Abstract
The spread of zoonotic infectious diseases may increase due to climate factors such as temperature, humidity and precipitation. This is also true for hantaviruses, which are globally spread haemorrhagic fever viruses carried by rodents. Hantaviruses are frequently transmitted to humans all over the world and regarded as emerging viral diseases. Climate variations affect the rodent reservoir populations and rodent population peaks coincide with increased number of human cases of hantavirus infections. In northern Sweden, a form of haemorrhagic fever called nephropathia epidemica (NE), caused by the Puumala hantavirus (PUUV) is endemic and during 2006–2007 an unexpected, sudden and large outbreak of NE occurred in this region. The incidence was 313 cases/100,000 inhabitants in the most endemic areas, and from January through March 2007 the outbreak had a dramatic and sudden start with 474 cases in the endemic region alone. The PUUV rodent reservoir is bank voles and immediately before and during the peak of disease outbreak the affected regions experienced extreme climate conditions with a record-breaking warm winter, registering temperatures 6–9°C above normal. No protective snow cover was present before the outbreak and more bank voles than normal came in contact with humans inside or in close to human dwellings. These extreme climate conditions most probably affected the rodent reservoir and are important factors for the severity of the outbreak.
Keywords: hantavirus, climate, bank vole, Sweden
Viewpoint
Climate change may affect the spread of zoonotic infectious diseases in several ways. The geographic distribution and thereby the population at risk as well as the transmission of these diseases may increase due to climate factors such as temperature, humidity and precipitation (1). The group of zoonotic infectious diseases includes large and increasing number of bacterial, viral and parasitic infections, each with its specific characteristics, reservoir host and mode of transmission (2–6). Several of these infections are recognised as emerging diseases and pose a serious threat to human public health (7). These diseases are most commonly transmitted to humans either directly from animals (e.g. rodents) (4) or by vectors (e.g. mosquitoes and ticks) (2, 3, 8).
Hantaviruses are globally spread haemorrhagic fever viruses carried by various rodents (9). These viruses are frequently transmitted to humans globally and are recognised as emerging viral diseases. Hantaviruses can cause two febrile illnesses in humans: haemorrhagic fever with renal syndrome (HFRS) in Asia and Europe and hantavirus cardiopulmonary syndrome (HCPS) in the Americas, depending on the species of hantavirus (9). HFRS accounts for more than 200,000 cases and thousands of deaths annually, whereas HCPS is much less frequent although more severe with a mortality rate above 40% (9). Severe forms of HFRS are prevalent in China and Korea (Haantan virus) and the Balkans (Dobrava virus) and a milder form, nephropathia epidemica (NE), is most prevalent in Russia, Finland, Sweden, Germany, France, Belgium and Norway. NE is caused by Puumala virus (PUUV) and is transmitted to humans via inhalation of infectious aerosols containing rodent saliva, urine and/or faeces from bank voles (Myodes glareolus) (10).
The bank vole is one of the most abundant mammal species in Europe. Variations in availability of plants and berries due to climate variations affect the vole populations, and in Central Europe these abundance peaks are often related to high tree seed production, which is supposedly triggered by specific weather conditions (11, 12). In northern Fennoscandia, the bank vole population shows pronounced seasonal and multi-annual fluctuations in population density (13). The cyclic activity of the population dynamics has been explained by variations in predator populations and availability of food (14). The voles serve as prey for several predators, making them an important part in the ecological web in Fennoscandia (15). The bank vole population peaks coincide with increased number of human cases of hantavirus infections (16). In contrast to humans, rodent reservoirs become persistently infected showing no signs of disease or clinical pathology. In humans, after exposure to PUUV there is a 1–5 week incubation period before the NE disease symptoms appear. The disease is characterised by acute onset of high fever, headache, backache, myalgia and abdominal pain (17). Renal impairment is common and there is increasing evidence for a correlation between previous PUUV infection and later chronic renal affection and hypertension. One-third of the patients in Sweden have haemorrhagic symptoms and severe bleedings may occur. Some patients with more severe forms of HFRS (5–10%) die from hypotensive shock or bleeding.
