Abstract
Fibromyalgia is a condition with widespread muscle pain. Prevalence studies showed that 2% to 7% of the population have fibromyalgia, which affects approximately one million Canadians. Fibromyalgia is most common in women, but it also involves men and children. As with most chronic illnesses, the causes of fibromyalgia are unknown. However, recent research supports underlying abnormalities in the central nervous system, which supports fibromyalgia as a chronic disease state and valid clinical entity. Pain is the primary symptom, often accompanied by overwhelming fatigue, sleep dysfunction and cognitive impairment. In 1990, the American College of Rheumatology developed diagnostic criteria for the diagnosis of fibromyalgia. Lifestyle changes, including pacing of activities and aerobic exercise, are very important in managing fibromyalgia symptoms. Emotional and behavioural therapy can also be helpful. Controlled trials of antidepressants, gabapentinoids, tramadol, zopiclone and sodium oxybate have shown effectiveness in fibromyalgia patients. Pregabalin and duloxetine were recently approved in the United States. Effective management of fibromyalgia is complex and requires a multidisciplinary treatment approach. Response and tolerance of different therapeutic interventions vary from patient to patient. Recent advances in the pathophysiology of fibromyalgia offer hope for new and improved therapies in the management of this disabling condition.
Keywords: Fatigue, Fibromyalgia, Pain, Treatment
Abstract
La fibromyalgie est une maladie qui s’accompagne d’importantes douleurs musculaires. Les études sur sa prévalence ont montré que de 2 % à 7 % de la population souffrirait de fibromyalgie; c’est donc dire qu’elle affecterait jusqu’à trois millions de Canadiens. La fibromyalgie est plus fréquente chez les femmes, mais elle touche également les hommes et les enfants. Comme dans la plupart des maladies chroniques, on ignore quelle en sont les causes. Toutefois, de récentes recherches appuient la thèse voulant que des anomalies du système nerveux central sous-tendent la fibromyalgie en tant que maladie chronique et entité clinique valide. La douleur en est le principal symptôme, souvent accompagnée d’une énorme fatigue, de troubles du sommeil et d’une atteinte cognitive. En 1990, l’American College of Rheumatology a établi les critères diagnostiques pour confirmer la présence de fibromyalgie. Certains changements au mode de vie, notamment un dosage des activités et la pratique d’exercices aérobiques jouent un rôle très important dans la prise en charge des symptômes de fibromyalgie. Une thérapie émotivo-comportementale peut également être utile. Des études contrôlées sur les antidépresseurs, les gabapentinoïdes, le tramadol, le zopiclone et l’oxybate de sodium ont fait état de leur efficacité chez les patients atteints de fibromyalgie. La prégabaline et la duloxétine ont récemment été approuvée aux États-Unis. Le traitement efficace de la fibromyalgie est complexe et requiert une approche pluridisciplinaire. La réponse et la tolérance aux diverses approches thérapeutiques varient d’un patient à l’autre. Les progrès récemment réalisés dans le domaine de la physiopathologie de la fibromyalgie permettent d’espérer la découverte de nouvelles thérapeutiques améliorées pour le traitement de cette maladie invalidante.
The purpose of the present article is to review the pathophysiology and clinical presentation of fibromyalgia and itsmanagement. Recent data has provided a clearer understanding of the pathophysiology and this has lead to new treatments in the management of this disabling condition.
The prevalence of chronic musculoskeletal pain in most industrialized nations is estimated to be approximately 35% of the general population (1). Fibromyalgia is a condition with widespread muscle pain (2,3). Epidemiological studies show that 2% to 7% of the population in the developed world have fibromyalgia (4). This is responsible for 5% to 6% of adult patients presenting to general medical and family practice clinics, and 10% to 20% of adult patients in rheumatology clinics (5). It is estimated that approximately one million Canadians have this affliction (6).
