Climate change is incontestably a phenomenon of global causes and impacts. However, as much as the contribution of different regions and countries to climate change differs, as much differ the impacts. This paper examines the current and potential impact of climate change on infectious diseases in regions that could not be more different: the Arctic and the tropics (The Arctic is the area north of the Arctic Circle (66.6°N), while the tropics lie between the Tropic of Cancer (23.4°N) and the Tropic of Capricorn (23.4°S)) (Fig. 1). Despite obvious differences in environmental and socio-economic contexts, there are commonalities between these areas, both in the mechanisms through which climate change influences disease transmission and in the adaptation responses health systems can and should mount. We hope that the lessons in this comparison can be distilled both by policy makers and researchers in both regions.
Fig. 1.
The Arctic, looking down to the North Pole.
The purpose of this article is ‘to join the dots’ and thus stimulate discussion. Inevitably, the different dots (issues) themselves cannot be elaborated on in detail here. For this, we refer the interested reader to a wide-ranging list of references.
Climate-sensitive infectious diseases in the Arctic
Of all regions in the world, the Arctic is particularly affected by global warming. The proportional increase in surface air temperature has been twice the average global increase. Importantly, warming since 1980 has been strongest in the wintertime at 1°C per decade. It comes as no surprise that the sea-ice cover has decreased by 10% during this period (1). Warming is projected to an increase of 2–9°C by the end of the century, a much higher increase than the projected global mean of 1.1–6.4°C (1). Thus, models project a substantial decrease in snow and sea-ice cover over most of the Arctic by the end of the 21st century (2). An already noticeable change was the opening of both the Northwest and the Northeast passages for traffic in 2008. The Barent Sea has been ice-free and open during summer for the past four years.
The health effects of these changes in the far North, particularly in the distribution and incidence of infectious diseases, have received little attention so far both in the published literature (3, 4) and in the media. Climate change will affect the distribution of infectious diseases directly as well as indirectly.
Disruptions in the operation of water supply and sewage facilities increase the risks for intestinal infections through the spread of water and food-borne infections. Some recent examples include a 30-fold increase in hepatitis A in one Russian region after flooding. Also, reduced access to clean water may increase skin infections, many of which are caused by multi-resistant bacteria.
As the boreal forest extends north, areas for certain animals such as foxes carrying rabies or echinococcosis and beavers carrying Giardia intestinalis will expand.
Another effect of warmer average temperatures and changes in the relative length of seasons is the changing pattern in vector-borne diseases. Insects that exist today in the south of the region transmit different rickettsia species such as Mediterranean spotted fever and Q-fever, and viruses such as West Nile virus. These and the Chikungunya virus, recently established in Europe, could potentially move northwards, although this is less likely, as temperatures needed are higher than most models predict. However, the transmitting vector can change, as has occurred with the transmission of Dengue virus and Chikungunya virus from Aedes egyptii to Aedes albopictus, an insect that thrives in colder temperatures.
Ticks transmit both Lyme disease (5) and tick-borne encephalitis (TBE) (6), see Box 1. While the incidence of TBE has halved since the late 1990s in Russia, the incidence rate tripled in Arkhangelsk Oblast, in the North, during the same period. In the 1980s, there were relatively few cases of TBE in the Russian Federation, including 300 cases per year in Arkhangelsk Oblast, but rising temperatures from 2001 increased incidence to 2,300 cases per year in 2007, a 10-fold increase within a decade.
Box 1. Tick-borne encephalitis (TBE) in Sweden.
TBE was first discovered in Sweden in the 1950s and the reported annual numbers of cases have increased ever since. Up to 1979 approximately 25 cases were reported each year. During the 1980s around 40 cases were reported annually and during the 1990s the number rose to 60–80 (7). In the last 10 years the incidence increased further to about 140 cases annually. About 224 cases were reported in 2008 (8). All cases of encephalitis admitted in Stockholm County have been serologically tested for TBE since the late 1950s (9). Viral meningo-encephalitis has been a notifiable disease in Sweden since 2004. TBE causes more than half of the cases of viral meningo-encephalitis reported to the Swedish Institute for Infectious Disease Control (SMI), with most of the patients contracting TBE in Sweden. Only a few patients were infected in other European countries (8).