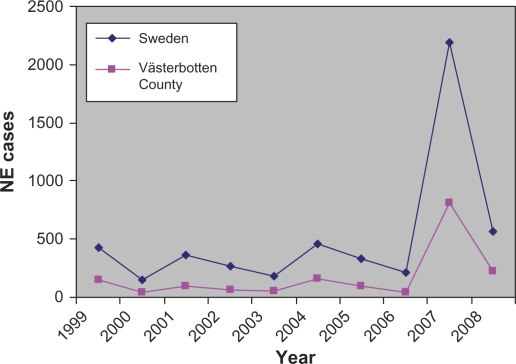
During the last days of December 2006 and the first months of 2007, northern Sweden experienced a sudden and large outbreak of NE with high numbers of NE patients that resulted in a considerable burden on public health services (18). According to the Swedish Communicable Disease Act, NE must be reported and records show that the outbreak peaked during the first three months of 2007 (Fig. 1). In Sweden during 2007 and during 2008, a record-breaking 2,195 NE cases were recorded and more NE cases were diagnosed although not in the similar frequencies as in early 2007. The incidence in one of the most endemic areas – Västerbotten County for 2007 – was 313 recognised cases/100,000 inhabitants. In Sweden during the peak (January–March 2007), 972 cases were recorded, 474 cases in Västerbotten County alone. The NE patients showed all the classical HFRS symptoms and displayed the whole spectra from mild to severe disease requiring hospitalisation and occasionally intensive care. Of the diagnosed patients, 30% were hospitalised and three known fatalities (0.24% case fatality) were recorded in the two most northern counties in Sweden during 2007. Previous NE peaks in 1999 (73/100,000), 2002 (38/100,000) and 2005 (61/100,000) had much lower incidence compared to the record year 2007 (313/100,000) (Fig. 1) even though the bank vole prevalence was similar (18, 19).
Fig. 1.
Annual incidence of human hantavirus infection in Sweden and Västerbotten County from 1999 through 2008.
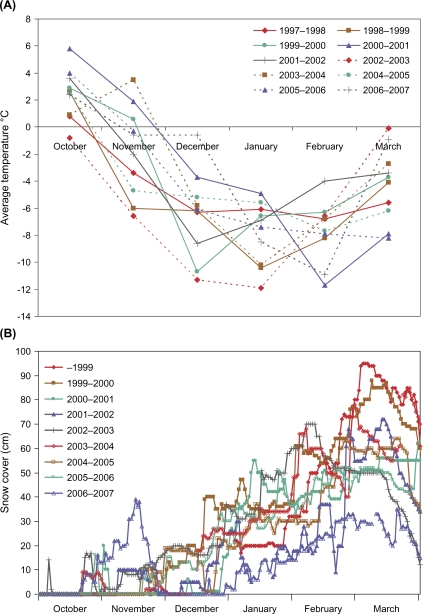
One important factor that influences hantavirus transmission to humans is the increased exposure of humans to infected rodent excreta. Several reports from inhabitants in areas where bank voles normally dwell indicated that more bank voles were found in traps inside houses than normal (18). This led to an investigation regarding the weather conditions during this period: interestingly, December 2006 was exceptional with respect to the mild weather with no or very little snow and hard ice-cover in the coastal area of northern Sweden (Fig. 2A). During two previous NE peak periods (2001–2002 and 2004–2005), when records of snow cover were available, the ground was already covered in early winter (Fig. 2A). In December 2006, Sweden experienced an average temperature 4.5–9.5°C warmer than normal and in the most endemic region, Västerbotten County, the average temperature for December was 6.0–9.0°C warmer than normal (Fig. 2B). In previous peak years (December 2001–2002 and 2004–2005), the average temperature was normal (18).
Fig. 2.
Climate conditions December 1999–2007 in the NE outbreak area of Västerbotten County. The figures show number of days with snow cover and the average temperature. Snow cover was defined as snow depth >0 cm. The measurements were made in locations approximately 30 km from the coast.
In Sweden during 2007, the NE outbreak seemed to have at least two main determinants, a peak year for bank voles and an extreme climate in the affected regions with a record-breaking warm winter that registered temperatures 6–9°C above normal. The number of NE cases depends on the size of the vole population, which peaks every third to fourth year (16, 20) and an increase in the bank vole population was reported in northern Sweden in the autumn of 2006, similar to the preceding autumn of two NE peaks (1998–1999 and 2004–2005) (19). Thus the bank vole population was high, but not more than previous peak years and could not alone explain the very high number of NE cases in early 2007.
Snow cover, an important factor for bank vole survival, provides insulation from the cold, protection from predators and access to food below the snow (13). Lately, the winters in the regions in northern Sweden where most cases of NE occur have been unusually mild (21). They have been characterised by late snowfall, periods with no snow and sometimes rain followed by freezing temperatures (21) destroying the protective effect of the snow cover. These extreme climate conditions with absence of the snow cover in northern Sweden during December 2006 and January 2007 probably contributed to the transmission of PUUV from bank voles to humans. The number of NE cases among humans as well as the PUUV prevalence among bank voles is linked to variables favouring the survival of the virus in the environment, such as indirect transmission facilitated by low winter temperatures (22). The increased PUUV transmission in northern Sweden seemed to be facilitated by more frequent contact between bank voles and humans. It is conceivable that during December 2006, when there was no snow cover for a long period, bank voles sought refuge in barns and houses and other buildings, a behaviour that increased the exposure for humans. In 2007 and 2008, there were still rather high numbers of diagnosed NE patients (Fig. 2), but these increases most probably were related to the bank vole population peak during this period (23). Furthermore, the food availability for bank voles could also be affected by climate conditions. In Belgium, the relationship between tree seed production, climate and NE incidence has been analysed and NE epidemics are preceded by abundant tree seed production (24). Moreover, a direct link between climate and NE incidence was found, with high summer and autumn temperatures, two years and one year, respectively, before NE occurrence, related to high NE incidence (24). Of course, another possible factor for the outbreak could be that the virus itself has mutated and to study this we have compared the PUUV genome from 2007 with previous years. Virus RNA from three patient samples were sequenced and revealed that the obtained sequences were highly homologous to previous rodent PUUV isolates from the area (18, 25).
