), and a body mass index (BMI) of 41.2 kg/m2 with an extremely muscular, highly toned physique (Figure 1). BP was 145/80 mmHg, and there was 2+ bilateral lower extremity edema. The patient was found to have a serum creatinine of 2.7 mg/dl, blood urea nitrogen of 24 mg/dl, serum albumin of 1.9 g/dl, total serum protein of 5.7 g/dl, serum cholesterol of 212 mg/dl, hematocrit of 45%, and white blood cell count of 10.3 × 109/L with a normal differential and platelet count of 254 × 109/L. Serum glucose and electrolytes including sodium, potassium, bicarbonate, chloride, and calcium were within normal limits. Serologic evaluation revealed a borderline positive ANA (titer 1:80 with a homogeneous pattern) with negative anti–double-stranded DNA antibody and negative viral serologies including HIV, hepatitis B surface and core antigens, and hepatitis C antibody. Serum complement levels including C3, C4, and CH50 were within normal limits. Urinalysis revealed 4+ protein, and microscopic examination showed fewer than five red blood cells per high-power field and no white blood cells or casts. Twenty-four-hour urine collection revealed proteinuria of 26.3 g/d and creatinine clearance (CrCl) of 91 ml/min. A renal biopsy was performed in August 2004 to determine the cause of the patient's nephrotic syndrome.
Figure 1.
Shown is patient 1, the index case (published with patient's permission).
Light microscopic examination showed two cores of renal cortex with overlying capsule and two additional fragments of cortex only. Sampling for light microscopy was predominantly subcapsular with 16 of the 22 glomeruli present showing global sclerosis. Of the remaining six glomeruli, four displayed lesions of FSGS with hyaline insudation, increased matrix material, and focal tuft collapse with hypertrophy and hyperplasia of overlying visceral epithelial cells. The remaining two glomeruli were hypertrophied but normocellular. There were severe tubular atrophy and interstitial fibrosis involving approximately 80% of the cortex, accompanied by a patchy mild to moderate mononuclear inflammatory infiltrate. Vessels exhibited mild arterio- and arteriolosclerosis. Tissue sampled for immunofluorescence contained one globally sclerotic glomerulus that was negative for IgG, IgM, IgA, C3, C1q, and κ and λ light chains.
Ultrastructural evaluation revealed one segmentally sclerotic glomerulus with wrinkling of the glomerular basement membrane (GBM), inframembranous hyalinosis, and adhesion to Bowman's capsule. No immune-type electron-dense deposits or tubuloreticular inclusions were identified. Podocytes displayed 95% estimated foot process effacement (FPE) and microvillous transformation.
The differential diagnosis included primary and secondary forms of FSGS. Importantly, the cortical sampling in the biopsy was disproportionately subcapsular, and the degree of glomerulosclerosis and tubular atrophy did not seem to correlate with the reported CrCl of 91 ml/min, suggesting overestimation of the chronic renal injury. The clinical history of markedly elevated BMI, extremely muscular body habitus, AAS use, creatine supplementation, and high-protein diet supported a form of postadaptive FSGS related to bodybuilding, whereas the unusual features of full nephrotic syndrome, focal collapsing features, and severe FPE suggested the possibility of a toxic form of secondary FSGS.1
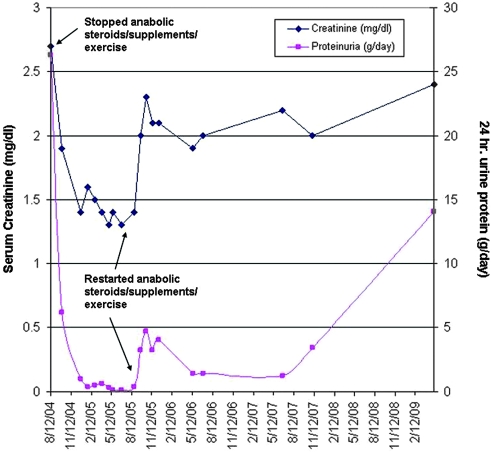
After biopsy, the patient was started on ramipril 5 mg/d and valsartan 160 mg/d and was strongly advised to desist from bodybuilding. His serum creatinine and 24-h urine protein measurements over the ensuing 4.73 yr are graphed in Figure 2. After discharge from the hospital, he discontinued bodybuilding, AAS use, and dietary supplements, resulting in the loss of 40 lbs in the subsequent 2 mo. He reported improved energy levels and a general sense of greater well-being. At 2 mo after biopsy, BP fell to 110/75 mmHg and edema had resolved. Laboratory testing revealed a decrease in serum creatinine to 1.9 mg/dl, decreased urinary protein excretion (down to 6.2 from 26.3 g/d), and normalization of albumin to 4.2 g/dl. Eight months later (10 mo after initial presentation), the patient had lost an additional 40 lbs (weighing 215 lbs) and was not performing strenuous exercise or taking any supplements or AASs. His creatinine further decreased to 1.3 mg/dl, his 24-h urine protein excretion fell to 146 mg/d, and his 24-h CrCl was 111 ml/min.
Figure 2.
Serum creatinine (left axis) and proteinuria (right axis) over time are shown for patient 1. Note the dramatic initial improvement in both parameters followed by relapse after restarting training with AASs and supplements.