Most cases occur in the central-eastern parts of the country, particularly in the archipelagos surrounding Stockholm (10). In the eastern parts of the country the risk areas have remained unchanged (7). In the 1990s new TBE-endemic areas became established around lake Vänern and lake Vättern, mainly close to the water where the climate is milder (7). Other areas where the disease seems to be emerging is in the south (Skåne and Blekinge) and in the west, the Bohuslän and Dalsland areas (10). During the last few years, additional foci have appeared. The virus has spread north along the east coast and cases have been reported from Dalarna in central Sweden, and Bohuslän on the west coast (10).
Climatic factors such as mild winters and early arrival of spring are thought to have contributed to the spread of the tick Ixodes ricinus further north and to an increase in tick density (10, 11). Other factors contributing to the increase in TBE may include increasing host animal populations such as roe deer, and more people spending time in endemic areas due to an increase in summer cottages (12). In addition, an increased awareness of the disease amongst health care workers and the general population leads to a higher number of diagnosed cases (7, 9). The cause of the increase in TBE in Sweden is most likely multi-factorial.
During the last ten years SMI has diagnosed more than 20 cases with serologically verified TBE despite complete active vaccination against the disease (8). The majority of these cases were diagnosed in 2007. The diagnosis of acute TBE is hard to establish using only a single serum among patients with prior vaccination. For these patients complementary laboratory tests are needed.
Climate-sensitive infectious diseases in the tropics
There have been several excellent recent reviews on this topic, to which we refer the reader (13–18). Table 1 compares the major climate-sensitive infectious diseases in the tropics with those in the Arctic. The list is neither exhaustive nor exclusive. Furthermore, the listed diseases are not necessarily limited in their prevalence to the tropics or the Arctic.
Table 1.
Climate-sensitive infectious diseases in Arctic's and tropics by type of pathogen
| Climate-sensitive diseases | ||
|---|---|---|
| Infective agents | Tropics | Arctic |
| Parasite | Malaria | Giardiasis |
| Leishmaniasis | Cryptosporidiosis | |
| Schistosomiasis | Echinococcosis multilocularis | |
| Trypanosomiasis | Toxoplasmosis | |
| Bacteria | Meningococcal meningitis | Lyme borreliosis |
| Relapsing fever | ||
| Tularaemia | ||
| Vibrio parahemolyticus infections | ||
| Brucellosis | ||
| Multi-resistant Staphylococcus aureus | ||
| Haemophilus influenza, Streptococcus pneumoniae and Mycobacterium tuberculosis | ||
| Rickettsia | Rocky mountain spotted fever, South African tick typhus, Queensland tick typhus and more | Mediterranean spotted fever |
| Virus | Dengue fever | Nephropathia epidemica (Puumala virus) |
| Yellow fever | Tick-borne encephalitis | |
| Rift Valley fever | Russian summer and spring encephalitis | |
| West Nile fever | West Nile fever | |
| Hantavirus cardio-pulmonary syndrome | Chikungunya | |
| Ross river virus fever | Dengue fever | |
| Chikungunya | Rabies | |
| Crimean-Congo haemorrhagic fever | Hepatitis A | |
Commonalities between climate-sensitive infectious diseases in the Arctic and the tropics
A large proportion of the populations in both these extreme climate zones share the characteristic of living in close proximity to their ecosystems, in fact living on them (3, 19). This makes these populations highly vulnerable to the health effects of climate change.
We consider here five main common characteristics and requirements, respectively, regarding climate-sensitive infectious diseases:
Exposure to new patterns of climate-sensitive infectious diseases.
Disease surveillance and early warning systems.
Health system preparedness.
Enhanced global efforts towards developing drugs and vaccines.
Common challenges for research.
Exposure to new patterns of climate-sensitive infectious diseases
The pathways leading from the presence of pathogens, vectors and host animals to manifestations of infectious disease in humans are non-linear and complex. Apart from climate change, they are influenced by human immune response, human behaviour (particularly regarding land use (20, 21), the quality of social and health systems, the development of drug resistance and many more. They all have a strong influence on whether or not diseases will manifest in populations and how they will spread.
