It has become increasingly clear that stressful early life events adversely affect a person's physical and mental well being in later life, and can eventually lead to the development of mental disorders such as depression, anxiety, or drug dependence. Such early life events are hypothesized to trigger long-lasting changes in specific neurocircuits, but the nature of this neuroplasticity is not fully understood. Because both the corticotropin-releasing factor (CRF) and serotonin brain systems are strongly implicated in anxiety-related behaviors, their interaction may play a crucial role in the induction of persistent behavioral changes by stress.
The dorsal raphe nucleus (dRN) is one of the brain sites where critical interactions between the CRF and serotonin systems may occur. CRF afferent fibers are present at all rostrocaudal levels of the dRN, where their presynaptic terminals contact dendrites of both GABAergic and serotonergic neurons [see the study by Waselus et al. (2009) and references therein]. However, CRF does not activate the same type of receptors on these two populations (Fig. 1). Activation of CRF2 receptors stimulates serotonergic neurons, whereas activation of CRF1 receptors intensifies the tonic inhibition of serotonergic neurons by GABAergic afferents (Kirby et al., 2008). Accordingly, low doses of CRF, which preferentially activate CRF1 receptors, reduce discharge rates of dRN serotonergic neurons and thereby reduce serotonin release in dRN target regions. In contrast, higher doses of CRF activate CRF2 receptors, resulting in increased serotonin outflow [see the study by Waselus et al. (2009) and references therein] (Fig. 1).
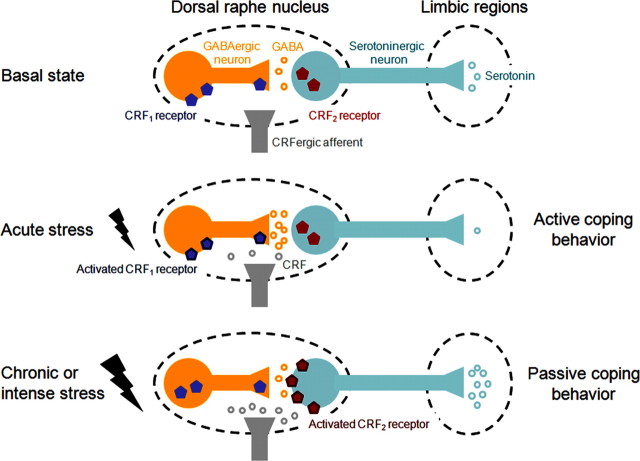
Figure 1.
Schema representing CRF-serotonin interactions in the dRN and their regulation by stress exposure. In the basal state (top diagram), dRN serotonergic neurons are tonically inhibited by GABAergic afferents, which limit the release of serotonin into dRN target brain regions. Exposure to an acute stressor (middle diagram) triggers the release of low levels of CRF into the dRN, which activate CRF1 receptors and potentiate GABAergic transmission, thereby resulting in reduced serotonin levels in dRN limbic targets and active behavioral responses to stress. Exposure to stress also produces a redistribution of the CRF receptors expressed by the different neuronal populations of the dRN: CRF1 receptors carried by nonserotonergic neurons internalize while CRF2 receptors are recruited at the surface of serotonergic neurons and their level of expression increases (middle vs bottom diagrams). Repetition or intensification of the stressor triggers the release of higher levels of CRF into the dRN, which now activate CRF2 receptors and stimulate the activity of serotonergic neurons, thereby leading to a massive serotonin efflux in targeted limbic structures and passive stress-coping strategies (learned helplessness).
Interestingly, the dual action of CRF on dRN serotonergic neurons has behavioral correlates, as revealed by the differential response to stressors engaging distinct CRF receptor subtypes. For instance, exposure to an acute swim stress activates dRN GABAergic neurons through CRF1 receptors, inhibits serotonin efflux in dRN projection areas, and promotes an active coping strategy (swimming). In contrast, repetition of the swim stress or exposure to a more intense stressor (e.g., uncontrollable shock, or social defeat) directly activates dRN serotonergic neurons through CRF2 receptors, stimulates serotonin release in limbic targets, and promotes passive coping strategies (floating, escape deficits, or freezing) [see the study by Waselus et al. (2009) and references therein and the study by Cooper and Huhman (2007)]. The limbic regions in which extracellular serotonin levels increase following activation of dRN serotonergic neurons by CRF include the lateral septum, the basolateral and the central nuclei of the amygdala, and the nucleus accumbens [see the study by Lukkes et al. (2009a) and references therein]. In addition, dRN activation leads to increased serotonin release in the medial prefrontal cortex (mPFC), which is strongly connected to these limbic regions (Forster et al., 2008). Differential serotonin outflow into limbic and associated structures could therefore play a role in the expression of distinct coping strategies (Fig. 1).