Although his renal function and proteinuria had significantly improved, the patient reported symptoms of severe depression related to changes in body image. He perceived himself as being too “skinny and weak” and complained of decreased libido. He wanted to resume bodybuilding but agreed to a reduced exercise regimen without supplements or hormones. Two months after restarting his exercise regimen, his weight increased to 267 lbs, his serum creatinine was 1.4 mg/dl, 24-h urine protein was 395 mg/d, and CrCl was 200 ml/min. At his next office visit, the patient reported feeling well but was dissatisfied with the muscle mass that he was able to achieve without supplements and hormones. Against his nephrologist's advice, he resumed his high-protein diet as well as the dietary supplements, testosterone, and growth hormone but at lower levels than previously. Six weeks after restarting supplements and hormones, his serum creatinine increased to 2.3 mg/dl and proteinuria increased to 4.7 g/d. He was advised to stop using supplements and hormones, decrease his protein intake, and decrease his workout regimen. The patient failed to comply, and 3.5 yr after restarting bodybuilding with hormones and supplements, the patient weighed 296 lbs (BMI 41.3 kg/m2) with creatinine of 2.4 mg/dl and 24-h urine protein of 14.1 g/d.
The dramatic improvement in renal parameters (including complete remission of proteinuria) achieved by stopping AASs, decreasing exercise, losing weight, and renin-angiotensin system (RAS) blockade, without resorting to immunosuppressive therapy, followed by relapse of nephrotic-range proteinuria and worsening of renal function after resumption of a full bodybuilding regimen strongly supports a secondary form of FSGS. We propose that abuse of AASs causes a secondary form of FSGS both by increasing lean body mass and by potential direct toxic effects on glomeruli.
Introduction
AASs are taken by athletes to improve performance. In certain sports, such as bodybuilding and powerlifting, AAS abuse is quite common, although few reliable studies have addressed prevalence of abuse because these agents are generally taken surreptitiously. One study of bodybuilders in Sweden found that 75% of competitive bodybuilders and 24% who engaged in bodybuilding solely to improve their sense of well-being used AASs.2 The endocrine effects of AAS abuse are well established and commonly include testicular atrophy, decreased fertility, and gynecomastia.3 Additional adverse effects associated with AASs include changes in blood lipid levels (increase in LDL and decrease in HDL),4 various forms of hepatotoxicity,5 and neuropsychiatric disturbances.6,7 We present the first series of patients who developed FSGS after long-term use of AASs.
FSGS is a pattern of glomerular injury seen in primary podocytopathies as well as in secondary forms of glomerular injury caused by adaptive responses to elevated glomerular capillary pressures and flow rates. Conditions associated with either decreased nephron number or increased demand on a normal endowment of nephrons will result in increased single-nephron GFRs. These increased demands first manifest morphologically as glomerular hypertrophy but eventually become maladaptive, producing glomerulosclerosis. Multiple causes of secondary FSGS as a result of decreased nephron number have been described, including unilateral renal agenesis,8 surgical ablation, and low birth weight.9,10 Patients with normal numbers of nephrons may develop secondary FSGS as a result of morbid obesity, which causes an increased demand for glomerular filtration that parallels increased body mass.11,12 Secondary forms of FSGS typically have a lower incidence of nephrotic syndrome and a better overall prognosis when compared with primary (idiopathic) FSGS.13 Whereas the mainstay of treatment for primary FSGS is immunosuppression, secondary (postadaptive) forms of FSGS are treated with RAS blockade and treatment of the underlying cause whenever possible (e.g., weight loss in the obese patient).14
The link between obesity and FSGS is well established.11 In the process of studying patients with obesity-related glomerulopathy (ORG), we identified several highly muscular, nonobese patients with elevated lean body mass and FSGS, whose case histories and biopsy findings have been published.15 These patients had no documented AAS abuse and developed a form of secondary FSGS with strong similarities to ORG. We now enlarge our experience to patients with FSGS in the setting of elevated lean body mass and long-term AAS abuse.
Results
Patient demographics and clinical features are presented in Table 1. The cohort included six white and four Hispanic men with a mean age of 37 yr (range 28 to 49 yr) and an average BMI of 34.7 kg/m2 (range 27 to 43 kg/m2). All engaged in strenuous weightlifting for the purpose of bodybuilding or as training for participation in strength competitions. Each man used at least one AAS, often in combination with dietary supplements such as creatine monohydrate and a high-protein diet. Six of 10 patients had systemic hypertension at clinical presentation, and three patients had a documented history of hypertension, ranging in duration from 3 mo to 5 yr. One white patient was HIV positive, had been on long-term antiviral therapy, and had recent onset of type 2 diabetes, but he had an undetectable viral load, no evidence of diabetic nephropathy, and no features of HIV-associated nephropathy (collapsing glomerulopathy or tubular microcysts). In situ PCR for HIV DNA, typically positive in HIV-associated nephropathy, was negative.16 Two patients, including our index case, had sleep apnea attributed to airway obstruction related to increased muscle mass in the neck.
Table 1.