With some caveats, it is generally true that some pathogens such as salmonella respond directly to higher temperatures in terms of their proliferation. Vector-borne diseases react indirectly to changes in temperature and humidity, as insect vectors and their associated pathogens undergo possibly accelerated life cycles as ambient temperature increases (up to a certain point). They, in turn, frequently depend on host vertebrates, which have their own climate-dependent migration patterns. We would therefore expect some of these diseases to expand pole-wards and upwards in terms of altitude.
Certain vectors have a particular potential to act in joining the dots between the south and the north. The species mentioned below exist today in southern Europe:
Mosquitoes (Anopheles, Culex and Aedes) can give rise to malaria (different species), West Nile virus, Dengue virus and Chikungunya virus, respectively.
Ticks (Ixodes ricinus, Dermacentor reticulates, Hyalomma marginatum and Rhipecephalus sanguineus) transmit Borrelia burgdoferi (leading to Lyme borreliosis), TBE virus (TBE-encephalitis), Tularaemia franciscella (tularaemia), Coxiella burnetii (Q-fever), Bunyaviridae viruses (Crimean-Congo haemorrhagic fever), Rickettsia conorii (Mediterranean-spotted fever).
Sandflies (Phlebotominae) transmit Leishmania donovani and Leishmania infantum.
Autochthonous (locally transmitted) cases of most of these infectious diseases have been reported in Europe.
Changing spatial range of diseases
Higher latitudes
The last case of indigenous malaria in Sweden was reported during the 1930s. Even if other parameters, such as socio-economic situations and well-performing health systems, influence the epidemiology of these infections, higher temperatures could - in a longer-term perspective - move the northern limits of these infections as locally transmitted malaria cases occurring in Italy have shown. West Nile virus has expanded to Canada, and the northward movement of TBE is described in Box 1.
A particularly instructive case, albeit from a veterinary infectious disease, is Blue Tongue, a frequently fatal disease of cattle, goats, sheep and deer. Between 1998 and 2005, this insect-borne disease (spread by the biting midge Culicoides imicola) spread northwards in Europe. In 2006, it reached central and northern Europe including Switzerland, the Netherlands, Belgium and Germany. The spread of the disease could be explained by the basic reproductive number R 0, which is temperature-dependent (22, 23). In 2007, Blue Tongue disease appeared for the first time in Britain. Gubbins et al. (23) reported the greatest risk of spread in Britain (R0 between 2 and 4) in a temperature range from 15 to 25°C.
Higher altitudes
The case of highland malaria in Africa has been hotly debated in the literature (24–27). The emerging consensus (28) seems to reflect many factors influencing the upward move of malaria transmission in the Kenyan highlands (Fig. 2), but shows that climate change is among them (29).
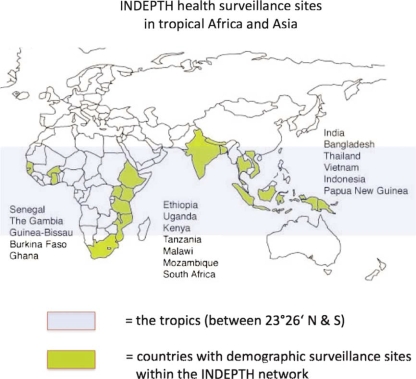
Fig. 2.
The world, showing the tropics (blue zone) and countries containing INDEPTH health and demographic surveillance sites (yellow). Source: www.indepth-network.org
Note: There is one INDEPTH site Latin America, in Nicaragua (not shown on the map).
Changing temporal pattern of diseases
Changing seasonal patterns of infectious diseases have been reported for many climate-sensitive infectious diseases including malaria (30, 31), tularaemia (32) and TBE (33).
Not surprisingly, inter-annual variability of climate, too, has been shown to have an influence on infectious diseases: increases in the incidence of malaria, dengue fever, Rift Valley fever, hantavirus infections, cholera and other diseases have been associated with the El-Nino-Southern Oscillation (ENSO). For an overview, see Kovats et al. (34) and Anyamba et al. (35).