A related mechanism for the switch from inhibitory to excitatory effects of CRF on dRN serotonergic neuron activity, and accordingly for the transition from active to passive stress coping strategies, has been recently proposed. Exposure to acute swim stress produces a redistribution of CRF receptors within dRN neurons, in which CRF1 receptors are internalized and CRF2 receptors are recruited at the plasma membrane (Waselus et al., 2009). This trafficking requires the initial activation of CRF1 receptors, but ultimately potentiates CRF2-mediated signaling and decreases CRF1-mediated signaling. In accordance with this finding, early life social isolation alters the serotonergic response to CRF infusions into the dRN of adult rats. Isolation stress not only eliminates inhibition of dRN serotonergic neurons by low doses of CRF that activate CRF1 receptors in group-reared rats, but also potentiates stimulation of dRN serotonergic neurons by high doses of CRF that activate CRF2 receptors (Lukkes et al., 2009b).
An elegant study by Lukkes et al. (2009a) published recently in The Journal of Neuroscience provides evidence that the recruitment of dRN CRF2 receptors induced by severe stress is long-lasting and has behavioral repercussions that can be detected long after the stressor has terminated. Lukkes et al. (2009a) show that microinjection of a CRF antagonist (D-Phe-CRF(12-41)) into the dRN reverses the social anxiety-like behavior displayed by adult rats (postnatal day 61) that had been subjected to social isolation during adolescence (between postnatal days 21 and 42), as assessed by three measures of social interaction [Lukkes et al. (2009a), their Fig. 3]. Vehicle-pretreated isolates took longer to approach an unfamiliar rat, spent less time in social contact and spent more time freezing than their group-reared counterparts. In isolated rats, D-Phe-CRF(12-41) microinjected into the dRN reduced the latency to approach the unfamiliar rat, increased the duration of social contact, and decreased the duration of freezing. These findings were specific to the dRN, because infusion of the CRF antagonist into adjacent sites was not effective [Lukkes et al. (2009a), their Table 1]. Because D-Phe-CRF(12-41) has a 2–10 times higher affinity for the CRF2 receptor compared with the CRF1 receptor [see the study by Lukkes et al. (2009a) and references therein], these data indicate that the social anxiety observed in rats exposed to a severe stress at a young age results from the persistent recruitment of dRN CRF2 receptors in adulthood. While CRF2 receptors had never been clearly linked to social anxiety-like behaviors [see the study by Zhao et al. (2007) and references therein], Lukkes et al. (2009a) show that they reduce social interaction by mediating passive submissive behaviors, such as freezing.
Interestingly, microinjection of the CRF antagonist into the dRN did not alter the behavior of the group-reared rats [Lukkes et al. (2009a), their Fig. 3], which indicates a minimal contribution of CRF neurotransmission within the dRN to social interaction under basal conditions. According to the literature, inhibition of dRN CRF receptors in unstressed animals would mostly block CRF1 signaling and thereby release the tonic inhibition of serotonergic neurons by GABAergic afferents. The associated increase in serotonin release in dRN projection areas would be expected to promote social anxiety-like behavior. The lack of effect of the CRF antagonist in group-reared rats suggests that the social interaction test does not trigger CRF release in the dRN of unstressed animals.
CRF afferents to the dRN may be part of a complex neurocircuit involving the amygdala, dRN, and mPFC. CRF afferents to the dRN from the central nucleus of the amygdala (CeA) control serotonin release in the mPFC through activation of CRF2 receptors (Forster et al., 2008). Repeated, chronic, and/or intense stressors may produce neuroadaptations in the CeA that would lead to a sustained release of CRF into the dRN, thereby activating dRN CRF2 receptors and increasing serotonin signaling in limbic and associated structures. When a subject experiences a controllable stress, the mPFC represses the activation of dRN serotonergic neurons, thereby limiting the behavioral impact of stress exposure. However, in the case of an uncontrollable stressor the mPFC does not exert this inhibitory control (Amat et al., 2005). Repeated, chronic, and/or intense stressors may be perceived as uncontrollable, which may in turn lead to mPFC inactivation, increased dRN serotonin activity, and passive coping strategies (learned helplessness).