The bank vole and other rodents play an important role as reservoir for many pathogens that may cause disease in humans and domestic animals (26). Many of these pathogens are recognised as emerging zoonotic infections (7). Climatic changes may greatly affect the rodent population size and behaviour, increasing the risk for transmission of several human pathogens (1, 4). Similarly, other vector-borne infections may increase due to climate change (1, 2, 5, 8). To minimise threats to public health and to maximise preparedness and proper actions, surveillance of reservoirs, vectors and pathogens are of the greatest importance. Attempts to forecast disease outbreaks have focused on the impact of climate variability on infectious diseases (27). Using El Niño/Southern Oscillation-related climate anomalies, vector-borne infections – such as dengue fever, malaria, Rift Valley fever, West Nile fever and hantavirus disease – have been predicted to occur in several parts of the world (28). For the vector-borne Rift Valley fever virus, the risk-mapping model using these climate data predicted areas where outbreaks of Rift Valley fever in humans and animals were expected and occurred in the Horn of Africa from December 2006 to May 2007 (29).
Conclusion
A combination of a mild climate at the beginning of winter, loss of protective snow cover and high rodent reservoir numbers likely caused the sudden and dramatic increase of NE cases in the winter 2006/2007 in northern Sweden. The globally spread, rodent-borne hantaviruses should be regarded as an increasing threat to health since future climate change scenarios predict higher temperatures.
Acknowledgements
This project was supported by the County Councils of northern Sweden and the Medical Faculty of Umeå University.
Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this study.
References
- 1.Semenza JC, Menne B. Climate change and infectious diseases in Europe. Lancet Infect Dis. 2009;9:365–75. doi: 10.1016/S1473-3099(09)70104-5. [DOI] [PubMed] [Google Scholar]
- 2.Gould EA, Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg. 2009;103:109–21. doi: 10.1016/j.trstmh.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaenson TG, Eisen L, Comstedt P, Mejlon HA, Lindgren E, Bergström S, et al. Risk indicators for the tick Ixodes ricinus and Borrelia burgdorferi sensu lato in Sweden. Med Vet Entomol. 2009;23:226–37. doi: 10.1111/j.1365-2915.2009.00813.x. [DOI] [PubMed] [Google Scholar]
- 4.Klempa B. Hantaviruses and climate change. Clin Microbiol Infect. 2009;15:518–23. doi: 10.1111/j.1469-0691.2009.02848.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakazawa Y, Williams R, Peterson AT, Mead P, Staples E, Gage KL. Climate change effects on plague and tularemia in the United States. Vector Borne Zoonotic Dis. 2007;7:529–40. doi: 10.1089/vbz.2007.0125. [DOI] [PubMed] [Google Scholar]
- 6.Patz JA, Graczyk TK, Geller N, Vittor AY. Effects of environmental change on emerging parasitic diseases. Int J Parasitol. 2000;30:1395–405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 7.Kuiken T, Leighton FA, Fouchier RA, LeDuc JW, Peiris JS, Schudel A, et al. Public health. Pathogen surveillance in animals. Science. 2005;309:1680–1. doi: 10.1126/science.1113310. [DOI] [PubMed] [Google Scholar]
- 8.Zeman P, Beneś C. A tick-borne encephalitis ceiling in Central Europe has moved upwards during the last 30 years: possible impact of global warming? Int J Med Microbiol. 2006;293:48–54. doi: 10.1016/s1433-1128(04)80008-1. [DOI] [PubMed] [Google Scholar]
- 9.Schmaljohn C, Hjelle B. Hantaviruses a global disease problem. Emerg Infect Dis. 1997;3:95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vapalahti O, Mustonen J, Lundkvist Å, Henttonen H, Plyusnin A, Vaheri A. Hantavirus infections in Europe. Lancet Infect Dis. 2003;3:653–61. doi: 10.1016/s1473-3099(03)00774-6. [DOI] [PubMed] [Google Scholar]
- 11.Piechotowski I, Brockmann SO, Schwarz C, Winter CH, Ranft U, Pfaff G. Emergence of hantavirus in South Germany: rodents, climate and human infections. Parasitol Res. 2008;103:S131–7. doi: 10.1007/s00436-008-1055-8. [DOI] [PubMed] [Google Scholar]