The few studies on possible relationships between infectious diseases and the North Atlantic Oscillation (NAO) have been inconclusive so far. Palo (36) performed time-series analyses between the frequency of cases of nephropathia epidemica and the NAO index and did not find a significant association. Hubalek (37) found an association between the NAO index and some infectious diseases such as toxoplasmosis. Intriguingly, Lyme Borreliosis and TBE showed no relationship at all. More research is certainly needed in this arena.
In conclusion, in spite of the extremely different ecosystems in the Arctic and the tropics, the nature of transmission frequently via vectors, generates similarities between climate-sensitive diseases in both areas. This is illustrated in Table 2 using the example of tularaemia and malaria.
Table 2.
Common effects of climate change on disease transmission, taking the examples of tularaemia and malaria
| Common effects on diseases transmission | Tularaemia | Malaria |
|---|---|---|
| Incidence sensitive to inter-annual climate changes | NAO | ENSO (El Nino) |
| Length of transmission period | Increased | Increased |
| Expansion of geographical coverage | Yes | Yes, both latitudinal and altitudinal |
| Human land-use important | Yes | Yes |
Source: Rydén et al. (32).
Disease surveillance and early warning systems
Given the dynamics and the complexity of climate-sensitive infectious diseases, particularly those transmitted by mosquitoes or rodents, both regions need to develop and sustain surveillance and early warning systems. While a global early warning system, such as the Global Alert and Response Network (GOARN), which was developed by the World Health Organization (38), is impressive, it cannot achieve the task of monitoring the imperceptible extension of infectious diseases into new areas. We discuss below some specific issues pertaining to the Arctic and the tropics.
The Arctic
As populations are relatively small and scattered over a large area, it makes sense to develop a region-specific surveillance system to detect significant trends in infectious diseases. Linking existing national monitoring systems is the basis for appropriate and coordinated actions. Standardising laboratory methods and clinical surveillance definitions across borders facilitates comparing and analysing regional epidemiological data.
An example of such a network is the ‘International Circumpolar Surveillance System for Emerging Infectious Diseases’ (39). This network links hospital and public health laboratories for the purposes of monitoring invasive bacterial diseases and tuberculosis in Arctic populations (39). It is planned to extend this surveillance network to include climate-sensitive infections.
The tropics
There are three main weaknesses in most developing countries with regard to disease reporting: Firstly, health services ‘see’ only a fraction of those suffering from diseases, typically less than one fifth. Those who do visit health services are certainly not representative of the entire population. It is therefore quite possible to miss an increase in cases, if those occur in remote (geographically barred) or in poor (financially barred) populations. Secondly, incomplete reporting may generate fluctuations in case numbers, which are reporting artefacts, rather than actual changes in case frequencies. Thirdly, case verification is difficult and lengthy, given the dearth of reference laboratories.
Population-based health surveillance on a national scale (40) would be ideally implemented using a random sample of sentinel sites. This is however quite expensive and out of reach for many low-income countries.
Another possibility would be to use existing population-based surveillance systems, such as the INDEPTH network (www.indepth-network.org) as a tool for the surveillance of climate-sensitive infectious diseases in the tropics. This network currently covers about two million people in the tropics under demographic and health surveillance in some 30 sites, mainly in Africa and Asia (see Fig. 2). Ascertainment of cause of death is standardised across sites and as valid as possible in the absence of facility-based biomedical exams. Many of these research centres include high quality laboratories, which could be included in systematic surveillance and early warning systems. Although certainly not strictly representative of their countries’ general population, these sites provide population-based data which is unbiased by the different barriers associated with health care utilisation. In addition, they are certainly faster and more valid in their case ascertainment than the average local health service.
Health system preparedness
Particularly in low-income countries in the tropics, under-performing health services must be strengthened to meet the additional challenge of network to include climate-sensitive infectious diseases. In the language of climate policy, this would be called a ‘no regrets’ strategy, something ‘… whose benefits equal or exceed their costs to society, excluding the benefits of avoided climate change’ (41). Hence, strengthening health systems is a sensible policy even in the absence of climate change.
Priority action should be considered in the following areas:
Protection of the infrastructure, particularly communications networks, against extreme weather events such as floods, storms and heat waves.