Demographics and clinical history
| Patient | Age | Race/Gender | Height (in) | Weight (kg) | BMI | Exercise | Hormone Use | Supplements/Diet | Hypertension? | Other PMHx |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | W/M | 71 | 134 | 41 | Bodybuilding | AASs including M-1-T (testosterone prohormone), GH, and insulin for >10 yr | Creatine, amino acid supplements, glutamate, >550 g/d protein diet | HTN (duration unknown) | Sleep apnea |
| 2 | 31 | W/M | 63 | 102 | 40 | Bodybuilding | AASs including stanzolol and durabolin for 8 yr | High-protein diet (300 to 400 g/d) and protein shakes | HTN for 3 mo | None |
| 3 | 41 | W/M | 68 | 93 | 31 | Bodybuilding | AASs and GH for 20 yr | High-protein shakes | HTN for 5 yr | None |
| 4 | 28 | H/M | 66 | 100 | 36 | Bodybuilding | AASs and GH, “for years” | Creatine for 5 yr | No | None |
| 5 | 49 | W/M | 72 | 114 | 34 | Bodybuilding | AASs “for years” | High-protein diet | No | HIV for 21 yr, on HAART, undetectable viral load, diabetes |
| 6 | 38 | H/M | 71 | 96 | 30 | Bodybuilding | AASs including sustanon durabolin, primobolan, equipoise, and winstrol “for years” | Unknown | No | None |
| 7 | 38 | H/M | 71 | 107 | 33 | Bodybuilding | AASs and GH for 8 to 10 yr | Creatine, amino acid supplements, 500 g/d protein diet | HTN (duration unknown) | Sleep apnea |
| 8 | 33 | H/M | 69 | 81 | 27 | Bodybuilding | AASs “for years” | High-protein diet | HTN (duration unknown) | None |
| 9 | 45 | W/M | 68 | 130 | 43 | Powerlifting | AASs including testosterone ethanate and deca-durabolin for 15 yr | Amino acid supplements, 300 g/d protein diet | HTN for 18 mo | None |
| 10 | 40 | W/M | 67 | 95 | 33 | Bodybuilding | Intramuscular testosterone injections for “many years” | High-protein diet with 5 protein shakes/d | No | Cocaine use, occasional UTIs |
GH, growth hormone; H, Hispanic; HAART, highly active antiretroviral therapy; HTN, hypertension; PMHx, past medical history; UTI, urinary tract infection; W, white.
Laboratory data and physical findings are presented in Table 2. At the time of renal biopsy, patients had variable elevations in serum creatinine, with mean serum creatinine of 3.0 mg/dl (range 1.3 to 7.8 mg/dl) and mean 24-h protein of 10.1 g/d (range 1.3 to 26.3 g/d). Among the 10 patients, five had recorded values for 24-h urine creatinine excretion, which ranged from 2.8 to 3.95 g/d. CrCl ranged from severely decreased (17.0 ml/min) to supranormal (196 ml/min) with an average of 96.2 ml/min. Because of the unusually large body surface area (BSA) of our patient population, values for 24-h urine protein excretion and CrCl that have been corrected for BSA are also presented in Table 2. Three of our 10 patients presented with full nephrotic syndrome, and two additional patients had nephrotic-range proteinuria and hypoalbuminemia in the absence of edema. Microhematuria was present in three of 10 patients, but no significant leukocyturia or casts were detected. Cholesterol levels were elevated in six of eight patients when these were measured (total cholesterol >200 mg/dl); of note, these elevations did not seem to correlate with the presence or absence of full nephrotic syndrome, reflecting known effects of anabolic steroid use on hepatic synthesis of cholesterol.17 Hematocrit at presentation was available for six of 10 patients. Mean hematocrit was 45.2% (range 41 to 50%), at the upper limit of normal range. Erythrocytosis is known to be an adverse effect of AAS use18 and has rarely been reported as a potential cause in development of FSGS.19
Table 2.
Clinical presentation
| Patient | BSA (m2) | Serum Cr (mg/dl) | 24-H Urine Cr (g/d) | 24-H CrCl (ml/min) | CrCl (ml/min per 1.73 m2) | 24-H Urine Protein (g/d) | 24-H Urine Protein (g/d per 1.73 m2) | Albumin (g/dl) | Edema? | Microhematuria? | Cholesterol (mg/dl) | Full Nephrotic Syndrome? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.49 | 2.7 | 3.55 | 91 | 63 | 26.3 | 18.30 | 1.9 | Yes | No | Normal | Yes |
| 2 | 2.03 | 1.5 | NA | 103a | 88a | 6.0 | 5.11 | 2.5 | Yes | Yes | 174 to 231 | Yes |
| 3 | 2.07 | 5.7 | NA | 22a | 19a | 16.0 | 13.40 | 2.2 | No | Yes | 202 | No |
| 4 | 2.08 | 4.2 | NA | 37a | 31a | 17.0 | 14.10 | 1.7 | Yes | No | 190 | Yes |
| 5 | 2.34 | 1.4 | 3.95 | 196 | 145 | 3.9 | 2.88 | 3.7 | No | Yes | Elevated | No |
| 6 | 2.16 | 1.3 | 2.80 | 150 | 120 | 1.3 | 1.04 | wnl | No | No | Elevated | No |
| 7 | 2.26 | 2.2 | 3.23 | 119 | 91 | 9.0 | 6.89 | 3.7 | No | No | 218 | No |
| 8 | 1.97 | 1.4 | NA | 86a | 76a | 4.2 | 3.69 | wnl | No | No | NA | No |
| 9 | 2.38 | 1.6 | 3.20 | 139 | 100 | 5.79 | 4.19 | 4.4 | No | No | Elevated | No |
| 10 | 2.07 | 7.8 | NA | 17a | 14a | 11.1 | 9.28 | 2.5 | No | No | NA | No |
NA, not available; wnl, within normal limits.
aCrCl based on Cockcroft-Gault estimate.
