Abstract
Primary effusion lymphomas (PEL) form a subset of AIDS-related lymphomas and usually have a poor prognosis. Although Kaposi’s sarcoma–associated herpes virus (KSHV) is often associated with PEL, very little is known about the exact mechanisms or causative effects of these associations. We investigated the chromosomal imbalances in six KSHV-positive PEL cell lines using comparative genomic hybridization analysis. We defined the shortest regions of overlaps for genomic gains on six chromosomes: 1q31, 4q31~q33, 7q10~q21, 8q21.1, 12q0~q23, and Xp11~q21. The recurrent nature of the gains found in these chromosomal regions suggests that these imbalances play roles in the pathogenesis of PEL.
1. Introduction
Primary effusion lymphoma (PEL), also known as body cavity–based lymphoma, is a very rare AIDS-related malignancy with poor prognosis [1]. PEL has also been found in patients without HIV infection. The clinicopathology is usually characterized by the presence of malignant body cavity effusions in the absence of a solid tumor mass. Etiopathologically, PEL is often linked to Kaposi’s sarcoma–associated herpesvirus (KSHV), also known as human herpesvirus-8 (HHV-8) [2]. The majority of PELs are also coinfected with Epstein–Barr virus (EBV); however, a subset of PELs are EBV negative. Unlike EBV-associated Burkitt lymphomas, EBV-positive PELs do not manifest MYC rearrangements [2]. Recent studies have shown that KSHV infection and some of the resulting altered signaling pathways (e.g., constitutively active NF-kappa B pathway) are essential for the survival of PEL cells [3,4]. Nevertheless, the precise mechanisms underlying the malignancies remain unclear.
Chromosomal imbalances play a major role in the pathogenesis of most malignant diseases. Recent study has shown that KSHV infection can cause chromosome instability and thus may contribute to the pathogenesis of KSHV-induced malignancies [5]. To date, however, DNA copy number alterations have been characterized in only a very limited number of PEL cases, because of the rarity of this disease [6–9]. It is therefore impossible, for now, to definitively determine whether there are patterns of genomic imbalance that are specifically associated with this malignancy. We have performed comparative genomic hybridization (CGH) analysis of six KSHV-positive PEL cell lines to gain further insight into the genomic alterations that characterize this disease.
2. Materials and methods
2.1. Cell lines
The six PEL cells lines (BC-1, BC-2, BC-3, PK-1, BCP-1, and BCBL-1) used in the present study have been described previously [10]. BC-1 and BC-2 are coinfected with KSHV and EBV, whereas BC-3, BCP-1, BCBL-1, and PK-1 are infected with KSHV but not EBV.
2.2. CGH analysis
Comparative genomic hybridization analysis was performed on DNA samples as previously described [11]. The CGH nick-translation kit (Vysis, Downers Grove, IL) was used for the labeling reaction. Normal female DNA (Promega, Madison, WI) was used as a reference for all analyses and was labeled with SpectrumRed dUTP. The test DNA from PEL cells was labeled with SpectrumGreen dUTP. The labeled DNAs were mixed, precipitated in the presence of 50× human Cot-1 DNA, and resuspended in hybridization buffer containing 50% formamide, 1× saline sodium citrate (SSC), and 10% dextran sulfate; they were then hybridized onto metaphase chromosome slides (Vysis) for 48 h. After hybridization, the slides were washed for 5 min in 0.4 × SSC–0.3% NP-40 at 75°C and for 5 min in 2 × SSC–0.1% NP-40 at room temperature. DNA was counterstained with 4′,6-diamidino-2-phenylindole (DAPI) in antifade solution and the images quantified using Genus software (Applied Imaging, Santa Clara, CA) and the Olympus BX51 fluorescent microscope.