Training health staff about diseases which might be expected to occur or increase and the local measures that should be taken. This applies, for example, as much to Lyme disease in northern Sweden as to malaria in the latitudinal and altitudinal fringes of its current distribution.
Establishing decentralised stocks of drugs, vaccines and equipment for preventing or treating climate-sensitive diseases.
Establishing fast routine reporting systems for climate-sensitive infectious diseases.
Part of preparedness pertains to the active cooperation of health sector staff with colleagues from other relevant sectors, such as forestry, agriculture and meteorology. The need for such inter-sectoral cooperation is obvious, as the control of climate-sensitive infectious diseases, particularly those borne by vectors, strongly depends on information and action from these sectors. Table 3 illustrates the common characteristics of adaptation measures, using the example of two otherwise unrelated diseases, tularaemia in the Arctic and malaria in the tropics.
Table 3.
Common adaptation mechanisms, illustrated by two climate-sensitive diseases: tularaemia and malaria.
| Common adaptation | Tularaemia | Malaria |
|---|---|---|
| Early warning systems based on meteorology | In place (e.g. in Sweden) | At a research stage |
| Early warning systems based on case detection | Active and passive | Passive |
| Surveillance possible | Yes | Yes |
| Preparedness of health services | Moderate | Low |
| Involvement of other sectors (forestry, agriculture and infrastructure) | Yes | Beginning |
Source: Rydén et al. (32).
Enhanced global efforts towards developing drugs and vaccines for climate-sensitive infectious diseases
The development of vaccines against dengue fever and malaria is an important global research priority per se. The importance is accentuated by the projected increase in the burden of these diseases burden due to climate change. In principle, the need for vaccine development arises also in the case of Lyme disease (42). The debate should be re-opened in view of its anticipated spread northwards into the Arctic.
Drug treatment of malaria currently hinges primarily on one single type of drug, the artemisinin derivates. Given that we have evidence of in-vitro markers of resistance, particularly in south-east Asia (43), clinical resistance is imminent (44). The need for research and development of anti-malarial drugs is highlighted in view of potential effects of climate change on malaria transmission.
The same holds for drugs against other tropical climate-sensitive diseases, such as leishmaniasis and trypanosomiasis against which we urgently need new, less toxic and more effective drugs.
Common challenges for research
Surprisingly, research output on the links between climate and infectious diseases is limited for both regions, particularly as far as intervention-oriented research is concerned.
In both regions, researchers essentially face a similar agenda:
-
The attribution of changes in infectious diseases to climate change.
This almost invariably involves modelling techniques, linking regional climate models or at least time-series of meteorological data to models of infectious diseases.
For example, the Arctic is a region characterised by complex and incompletely understood climate processes, making climate modelling for this region challenging. Local climate data need to be coupled to large-scale data flows. There is a rapid development in the field, but, so far, regional models have not been used for the Arctic Climate Impact Assessment (ACIA). The validation of early warning and surveillance systems for climate-sensitive infectious diseases.
The development and evaluation of evidence-based adaptation strategies, their protective effectiveness and costs.
-
The identification of most vulnerable population sub-groups and subsequent targeting of adaptation interventions to them.
For example, there are 40 different ethnic groups in northern Russia. These minorities speak different languages as well as the language of the nation they live in and they have their own customs and cultures. Adapting the research methodology used according to these differences is important and needs special consideration. Research training.
Few health researchers are comfortable with modelling infectious diseases, let alone with using climate models and linking them to disease data (45). Many of the required techniques, such as time series analysis and analysis of remote sensing data are new, particularly to public health researchers and epidemiologists. Techniques for working with multi-sectoral data sets, e.g. from meteorology and agriculture, need to be learnt. While we need to raise interest for this issue within the global research community, a special effort in training and capacity building is needed in low income countries, where climate effects will be first and most strongly felt (46).