Renal biopsy findings are listed in Table 3. Sampling for light microscopy ranged from six to 61 glomeruli. Nine of 10 patients in our cohort had FSGS, four of whom displayed lesions of perihilar sclerosis and three of whom had collapsing lesions (Figure 3, A through C). One patient had no discrete lesions of segmental sclerosis but did have glomerulomegaly. Global glomerulosclerosis involved a mean of 32% of glomeruli (range 0 to 73%), and segmental sclerosis involved a mean 24% (range 0 to 47%). Tubular atrophy and interstitial fibrosis occupied a mean of 49% of the cortical area sampled (range <5 to 90%), and arteriosclerosis ranged from absent to moderate. Immunofluorescence was performed in all cases. There was weak (trace to 1+) segmental staining for IgM and C3 in areas of sclerosis in seven patients. There was no glomerular positivity for IgG, IgA, C1q, or κ or λ light chains. By electron microscopy, no significant GBM thickening was observed. No immune-type electron-dense deposits were identified in the eight cases studied; however, one case contained rare intramembranous lucencies, possibly representing the sites of resorbed deposits or remodeled GBM. FPE ranged from mild (15%) to severe (95%; Figure 3D). Electron microscopy was available for four of five cases with nephrotic-range proteinuria and hypoalbuminemia, for whom mean 89% FPE was noted (range 80 to 95%).
Table 3.
Renal biopsy findings
| Patient | Light Microscopy |
Electron Microscopy | ||||
|---|---|---|---|---|---|---|
| Pattern | Global Sclerosis | Segmental Sclerosis | TA/IF (%) | Arteriosclerosis | ||
| 1 | FSGS with collapsing features | 16 of 22 | 4 of 22 | 80 | Mild | 95% FPE |
| 2 | FSGS, perihilar variant, glomerulomegaly | 4 of 17 | 8 of 17 | 40 | None | 90% FPE |
| 3 | FSGS NOS | 4 of 7 | 3 of 7 | 85 | Moderate | 80% FPE |
| 4 | FSGS NOS, glomerulomegaly | 4 of 6 | 1 of 6 | 60 | Mild | NA |
| 5 | FSGS NOS, glomerulomegaly | 0 of 13 | 2 of 13 | 15 | Mild | 50% FPE |
| 6 | Glomerulomegaly | 0 of 15 | 0 of 15 | <5 | Mild | Moderate FPE, rare intramembranous lucencies |
| 7 | FSGS with perihilar lesions, focal cellular and collapsing features | 9 of 15 | 3 of 15 | 40 | Mild to moderate | 85% FPE |
| 8 | FSGS with perihilar lesions, focal collapsing features | 7 of 61 | 15 of 61 | 15 | Mild | 15% FPE |
| 9 | FSGS, perihilar variant | 5 of 8 | 2 of 8 | 60 | Moderate | NA |
| 10 | FSGS NOS, glomerulomegaly | 9 of 17 | 6 of 17 | 90 | Moderate | 90% FPE |
NA, not available; NOS, not otherwise specified; TA/IF, tubular atrophy and interstitial fibrosis; FPE, foot process effacement.
Figure 3.
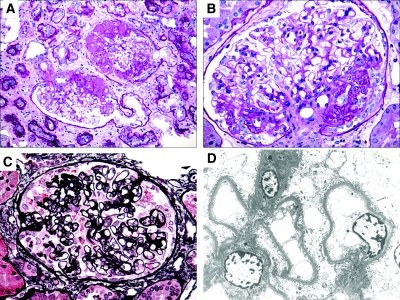
Representative light microscopic and ultrastructural findings are shown. (A) Two hypertrophied glomeruli contain discrete segmental lesions of sclerosis and hyalinosis with adhesions to Bowman's capsule. There is prominent surrounding tubular atrophy and interstitial fibrosis (periodic-acid Schiff). (B) A hypertrophied glomerulus demonstrates perihilar segmental sclerosis that has collapsing features with overlying podocyte hyperplasia (periodic-acid Schiff). (C) A collapsing lesion displays segmental wrinkling and retraction of the GBMs and hyperplasia of podocytes, which contain intracytoplasmic protein resorption droplets. There is a small adhesion to Bowman's capsule (Jones methenamine silver). (D) Electron micrograph from patient 8 with urine protein of 4.2 g/d shows mild FPE involving <20% of the glomerular capillary surface area. Magnifications: ×200 in A; ×400 in B and C; ×2000 in D.
Clinical follow-up was available for eight of 10 patients (mean follow-up time 799 d; range 26 to 2127 d; Table 4). Patient 10 had severe renal impairment and extensive cortical scarring at the time of biopsy and progressed to ESRD within 1 mo of biopsy. The remaining seven patients with available follow-up have received treatment directed to the RAS in the form of an angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, and/or renin inhibitor. Patient 3, who was treated with oral prednisone, was the only patient who received any immunosuppressive therapy. All patients were encouraged to discontinue use of AASs and supplements, decrease dietary protein intake, decrease muscle mass, and reduce their exercise regimens. All seven patients experienced stabilization or improvement in serum creatinine and a reduction in proteinuria. Excluding the patient who progressed to ESRD, average creatinine decreased from 2.34 to 1.61 mg/dl, and mean 24-h urine protein declined from 9.47 to 1.83 g after discontinuation of AASs. The single patient who subsequently resumed AAS abuse developed progressive renal insufficiency and marked increase in proteinuria.