The finding that early life stress persistently converts an inhibitory CRF–serotonin interaction into a stimulatory one (Lukkes et al., 2009a) has important implications for the currently debated question of how genes and environment interact in depression. An epidemiological study originally reported that adverse events in early life predict a diagnosis of depression in adulthood only in carriers of the short allele of the serotonin transporter-linked polymorphic region (5-HTTLPR), who express lower levels of the serotonin transporter (Caspi et al., 2003). Although a recent meta-analysis did not detect such an interaction between the 5-HTTLPR genotype and stressful life events on depression (Risch et al., 2009), the study of Lukkes et al. (2009a) indicates that the anxiogenic effects of early life stress are mediated by CRF-induced activation of the dRN, a major serotonergic nucleus. In line with this finding, early adversity in the form of peer rearing, instead of mother rearing, produces a stronger ACTH response and a lower cortisol response to separation stress in female rhesus monkeys carrying the short allele of the 5-HTTLPR (Barr et al., 2004). Stress-induced recruitment of the amygdala and subsequent activation of dRN CRF2 receptors could explain the latter observation, since the amygdala facilitates the activation of the hypothalamic-pituitary-adrenal axis by dRN serotonergic neurons (Weidenfeld et al., 2002). These findings, together with the data of Lukkes et al. (2009a), highlight the central role of serotonin neurotransmission in the vulnerability to the consequences of early life stress on adult physiology and behavior.
The study by Lukkes et al. (2009a) opens new perspectives for studying the interaction between the CRF and the serotonin systems in psychiatric disorders in which stress plays a triggering role, such as posttraumatic stress disorder, major depression, or drug addiction. Interestingly, preliminary findings suggest that a parallel can be drawn between the effect of early life social isolation and repeated amphetamine exposure, because they both induce a long-lasting increase in the level of CRF2 receptors expressed in the dRN (Pringle et al., 2008; Lukkes et al., 2009b). In addition, increased extracellular serotonin levels in limbic structures targeted by the dRN, as modeled in knock-out rats deficient in the serotonin transporter, is not only associated with anxiety- and depression-like phenotypes, but also with an increased motivation to self-administer cocaine (Homberg et al., 2008).
In conclusion, long-lasting facilitation of CRF2 signaling in the dRN may well be the missing link in the pathological mechanism bridging increased serotonin and CRF neurotransmission in stress-related psychiatric disorders. As discussed above, innate upregulation of the serotonin system could confer vulnerability to the effects of early life stress. Conversely, genetic factors in the CRF system may lead to increased recruitment of the serotonin system. Such interactions between brain systems may provide new paths for therapeutic treatment of stress-induced affective disorders.
Footnotes
Editor's Note: These short, critical reviews of recent papers in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to summarize the important findings of the paper and provide additional insight and commentary. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml.
J.R.H. and C.C. are funded by The Netherlands Organization for Scientific Research and the National Institute for Drug Abuse in a joint project.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, Taubman J, Thompson B, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci U S A. 2004;101:12358–12363. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Corticotropin-releasing factor receptors in the dorsal raphe nucleus modulate social behavior in Syrian hamsters. Psychopharmacology. 2007;194:297–307. doi: 10.1007/s00213-007-0849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. Eur J Neurosci. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, De Boer SF, Raasø HS, Olivier JD, Verheul M, Ronken E, Cools AR, Ellenbroek BA, Schoffelmeer AN, Vanderschuren LJ, De Vries TJ, Cuppen E. Adaptations in pre- and postsynaptic 5-HT1A receptor function and cocaine supersensitivity in serotonin transporter knockout rats. Psychopharmacology. 2008;200:367–380. doi: 10.1007/s00213-008-1212-x. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci. 2008;28:12927–12937. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes J, Vuong S, Scholl J, Oliver H, Forster G. Corticotropin-releasing factor receptor antagonism within the dorsal raphe nucleus reduces social anxiety-like behavior after early-life social isolation. J Neurosci. 2009a;29:9955–9960. doi: 10.1523/JNEUROSCI.0854-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Summers CH, Scholl JL, Renner KJ, Forster GL. Early life social isolation alters corticotropin-releasing factor responses in adult rats. Neuroscience. 2009b;158:845–855. doi: 10.1016/j.neuroscience.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle RB, Mouw NJ, Lukkes JL, Forster GL. Amphetamine treatment increases corticotropin-releasing factor receptors in the dorsal raphe nucleus. Neurosci Res. 2008;62:62–65. doi: 10.1016/j.neures.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry. 2009;66:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenfeld J, Newman ME, Itzik A, Gur E, Feldman S. The amygdala regulates the pituitary-adrenocortical response and release of hypothalamic serotonin following electrical stimulation of the dorsal raphe nucleus in the rat. Neuroendocrinology. 2002;76:63–69. doi: 10.1159/000064430. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, Weiss F, Zorrilla EP. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. J Pharmacol Exp Ther. 2007;323:846–854. doi: 10.1124/jpet.107.123208. [DOI] [PubMed] [Google Scholar]