Conclusions
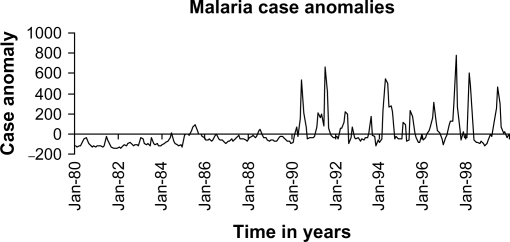
The task of understanding the nexus between climate change and infectious diseases is not made easier by the complex simultaneous ecological, social and health service influences, as the debate on highland malaria illustrates (28) Fig. 3.
Fig. 3.
Time trend for malaria in the Kenyan highlands. Source: Ref. (29).
The transition from knowledge to knowledge-based actions is another challenge. The key issue is: What degree of confidence or certainty do we need in order to act? The United Nations Framework Convention on Climate Change has clearly stated that action is needed in the presence of uncertainty if the potential adverse effects are severe and irreversible (‘Precautionary Principle’1 (47)).
In spite of obvious contrasts in geography and the respective biospheres, these challenges are common to researchers and policy makers both in the Arctic and in the tropics. Many lessons can be shared. Joining the dots essentially means connecting the people who work around these dots across different parts of the globe.
Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this study.
References
- 1.IPCC. Climate change: I. The scientific basis. Cambridge: Cambridge University Press; 2007. Fourth assessment report. [Google Scholar]
- 2.Katsov VM, Källén E. Arctic climate impact assessment. Cambridge: Cambridge University Press; 2005. Future climate change: modeling and scenarios for the Arctic; pp. 99–150. [Google Scholar]
- 3.Parkinson A, Evengård B. Climate change, its impact on human health in the Arctic and the public health response to threats of emerging infectious diseases. Global Health Action. 2009 doi: 10.3402/gha.v2i0.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkinson AJ, Butler JC. Potential impact on climate change on infectious disease emergence in the Arctic. Int J Circumpolar Health. 2005;64:478–86. doi: 10.3402/ijch.v64i5.18029. [DOI] [PubMed] [Google Scholar]
- 5.Jaenson TG, Eisen L, Comstedt P, Mejlon HA, Lindgren E, Bergström S, et al. Risk indicators for the tick Ixodes ricinus and Borrelia burgdorfTicks eri sensu lato in Sweden. Med Vet Entomol. 2009;23:226–37. doi: 10.1111/j.1365-2915.2009.00813.x. [DOI] [PubMed] [Google Scholar]
- 6.Randolph SE. Evidence that climate change has caused ‘emergence’ of tick-borne diseases in Europe? Int J Med Microbiol. 2004;293:5–15. doi: 10.1016/s1433-1128(04)80004-4. [DOI] [PubMed] [Google Scholar]
- 7.Haglund M. Occurrence of TBE in areas previously considered being non-endemic: Scandinavian data generate an international study by the International Scientific Working Group for TBE (ISW-TBE) Int J Med Microbiol. 2002;33:50–4. doi: 10.1016/s1438-4221(02)80010-8. [DOI] [PubMed] [Google Scholar]
- 8.Smittskyddsinsitute. Sjukdomsinformation om Tick Borne Encephalitis (TBE) (Disease information on TBE) 2009. Available from: http://www.smittskyddsinstitutet.se/sjukdomar/tbe/ [cited 21 September 2009]
- 9.Lindgren E, Gustafson R. Tick-borne encephalitis in Sweden and climate change. Lancet. 2001;358:16–18. doi: 10.1016/S0140-6736(00)05250-8. [DOI] [PubMed] [Google Scholar]
- 10.Fält J, Lundgren Å, Alsterlund R, Carlsson B, Eliasson I, Haglund M, et al. Tick-borne encephalitis (TBE) in Skåne, southern Sweden: a new TBE endemic region? Scand J Infect Dis. 2006;38:800–4. doi: 10.1080/00365540600664068. [DOI] [PubMed] [Google Scholar]
- 11.Lindgren E, Tälleklint L, Polfeldt T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus . Environ Health Perspect. 2000;108:119–23. doi: 10.1289/ehp.00108119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randolph SE. Tick-borne encephalitis virus, ticks and humans: short-term and long-term dynamics. Curr Opin Infect Dis. 2008:462. doi: 10.1097/QCO.0b013e32830ce74b. 467. [DOI] [PubMed] [Google Scholar]