Fibromyalgia is most common in women 30 to 55 years of age at symptom onset (7). It also effects men and children (2,6). School is very challenging for children with fibromyalgia due to its effects on cognition, fatigue and chronic pain (6). A study of 12-to 18-year-olds with fibromyalgia revealed that these teenagers were perceived as being isolated, less popular and less well-liked. This may impact on the psychological development of these adolescents (8).
Fibromyalgia is a complex multidimensional disorder that is unpredictable in its course (9,10). Management of this condition is a challenge for the patient and health provider alike.
ETIOLOGY
Similar to other chronic conditions, the exact causes of fibromyalgia are not known (2,11). The fact that a trial of prednisone versus placebo (12) showed no improvement and a trend toward deterioration in fibromyalgia patients strongly suggests that fibromyalgia does not have an inflammatory component. Some patients reported the initiating event as a psychological or physical trauma, or a viral infection (hepatitis B or C, or HIV) (3,4). Other patients described a slow, gradual onset of symptoms with no precipitating event (4,6). Genetics may also play a role (6).
CLINICAL PRESENTATION
Pain is the primary symptom, often accompanied by overwhelming fatigue, sleep dysfunction and cognitive impairment. Other symptoms may include stiffness, depression, anxiety, exercise intolerance (10), balance problems, irritable bowel syndrome, headaches, tingling and numbness, or restless legs syndrome (RLS). Patients can be highly sensitive to cold temperatures, noise, odours and light, and this may lead to sensory overload (6). Patients may also have chronic fatigue syndrome (CFS) (11). Despite the overwhelming number of symptoms, virtually all patients with fibromyalgia will experience pain, fatigue and sleep disturbance (5).
DIFFERENTIAL DIAGNOSIS
A thorough history and physical examination of the patient is paramount for making the diagnosis. Currently, there are no objective laboratory tests or imaging studies readily available that can produce positive results for fibromyalgia. One must rule out other diseases that can mimic fibromyalgia (13). These include rheumatoid arthritis, Sjogren’s syndrome, systemic lupus erythematosus, ankylosing spondylitis, polymyalgia rheumatica, inflammatory myositis and metabolic myopathies, hypothyroidism, peripheral neuropathies and osteomalacic myopathy (14). A Clinical Evaluation of Fibromyalgia tool for physicians can be found in the Overview of the Canadian Consensus Document on Fibromyalgia to assist in the evaluation and assessment of patients (6).
There is significant overlap of symptoms between fibromyalgia and CFS. These include muscle pain, fatigue, sleep disturbance, cognitive dysfunction, abdominal pain, muscle weakness, migratory arthralgias and decreased activity (5). A high proportion of patients meet the diagnostic criteria for both fibromyalgia and CFS (7). In general, the fibromyalgia patient will state pain as their worst symptom, whereas chronic fatigue patients report overwhelming fatigue.
DIAGNOSTIC CRITERIA FOR FIBROMYALGIA
In 1990, the American College of Rheumatology developed criteria for the diagnosis of fibromyalgia (3,5,6,13). In general terms, the patient must have widespread musculoskeletal pain for at least three months, and excessive tenderness in at least 11 of 18 defined tender points (6,7). A worksheet for diagnosis of fibromyalgia is available on-line (6).
PATHOGENESIS OF FIBROMYALGIA
Pain
Chronic widespread pain is the hallmark symptom of fibromyalgia (3,15). The pain may be described as widespread and exhausting, a bruised feeling, tingling, deep aching, throbbing, shooting, stabbing, sharp or burning (6). Fibromyalgia patients also have a lower pain threshold than healthy people (3). Allodynia (a normally nonpainful stimulus perceived as pain) and hyperalgesia (a painful stimulus is perceived as even more painful) are common responses in these patients (1,3). The pathophysiology of fibromyalgia is likely related to neurotransmitter and neuroendocrine abnormalities (1,3,13,15).