Table 4.
Clinical follow-up
| Patient | Follow-up (d) | Serum Cr at Biopsy (mg/dl) | Follow-up Serum Cr (mg/dl) | Δ Serum Cr (mg/dl) | Proteinuria at biopsy (g/d) | Follow-up Proteinuria (g/d) | Δ Proteinuria (g/d) | Medical Treatment | Lifestyle Changes |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 320 | 2.7 | 1.3 | −1.4 | 26.3 | 0.15 | −26.2 | RAS blockade | Stopped AASs, hormones, and supplements; decreased exercise; lost 80 lb |
| 1728 | 2.7 | 2.4 | −0.3 (+1.1)a | 26.3 | 14.1 | −12.2 (+14.0)a | Unable to tolerate changes because of poor body image; restarted AASs, supplements, and exercise | ||
| 2 | 427 | 1.5 | 1.4 | −0.1 | 6.0 | 4.5b | −1.5 | RAS blockade, verapamil | Decreased exercise, stopped AASs, decreased dietary protein, lost 20 lb |
| 3 | 948 | 5.7 | 2.4 | −3.3 | 16.0 | 6.0 | −10.0 | High-dosage prednisone, RAS blockade | Stopped AASs, growth hormone, and supplements |
| 4 | NA | 4.2 | NA | NA | 17.0 | NA | NA | NA | NA |
| 5 | NA | 1.4 | NA | NA | 3.9 | NA | NA | NA | NA |
| 6 | 308 | 1.3 | 1.3 | 0.0 | 1.3 | 0.3 | −1.0 | RAS blockade | Stopped AASs; lost 16 lb initially but unable to tolerate weight loss because of poor body image |
| 7 | 780 | 2.2 | 2.0 | −0.2 | 9.0 | 0.8b | −8.2 | RAS blockade | Mild decrease in exercise and dietary protein, stopped AASs; initially lost 20 lb but regained weight because he perceived himself as “too skinny” |
| 8 | 2127 | 1.4 | 1.3 | −0.1 | 1.9 | 0.05 | −1.8 | RAS blockade, fish oil | Stopped AASs, lost 15 lb |
| 9 | 49 | 1.6 | 1.6 | 0.0 | 5.8 | 1.0b | −4.8 | RAS blockade, amlodipine | Stopped AASs and supplements, decreased exercise, lost 20 lb |
| 10 | 26 | 7.8 | 9.1 | +1.3 | 11.1 | 3+ by dipstick | NA | Metoprolol | Continued exercise and protein supplements |
NA, not available.
aRepresents interval change after resumption of AASs.
bRepresents proteinuria estimated by spot ratio of urine protein/urine creatinine.
Discussion
The National Institutes of Health define obesity as BMI ≥30 kg/m2. The vast majority of people who meet this criterion have elevated body fat content; however, patients with dramatically increased lean body mass may have a significantly elevated BMI and subnormal body fat content. Obesity is a widely recognized risk factor for renal disease and a common cause of secondary FSGS, but reports of similar pathology in highly muscular patients are rare,15 as are reports of ESRD in bodybuilders.20
Clinically, patients with postadaptive FSGS typically develop subnephrotic proteinuria or nephrotic-range proteinuria without full nephrotic syndrome. They usually lack hypoalbuminemia and edema. Depending on the stage of disease at presentation, GFR in obese patients with FSGS ranges from supranormal (in early stages) to reduced. In our cohort, three of 10 patients presented with full nephrotic syndrome, and five of the remaining seven had nephrotic-range proteinuria. CrCl exceeded 110 ml/min in four of 10 patients, indicating relative renal hyperfiltration, whereas the remaining patients had CrCls ranging from minimally to severely decreased.
Importantly, the estimation of CrCl in this cohort is potentially limited by two factors. For patients without measured 24-h urine creatinine, the Cockcroft-Gault estimate was used. Although this equation has reasonable validity in most patients,21 its accuracy in patients with extremely muscular body composition has not been established. In addition, several patients reported taking creatine supplements, and because creatine is readily converted into creatinine, this presents a potential problem in using creatinine measurements to estimate GFR. Creatine supplementation is associated with a slight increase in both serum and urine creatinine levels, although these changes are negated in CrCl calculations that are based on 24-h urine creatinine measurement and a simultaneous serum creatinine. In patients whose clearance is estimated using serum creatinine only, creatine supplementation may cause a mild increase in serum creatinine and a corresponding mild underestimation of CrCl.22
The classic histologic findings in secondary FSGS mediated by adaptive responses are glomerular hypertrophy and predominantly perihilar lesions of segmental sclerosis with typically ≤50% glomerular FPE on ultrastructural examination.23 Whereas four of the patients in our cohort had perihilar lesions and five showed evidence of glomerulomegaly in nonsclerotic glomeruli, three patients had segmental lesions with collapsing features. The collapsing subtype is an unusual finding in postadaptive FSGS, and in a series of 71 patients with ORG, only one patient had collapsing lesions.11 Electron microscopy was available for eight of 10 patients in this study and showed highly variable podocyte FPE ranging from 15 to 95%. Five patients had FPE >50%, which is uncommon in cases of postadaptive FSGS.