- 13.Jaenisch T, Patz J. Assessment of associations between climate and infectious diseases. Global Change Human Health. 2002;3:67–72. [Google Scholar]
- 14.Epstein P. Detecting the infectious diseases consequences of climate change and extreme weather events. In: Martens P, McMichael AJ, editors. Environmental change, climate and health – issues and research methods. Cambridge: Cambridge University Press; 2002. pp. 172–96. [Google Scholar]
- 15.Sutherst RW. Global change and human vulnerability to vector-borne diseases. Clin Microbiol Rev. 2004;17:136–73. doi: 10.1128/CMR.17.1.136-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardenas R, Sandoval CM, Rodriguez-Morales AJ, Vivas P. Zoonoses and climate variability. Ann NY Acad Sci. 2008;1149:326–30. doi: 10.1196/annals.1428.094. [DOI] [PubMed] [Google Scholar]
- 17.Sauerborn R, Louis V, editors. Global environmental change and infectious diseases: impacts and adaptation strategies. Heidelberg: Springer Verlag; 2009. (forthcoming) [Google Scholar]
- 18.Zhang Y, Bi P, Hiller JE. Climate change and the transmission of vector-borne diseases: a review. Asia Pac J Public Health. 2008;20:64–76. doi: 10.1177/1010539507308385. [DOI] [PubMed] [Google Scholar]
- 19.Grassl H, Schubert R, Epiney A, Kulessa M, Luther J, Nuscheler F, et al. London and Sterling: German Advisory Council for Global Change (WBGU, Berlin), Earthscan; 2005. Fighting poverty through environmental policy. [Google Scholar]
- 20.Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Sánchez-Lozano W, et al. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11. [PubMed] [Google Scholar]
- 21.Afrane YA, Little TJ, Lawson BW, Githeko AK, Yan G. Deforestation and vectorial capacity of Anopheles gambiae Giles mosquitoes in malaria transmission, Kenya. Emerg Infect Dis. 2008;14:1533–8. doi: 10.3201/eid1410.070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Racloz V, Venter G, Griot C, Stärk KDC. Estimating the temporal and spatial risk of bluetongue related to the incursion of infected vectors into Switzerland. BMC Veter Res. 2008;4:60–8. doi: 10.1186/1746-6148-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gubbins S, Carpenter S, Baylis M, Wood JLN, Mellor PS. Assessing the risk of bluetongue to UK livestock: uncertainty and sensitivity analyses of a temperature-dependent model for the basic reproduction number. J R Soc Interface. 2008;5:363–71. doi: 10.1098/rsif.2007.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hay SI, Rogers DJ, Randolph SE, Stern DI, Cox J, Shanks GD, et al. Hot topic or hot air? Climate change and malaria resurgence in East African highlands. Trends Parasitol. 2002;18:530–4. doi: 10.1016/s1471-4922(02)02374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouma MJ. Methodological problems and amendments to demonstrate effects of temperature on the epidemiology of malaria. A new perspective on the highland epidemics in Madagascar, 1972–89. Trans R Soc Trop Med Hyg. 2003;97:133–9. doi: 10.1016/s0035-9203(03)90099-x. [DOI] [PubMed] [Google Scholar]
- 26.Zhou G, Minakawa N, Githeko AK, Yan G. Association between climate variability and malaria epidemics in the East African highlands. Proc Natl Acad Sci USA. 2004;101:2375–80. doi: 10.1073/pnas.0308714100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minakawa N, Omukunda E, Zhou G, Githeko A, Yan G. Malaria vector productivity in relation to the highland environment in Kenya. Am J Trop Med Hyg. 2006;75:448–53. [PubMed] [Google Scholar]
- 28.Pascual M, Cazelles B, Bouma MJ, Chaves LF, Koelle K. Shifting patterns: malaria dynamics and rainfall variability in an African highland. Proc Biol Sci. 2008;275:123–32. doi: 10.1098/rspb.2007.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Githeko A. African highland malaria. In: Sauerborn R, Louis V, editors. Global environmental change and infectious diseases: impacts and adaptation strategies. Heidelberg: Springer Verlag; 2009. (forthcoming) [Google Scholar]