3. Results and discussion
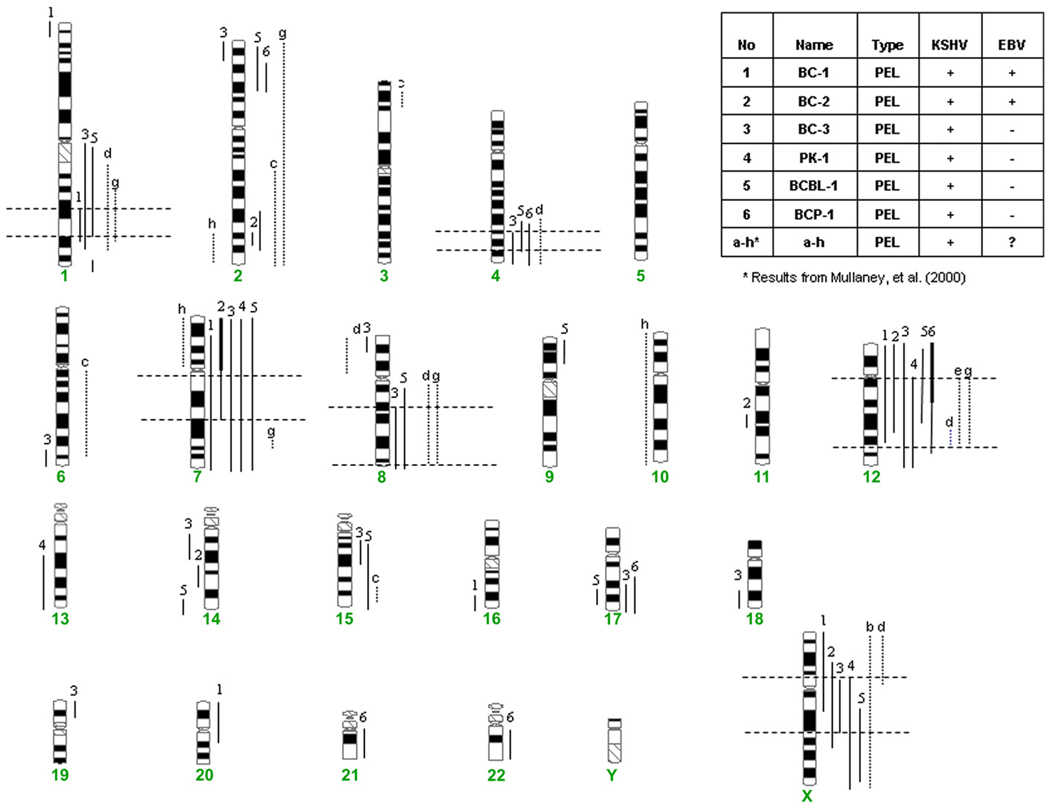
A total of six KSHV-positive PELs were analyzed by CGH to detect chromosomal imbalances. Chromosomal abnormalities were detected in all the samples analyzed and were spread throughout the genome. The chromosomal imbalances are summarized in Fig. 1. Numerous whole and partial chromosome imbalances were detected, with gain of chromosome 1q, 7, 12, and X materials being the most common abnormalities. Chromosomal deletions were less common than gains.
Fig. 1.
Comparison of the CGH results in the six KSHV-positive PELs with results from eight PEL cell lines analyzed by Mullaney et al. [6]. Dashed lines represent PELs examined by Mullaney et al. [6]. Six shortest regions of overlap for gains on chromosomes 1, 4, 7, 8, 12, and X are designated with horizontal lines.
Our results are in general consistent with a previous CGH study by Mullaney et al. [6] that analyzed eight PEL cases; in that study, genomic aberrations were identified in six of eight cases, including recurrent gain of sequence in chromosome 12 in three of eight cases and X in two of eight cases. In contrast, our results are less consistent with those of Ohshima and colleagues who analyzed five PEL-like cases by CGH [7]; the PEL-like cases that they used are all KSHV negative, so they could represent different pathological entities. Our results are also consistent with another study, using conventional cytogenetics and fluorescence in situ hybridization, by Gaidano et al. [8], who found aberrations in chromosomes 1, 7, and 12 in seven PEL cell lines. In that study, all seven PEL cell lines were found to have complete or partial trisomy 12, and four of the PEL cell lines were also found to have complete trisomy 7; abnormalities of chromosome 1 were also found in six of seven cell lines, of which four carried partial trisomy encompassing 1q21~q25 bands and another cell line had an inv(1)(p31q21) [8]. In another study, by Boulanger et al. [9], a complex karyotype was found in all seven PEL cases examined by RHG-banding, but no recurrent abnormalities were identified.
Comparison of our CGH results with those of Mullaney et al. [6] permits us to define the shortest regions of overlap for genomic gains on six chromosomes (Fig. 1): gain of 1q31, 4q31~q33, 7q0~q21, 8q21.1, 12q0~q23, and Xp11~q21. Some genes of interest located in these regions include ELK4, IL19, IL20, IL24, ATF3, and TRAF5 on chromosome 1; IL15, SMAD1, and PDGFC on chromosome 4; SEMA3A, SEMA3C, SEMA3D, SEMA3E, BCL7B, HGF, ADAM22, CYP3A4, and CYP3A43 on chromosome 7; HDAC7A, ATF7, HOXC6, HOXC8, MMP19, CDK2, ERBB3, MDM2, and IGF1 on chromosome 12; and TAF1, ATRX, IL2RG, and CXCR3 on chromosome X. The roles of these genes in the lymphomagenesis of PEL remain to be further elucidated.