The clinical features and biopsy findings in this cohort are unconventional for postadaptive FSGS because of the relatively high incidence of full nephrotic syndrome, presence of collapsing or cellular lesions of FSGS, advanced fibrosis and glomerulosclerosis, and the high degree of FPE in some patients. When compared with patients with ORG,11 our cohort of bodybuilders who used AASs had more severe proteinuria (mean 10.1 versus 4.09 g/d) and higher serum creatinine at biopsy (mean 3.0 versus 1.47 mg/dl) despite lower mean BMI (34.7 versus 41.7). In comparing renal biopsy findings between these two groups, the bodybuilders had more advanced global glomerulosclerosis (mean 32 versus 20%), a higher percentage of segmentally sclerotic glomeruli (mean 24 versus 10%), more tubulointerstitial scarring (mean 69 versus <25%), and more extensive podocyte FPE (mean 69 versus 40%). The greater severity of clinical and biopsy findings in bodybuilders suggests that additional factors are modifying the typical clinical and pathologic features seen in purely postadaptive FSGS, leading us to hypothesize AAS abuse also has direct nephrotoxic effects. The response in seven of eight patients to stopping AASs, decreasing exercise, and inhibiting the RAS provides strong evidence that these cases do indeed represent a secondary FSGS. Of note, none of our patients was black, the most common racial group to develop primary FSGS.
Postadaptive forms of FSGS are usually due to structural-functional adaptations driven by increased hemodynamic stress on the glomerulus. Animal models suggest that podocyte depletion plays a key role in postadaptive models of FSGS.24 Increased body mass requires an increase in glomerular filtration. In an attempt to meet these demands, individual glomeruli adapt to hyperfiltration through hypertrophy. Podocytes are terminally differentiated cells that cannot proliferate, and in the process of compensatory glomerular hypertrophy, podocyte connections to the GBM become mechanically strained. If these conditions persist, then podocytes eventually detach from the GBM, leading to development of a segmental scar.25
Aside from increased lean body mass, our cohort of bodybuilders has additional factors that could exert stress on glomeruli. Diets high in protein cause marked changes in renal hemodynamics, both acutely and chronically. Protein ingestion seems to cause an increase in renal blood flow and GFR by a variety of mechanisms.26 Although this is an appropriate adaptive response to the increase in nitrogenous waste that is the byproduct of protein metabolism, chronic hyperfiltration from a high-protein diet may accelerate progression to glomerulosclerosis.
Six of our 10 patients had elevated BP or a history of hypertension at the time of renal biopsy. Although chronic systemic hypertension may have contributed to the renal disease in our cohort, most of our patients had only mild arteriosclerosis on biopsy, arguing against hypertensive arterionephrosclerosis as the major process. In this unique population, the potentially extreme episodic elevations in BP that are experienced during heavy weightlifting may also have contributed to renal disease. Although cardiovascular benefits of exercise are seen with both aerobic and resistance training, weightlifting can elevate systolic BP up to 400 mmHg, which has the potential to inflict endothelial damage and impair endothelial function.27
Although the potential effects of AASs on renal function have not been well characterized in humans, several studies suggest that androgens may exert a direct toxic effect on glomerular cells, leading to mesangial matrix accumulation and podocyte depletion independent of structural-functional adaptations. Men are known to be at an overall increased risk for cardiovascular and renal disease compared with women, and prognosis in men is worse for various types of chronic kidney disease. The sources for these differences are under investigation, and there is mounting evidence that androgens may play a central role in both normal and diseased kidney function and that estrogens are protective. In a model of adriamycin nephropathy, male rats developed more glomerulosclerosis than did female rats, and treatment of female rats with either ovariectomy or exogenous testosterone administration caused increased albuminuria and accelerated mesangial sclerosis.28 Androgen receptors have been identified in microdissected murine glomeruli and cultured mesangial cells. Exogenous testosterone administration increased androgen receptor expression, as well as mRNA levels of the profibrotic cytokine TGF-β1.29 In addition, transgenic models support that overexpression of TGF-β1 provides a potent proapoptotic stimulus to podocytes, promoting FSGS.30 Androgens are also known to induce oxidative stress and upregulate components of the RAS.31,32 An animal model of hypertension-induced end-organ damage showed significant attenuation of renal damage by androgen receptor blockade with flutamide.33 In uninephrectomy models, male rats developed greater proteinuria and mesangial sclerosis than did female rats.34 Testosterone seems to amplify compensatory glomerular and tubular growth, which may promote glomerulosclerosis.35 As yet, no human studies have directly addressed the potential role of androgens in podocyte stress or as predisposing hemodynamic factors in the development of FSGS.