- 30.Yé Y, Sauerborn R, Simboro S, Hoshen M. Using modeling to assess malaria infection risk during the dry season on a local scale in an endemic area of rural Burkina Faso. Ann Trop Med Parasitol. 2007;101:375–89. doi: 10.1179/136485907X176490. [DOI] [PubMed] [Google Scholar]
- 31.Yé Y, Hoshen M, Kyobutungi C, Louis VR, Sauerborn R. Local scale prediction of Plasmodium falciparum malaria transmission in an endemic region: a meteorologically based dynamic model. Global Health Action. 2009 doi: 10.3402/gha.v2i0.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rydén P, Sjöstedt A, Johansson A. Effects of climate change on tularemia activity in Sweden. Global Health Action. 2009 doi: 10.3402/gha.v2i0.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray JS, Dautel H, Estrada-Peña A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infect Dis. 2009 doi: 10.1155/2009/593232. 593232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovats RS, Bouma MJ, Hajat S, Worrall E, Haines A. El Niño and health. Lancet. 2003;362:1481–9. doi: 10.1016/S0140-6736(03)14695-8. [DOI] [PubMed] [Google Scholar]
- 35.Anyamba A, Chretien JP, Small J, Tucker CJ, Linthicum KJ. Developing global climate anomalies suggest potential disease risks for 2006–2007. Int J Health Geogr. 2006;5:60–8. doi: 10.1186/1476-072X-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palo RT. Time series analysis performed on nephropathia epidemica in humans of northern Sweden in relation to bank vole population dynamic and the NAO index. Zoonoses Public Health. 2009;56:150–6. doi: 10.1111/j.1863-2378.2008.01162.x. [DOI] [PubMed] [Google Scholar]
- 37.Hubalek Z. North Atlantic weather oscillation and human infectious diseases in the Czech Republic, 1951–2003. Eur J Epidemiol. 2005;20:263–70. doi: 10.1007/s10654-004-6518-3. [DOI] [PubMed] [Google Scholar]
- 38.WHO. Global alert and response network (GOARN) 2009. Available from: http://www.who.int/csr/outbreaknetwork/en/ [cited 28 September 2009]
- 39.Parkinson AJ, Bruce M, Zultz T. International circumpolar surveillance, and Arctic network for surveillance of infectious diseases. Emerg Infect Dis. 2008;14:18–24. doi: 10.3201/eid1401.070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling HY, Rocklöv J, Ng N, Sauerborn R, Siang CT, Yin PF. Climate variability and increase in intensity and magnitude of dengue incidence in Singapore. Global Health Action. 2009 doi: 10.3402/gha.v2i0.2036. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.IPCC. Climate change: III. Mitigation of climate change. Cambridge: Cambridge University Press; 2007. Fourth assessment report. [Google Scholar]
- 42.NIAID. Lyme disease – the facts and the challenge. NIH publication No. 08-7045. 2008. Available from: http://www3.niaid.nih.gov/topics/lymeDisease/PDF/LymeDisease.pdf [cited 14 June 2009]
- 43.Jambou R, Legrand E, Niang M, Khim N, Lim P, Volney B, et al. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet. 2005;366:1960–3. doi: 10.1016/S0140-6736(05)67787-2. [DOI] [PubMed] [Google Scholar]
- 44.Noedl H, Socheat D, Satimai W. Artemisinin-resistant malaria in Asia. N Engl J Med. 2009;30:540–1. doi: 10.1056/NEJMc0900231. [DOI] [PubMed] [Google Scholar]
- 45.Sauerborn R. Global environmental change-an agenda for research and teaching in public health – invited editorial. Scand J Public Health. 2007;35:561–3. doi: 10.1080/14034940701671388. [DOI] [PubMed] [Google Scholar]
- 46.Sauerborn R, Kjellstrom T, Nilsson M. Health as a crucial driver for climate policy. Global Health Action. 2009 doi: 10.3402/gha.v2i0.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.UN Conference on Environment and Development. Rio declaration on environment and development. 1992. Available from: http://www.unep.org/Documents.Multilingual/Default.asp?documentID=78&articleID=1163 [cited 14 June 2009]