The genetic alterations in KSHV-infected PELs are quite complex, and their recurrent nature suggests that they have a significant role in the pathogenesis of PEL. Higher resolution studies, such as those achievable by oligonucleotide array CGH [12] are required to further narrow the genomic regions that are important in PEL pathogenesis.
Acknowledgments
This work was supported in part by an R01 grant from the National Institutes of Health–National Cancer Institute (CA096512) to S-J Gao.
References
- 1.Waddington TW, Aboulafia DM. Failure to eradicate AIDS-associated primary effusion lymphoma with high-dose chemotherapy and autologous stem cell reinfusion: case report and literature review. AIDS Patient Care STDS. 2004;18:67–73. doi: 10.1089/108729104322802498. [DOI] [PubMed] [Google Scholar]
- 2.Cesarman E. The role of Kaposi’s sarcoma–associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Recent Results Cancer Res. 2002;159:27–37. doi: 10.1007/978-3-642-56352-2_4. [DOI] [PubMed] [Google Scholar]
- 3.Sun Q, Matta H, Chaudhary PM. The human herpes virus 8-encoded viral FLICE inhibitory protein protects against growth factor withdrawal-induced apoptosis via NF-kappa B activation. Blood. 2003;101:1956–1961. doi: 10.1182/blood-2002-07-2072. [DOI] [PubMed] [Google Scholar]
- 4.Guasparri I, Keller SA, Cesarman E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J Exp Med. 2004;199:993–1003. doi: 10.1084/jem.20031467. [Erratum in: J Exp Med 2006;203:1383] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Pan H, Zhou F, Gao SJ. Kaposi’s sarcoma–associated herpesvirus induction of chromosome instability in primary human endothelial cells. Cancer Res. 2004;64:4064–4068. doi: 10.1158/0008-5472.CAN-04-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullaney BP, Ng VL, Herndier BG, McGrath MS, Pallavicini MG. Comparative genomic analyses of primary effusion lymphoma. Arch Pathol Lab Med. 2000;124:824–826. doi: 10.5858/2000-124-0824-CGAOPE. [DOI] [PubMed] [Google Scholar]
- 7.Ohshima K, Ishiguro M, Yamasaki S, Miyagi J, Okamura S, Sugio Y, Muta T, Sasaki H, Tuchiya R, Kawasaki C, Kikuchi M. Chromosomal and comparative genomic analyses of HHV-8-negative primary effusion lymphoma in five HIV-negative Japanese patients. Leuk Lymphoma. 2002;43:595–601. doi: 10.1080/10428190290012100. [DOI] [PubMed] [Google Scholar]
- 8.Gaidano G, Capello D, Fassone L, Gloghini A, Cilia AM, Ariatti C, Buonaiuto D, Vivenza D, Gallicchio M, Avanzi GC, Prat M, Carbone A. Molecular characterization of HHV-8 positive primary effusion lymphoma reveals pathogenetic and histogenetic features of the disease. J Clin Virol. 2000;16:215–224. doi: 10.1016/s1386-6532(99)00082-7. [DOI] [PubMed] [Google Scholar]
- 9.Boulanger E, Agbalika F, Maarek O, Daniel MT, Grollet L, Molina JM, Sigaux F, Oksenhendler E. A clinical, molecular and cytogenetic study of 12 cases of human herpesvirus 8 associated primary effusion lymphoma in HIV-infected patients. Hematol J. 2001;2:172–179. doi: 10.1038/sj.thj.6200096. [DOI] [PubMed] [Google Scholar]
- 10.Gao SJ, Zhang YJ, Deng JH, Rabkin CS, Flore O, Jenson HB. Molecular polymorphism of Kaposi’s sarcoma–associated herpesvirus (human herpesvirus 8) latent nuclear antigen: evidence for a large repertoire of viral genotypes and dual infection with different viral genotypes. J Infect Dis. 1999;180:1466–1476. doi: 10.1086/315098. [Erratum in: J Infect Dis 1999;180:1756] [DOI] [PubMed] [Google Scholar]
- 11.Breen CJ, Barton L, Carey A, Dunlop A, Glancy M, Hall K, Hegarty AM, Khokhar MT, Power M, Ryan K, Green AJ, Stallings RL. Applications of comparative genomic hybridisation in constitutional chromosome studies. J Med Genet. 1999;36:511–517. [PMC free article] [PubMed] [Google Scholar]
- 12.Selzer RR, Richmond TA, Pofahl NJ, Green RD, Eis P, Nair P, Broth-man AR, Stallings RL. Analysis of chromosome breakpoints in neuroblastoma at sub-kilobase resolution using fine tiling oligonucleotide array CGH. Genes Chromosomes Cancer. 2005;44:305–319. doi: 10.1002/gcc.20243. [DOI] [PubMed] [Google Scholar]