The increasing prevalence of AAS use among athletes has received extensive coverage in the lay press. A potential causative role for long-term AAS abuse in the development of secondary FSGS, beyond their ability to increase lean body mass, seems likely on the basis of their diverse systemic effects. Given the pervasive use of AASs among athletes, it is likely that additional genetic and environmental factors are required to produce clinically significant renal disease; however, proteinuria and mild renal insufficiency are often asymptomatic, and unless athletes who use AASs and other performance-enhancing drugs and supplements are regularly screened, the early stages of secondary FSGS are likely to be underdiagnosed. This is especially the case in bodybuilders, for whom serum creatinine levels would be predicted to rise as a normal physiologic response to increased muscle mass.
In summary, we present the first series of FSGS in bodybuilders who abused AASs. These patients may present with either asymptomatic proteinuria or full nephrotic syndrome and heterogeneous histologic variants of FSGS. Although these patients responded well to discontinuation of AAS use, weight loss, and RAS blockade, others may progress to ESRD. Elevated lean body mass and long-term AAS abuse should be added to the list of causes of secondary FSGS.
Concise Methods
A cohort of 10 patients (including the index case) were identified from the archives of the Columbia Renal Pathology Laboratory between 1999 and 2009. One of these cases was provided by the Department of Pathology of Massachusetts General Hospital. Approval for review of patient biopsies and medical records was received from the institutional review board of Columbia University Medical Center. All patients identified themselves as engaging in either bodybuilding or competitive weightlifting/strength competitions and admitted to taking AASs and various combinations of dietary supplements. Patient records were reviewed for age, gender, ethnicity, renal presentation, medical history, and AAS and nutritional supplement use.
The National Institutes of Health define overweight as BMI of 25.0 to 29.9 kg/m2 and obese as BMI ≥30.0 kg/m2 in the general population.36 Most patients with BMI >25.0 kg/m2 have a body fat >20%37; however, elevated BMI as a result of increased lean body mass is not associated with increased percentage of body fat. Entry criteria for this study included (1) BMI ≥25 kg/m2; (2) assessment of physical appearance as “highly muscular” by the referring nephrologist in association with patient participation in bodybuilding/weightlifting; (3) long-term AAS use for years; (4) proteinuria (≥1 g/d) with or without full nephrotic syndrome (defined as nephrotic-range proteinuria ≥3.5 g/d per 1.73 m2, serum albumin ≤3.5 g/dl, and edema); and (5) renal biopsy diagnosis of FSGS and/or glomerulomegaly. In all patients, other potential causes of secondary FSGS were evaluated, including history of vesicoureteral reflux, renal agenesis, and other chronic renal diseases.
BSA was calculated using the Gehan and George method.38 BMI was calculated using the formula BMI = weight (kg)/height (m)2. CrCl at the time of biopsy was calculated from a 24-h urine collection using the formula CrCl (ml/min) = (urine creatinine mg/d)/(plasma creatinine [mg/dl]) × 0.07. In the absence of a 24-h urine collection, CrCl was estimated using the Cockcroft-Gault equation: [(140 − age)/(plasma creatinine in mg/dl)] × (body weight in kg/72).
All renal biopsy samples were processed according to standard techniques for light microscopy, immunofluorescence, and electron microscopy. In two cases (patients 4 and 9), there was insufficient tissue for electron microscopy. Glomerulomegaly was defined as mean glomerular diameter >1.4 times normal age-matched control, as described previously.10 FPE was estimated as the percentage of glomerular capillary surface area of patent capillaries exhibiting effacement.
Disclosures
None.
Acknowledgments
We thank patient 1 for kindly submitting his photograph for Figure 1.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, Kuhn JA, Dratch AD, D'Agati VD: Collapsing focal segmental glomerulosclerosis following treatment with high dose pamidronate. J Am Soc Nephrol 12: 1164–1172, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Lindstrom M, Nilsson A, Katzman P, Janzon I, Dymling J: Use of anabolic-androgenic steroids among body builders: Frequency and attitudes. J Intern Med 225: 407–411, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Sjoqvist F, Garle M, Rane A: Use of doping agents, particularly anabolic steroids, in sports and society. Lancet 371: 1872–1882, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bonetti A, Tirelli F, Catapano A, Dazzi D, Dei Cas A, Solito F, Ceda G, Reverberi C, Monica C, Pipitone S, Elia G, Spattini M, Magnati G: Side effects of anabolic androgenic steroids abuse. Int J Sports Med 29: 679–687, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Martin NM, Abu Dayyeh BK, Chung RT: Anabolic steroid abuse causing recurrent hepatic adenomas and hemorrhage. World J Gastroenterol 14: 4573–4575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaver KM, Vaughn MG, DeLisi M, Wright JP: Anabolic-androgenic steroid use and involvement in violent behavior in a nationally representative sample of young adult males in the United States. Am J Public Health 98: 2185–2187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiblin I, Petersson A: Pharmacoepidemiology of anabolic androgenic steroids: A review. Fundam Clin Pharmacol 19: 27–44, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Kiprov DD, Colvin RB, McCluskey RT: Focal segmental glomerulosclerosis and proteinuria associated with unilateral renal agenesis. Lab Invest 46: 275–281, 1982 [PubMed] [Google Scholar]