- 12.Clement J, Vercauteren J, Verstraeten WW, Ducoffre G, Barrios JM, Vandamme AM, et al. Relating increasing hantavirus incidences to the changing climate: the mast connection. Int J Health Geogr. 2009;8:1. doi: 10.1186/1476-072X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansson I, Henttonen H. Gradients in density variations of small rodents: the importance of latitude and snow cover. Oecologia. 1985;67:394–402. doi: 10.1007/BF00384946. [DOI] [PubMed] [Google Scholar]
- 14.Crespin L, Verhagen R, Stenseth NC, Yoccoz NG, Prévot-Julliard A-C, Lebreton J-D. Survival in fluctuating bank vole populations: seasonal and yearly variations. Oikos. 2002;98:467–79. [Google Scholar]
- 15.Hörnfeldt B. Cycles of voles, predators, and alternative prey in boreal Sweden. Umeå, Sweden: Umeå University Dissertation; 1991. [Google Scholar]
- 16.Olsson GE, Dalerum F, Hörnfeldt B, Elgh F, Palo TR, Juto P, et al. Human hantavirus infections, Sweden. Emerg Infect Dis. 2003;9:1395–401. doi: 10.3201/eid0911.030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Settergren B. Clinical aspects of nephropathia epidemica (Puumala virus infection) in Europe: a review. Scand J Infect Dis. 2000;32:125–32. doi: 10.1080/003655400750045204. [DOI] [PubMed] [Google Scholar]
- 18.Pettersson L, Boman J, Juto P, Evander M, Ahlm C. Outbreak of Puumala virus infection, Sweden. Emerg Infect Dis. 2008;14:808–10. doi: 10.3201/eid1405.071124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsson GE, Hörnfeldt B, Hjertkvist M, Lundkvist Å. Nephropathia epidemica: high risk in Norrland during winter. Läkartidningen. 2007;104:3450–3. [In Swedish] [PubMed] [Google Scholar]
- 20.Brummer-Korvenkontio M, Vapalahti O, Henttonen H, Koskela P, Kuusisto P, Vaheri A. Epidemiological study of nephropathia epidemica in Finland 1989–96. Scand J Infect Dis. 1999;31:427–35. doi: 10.1080/00365549950163941. [DOI] [PubMed] [Google Scholar]
- 21.Alexandersson H. Temperature and precipitation in Sweden 1860/2002. Norrköping: SMHI; 2002. Meteorologi rapport 104. [In Swedish with English abstract] [Google Scholar]
- 22.Linard C, Tersago K, Leirs H, Lambin EF. Environmental conditions and puumala virus transmission in Belgium. Int J Health Geogr. 2007;6:55. doi: 10.1186/1476-072X-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsson GE, Hjertqvist M, Lundkvist A, Hörnfeldt B. Predicting high risk for human hantavirus infections, Sweden. Emerg Infect Dis. 2009;15:104–6. doi: 10.3201/eid1501.080502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tersago K, Verhagen R, Servais A, Heyman P, Ducoffre G, Leirs H. Hantavirus disease (nephropathia epidemica) in Belgium: effects of tree seed production and climate. Epidemiol Infect. 2009;137:250–6. doi: 10.1017/S0950268808000940. [DOI] [PubMed] [Google Scholar]
- 25.Pettersson L, Klingström J, Hardestam J, Lundkvist A, Ahlm C, Evander M. Hantavirus RNA in saliva from patients with hemorrhagic fever with renal syndrome. Emerg Infect Dis. 2008;14:406–11. doi: 10.3201/eid1403.071242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis S, Calvet E, Leirs H. Fluctuating rodent populations and risk to humans from rodent-borne zoonoses. Vector Borne Zoonotic Dis. 2005;5:305–14. doi: 10.1089/vbz.2005.5.305. [DOI] [PubMed] [Google Scholar]
- 27.Epstein P. Climate change and infectious disease; stormy weather ahead. Epidemiology. 2002;13:373–5. doi: 10.1097/00001648-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Anyamba A, Chretien JP, Small J, Tucker CJ, Linthicum KJ. Developing global climate anomalies suggest potential disease risks for 2006–2007. Int J Health Geogr. 2006;5:60. doi: 10.1186/1476-072X-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anyamba A, Chretien JP, Small J, Tucker CJ, Formenty PB, Richardson JH, et al. Prediction of a Rift Valley fever outbreak. Proc Natl Acad Sci USA. 2009;106:955–9. doi: 10.1073/pnas.0806490106. [DOI] [PMC free article] [PubMed] [Google Scholar]