- 9.Rennke HG, Klein PS: Pathogenesis and significance of nonprimary focal and segmental glomerulosclerosis. Am J Kidney Dis 13: 443–456, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Hodgin JB, Rasoulpour M, Markowitz GS, D'Agati VD: Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 4: 71–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD: Obesity-related glomerulopathy: An emerging epidemic. Kidney Int 59: 1498–1509, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Praga M, Hernandez E, Morales E, Campos AP, Valero MA, Martinez MA, Leon M: Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant 16: 1790–1798, 2001 [DOI] [PubMed] [Google Scholar]
- 13.D'Agati VD: The many masks of focal segmental glomerulosclerosis. Kidney Int 46: 1223–1241, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Praga M, Hernandez E, Andres A, Leon M, Ruilope LM, Rodicio JL: Effects of body-weight loss and captopril treatment on proteinuria associated with obesity. Nephron 70: 35–41, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Schwimmer JA, Markowitz GS, Valeri AM, Imbriano LJ, Alvis R, D'Agati VD: Secondary focal segmental glomerulosclerosis in non-obese patients with increased muscle mass. Clin Nephrol 60: 233–241, 2003 [PubMed] [Google Scholar]
- 16.Tanji N, Ross MD, Tanji K, Bruggeman L, Markowitz G, Klotman PE, D'Agati VD: Detection and localization of HIV-1 DNA in renal tissues by in situ polymerase chain reaction. Histol Histopathol 21: 393–401, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Thompson PD, Cullinane EM, Sady SP, Chenevert C, Sartelli AL, Sady MA, Herbert PN: Contrasting effects of testosterone and stanozol on serum lipoprotein levels. JAMA 19892611165–1168. [PubMed] [Google Scholar]
- 18.Rhoden EL, Morgentaler A: Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 350: 482–492, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Okuyama S, Hamai K, Fujishima M, Ohtani H, Komatsuda A, Sawada K, Wakui H: Focal segmental glomerulosclerosis associated with polycythemia vera: Report of a case and review of the literature. Clin Nephrol 68: 412–415, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Hartung R, Gerth J, Funfstuck R, Grone HJ, Stein G: End-stage renal disease in a body builder: A multifactorial process or simply doping? Nephrol Dial Transplant 16: 163–165, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Shoker A, Hossain MA, Koru-Sengul T, Raju DL, Cockroft D: Performance of creatinine clearance equations on the original Cockroft-Gault population. Clin Nephrol 66: 89–97, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Pline KA, Smith CL: The effect of creatine intake on renal function. Ann Pharmacol 39: 1093–1096, 2005 [DOI] [PubMed] [Google Scholar]
- 23.D'Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 43: 368–382, 2004 [DOI] [PubMed] [Google Scholar]
- 24.D'Agati VD: Podocyte injury in focal segmental glomerulosclerosis: Lessons from animal models (a play in five acts). Kidney Int 73: 399–406, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Woods LL: Mechanisms of renal hemodynamic regulation in response to protein feeding. Kidney Int 44: 659–675, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Phillips SA, Syed AQ, Syed AY, Pitt S, Weaver A, Gutterman DD: The effect of exertional hypertension evoked by weight lifting on vascular endothelial function. J Am Col Cardiol 48: 588–589, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Sakemi T, Ohtsuka N, Tomiyoshi Y, Morito F: The ovaries attenuate the aggravating effect of testosterone on glomerular injury in adriamycin-induced nephropathy of female rats. Kidney Blood Press Res 20: 44–50, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Elliot SJ, Berho M, Korach K, Doublier S, Lupia E, Striker GE, Karl M: Gender-specific effects of endogenous testosterone: Female α-estrogen receptor-deficient C57Bl/6J mice develop glomerulosclerosis. Kidney Int 72: 464–472, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Schiffer M, Bitzer M, Roberts I, Kopp J, Dijke P, Mundel P, Bottinger E: Apoptosis in podocytes induced by TGF-B and Smad7. J Clin Invest 108: 807–816, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iliescu R, Cucchiarelli VE, Yanes LL, Iles JW, Reckelhoff JF: Impact of androgen-induced oxidative stress of hypertension in male SHR. Am J Physiol Regul Integr Comp Physiol 292: R731–R735, 2007 [DOI] [PubMed] [Google Scholar]
- 32.McGuire BB, Watson RW, Perez-Barrioncanal F, Fitzpatrick JM, Docherty NG: Gender differences in the renin angiotensin and nitric oxide systems: Relevance in the normal and diseased kidney. Kidney Blood Press Res 30: 67–80, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Baltatu O, Cayla C, Iliescu R, Andreev D, Jordan C, Bader M: Abolition of hypertension-induced end-organ damage by androgen receptor blockage in transgenic rats harboring the mouse Ren-2 gene. J Am Soc Nephrol 13: 2681–2687, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Gafter U, Ben-Bassat M, Levi J: Castration inhibits glomerular hypertrophy and proteinuria in uninephrectomized male rats. Eur J Clin Invest 20: 360–365, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Zeier M, Schonherr R, Amann K, Ritz E: Effects of testosterone on glomerular growth after uninephrectomy. Nephrol Dial Transplant 13: 2234–2240, 1998 [DOI] [PubMed] [Google Scholar]
- 36.National Institutes of Health: Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults, Bethesda, National Institutes of Health, 1998. NIH Publication No. 98-4083 [Google Scholar]
- 37.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y: Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am J Clin Nutr 72: 694–701, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Gehan EA, George SL: Estimation of human body surface area from height and weight. Cancer Chemother Rep 54: 225–235, 1970 [PubMed] [Google Scholar]