The term ‘central sensitization’ describes the ‘amplification’ of pain and other sensory input (noise, odours, light) in fibromyalgia (1,3,13,15) and there is evidence that this ‘wind-up’ phenomena plays a role in the pathogenesis. ‘Wind-up’ results in perception of more intense pain when experiencing a subsequent equally painful stimulus (3). N-methyl-D-aspartic acid (NMDA) receptors in the dorsal horn of the spinal cord are activated after repeated neuronal depolarization (3) and are probably responsible for the clinical expression of temporal summation to pain. There is evidence that the NMDA receptor antagonists ketamine and dextromethorphan reduce temporal summation in patients with fibromyalgia (16,17). There is also emerging evidence that microglial activation within the central nervous system (CNS) as a result of the release of various pronociceptive cytokines plays a role in central sensitization (18). A report of significantly elevated levels of interleukin-1 receptor antibodies and interleukin-6 in patients with fibromyalgia provides support for the role of cytokines in this condition (19). Glial cells that surround pain neurons can enhance and alter the signalling and perception of pain (3).
There is also evidence that descending serotonergic and noradrenergic projections from the brain stem to the spinal cord that normally inhibit nociceptive transmission are deficient in fibromyalgia (20,21). Cerebrospinal fluid analyses have shown that substance P levels are elevated up to three times the normal level (22,23) and nerve growth factor is elevated up to four times the normal level (24). These neurochemical imbalances likely contribute to an increased perception of pain in fibromyalgia.
Fibromyalgia patients have a dysfunctional hypothalamicpituitary-adrenal axis. This results in low morning serum cortisol levels, increased adrenocortical trophic hormone and failed normal suppression with dexamethasone (7,13). This affects the patient’s physiological response to stress (3).
Sleep deprivation
Restorative sleep escapes up to 90% of patients (25). Stage 4 sleep (the deepest sleep) is markedly deficient in fibromyalgia patients and alpha-wave activity is over-represented (11). Repeated nights of nonrestorative sleep causes extreme fatigue (25) and exacerbates pain.
Cognitive dysfunction
This is commonly referred to by patients as ‘fibro fog’. Cognitive impairment worsens during periods of symptom flare-up. Deficient neurophysiology of the CNS may contribute to memory difficulties and impaired cognition (25). One study (26) looking at cognitive deficits in fibromyalgia patients compared with age-matched controls found that fibromyalgia patients functioned similar to control subjects 20 years their senior.
DIAGNOSTIC IMAGING FINDINGS
Diagnostic imaging studies support the contention that dysfunction of the CNS and altered sensory input processing explain many of the symptoms reported by fibromyalgia patients (25). Functional magnetic resonance imaging (MRI) showed augmented pain processing in fibromyalgia. These functional MRI differences were observed at a lower pain stimulus than the necessary pain stimulus needed in controls (27). Single photon emission computed tomography scans in fibromyalgia patients show a decrease in cerebral blood flow in brain structures that modulate pain perception (28). A recent study using voxel-based morphometric analysis of MRI brain images showed that patients had decreased grey matter (atrophy) and total brain volume compared with controls. The normal age-related grey matter reduction was accelerated in patients, and correlated with the number of years the patient had experienced fibromyalgia symptoms. There were clusters of grey matter with significantly lower density in fibromyalgia patients compared with controls, which matched structures involved in stress and pain processing, and areas consistent with cognitive difficulties (29). Cerebral abnormalities and increased neural recruitment during cognitive tasks have been demonstrated in neuroimaging studies (30).
MANAGEMENT OF THE FIBROMYALGIA PATIENT
There is no ‘magic bullet’ cure for fibromyalgia at present. Symptom control is the focus in the context of a multidisciplinary evaluation of pain, function and psychosocial issues. Decreasing pain, improving sleep and establishing a regular exercise program tailored to the patient are the major goals (25). Each patient has a different constellation of symptoms resulting in different responses to therapeutic interventions. A specific intervention will not work for every patient. An internet survey of 2596 fibromyalgia patients found that the most effective interventions in descending order were rest, heat modalities, prescription pain medications, prescription anti-depressants, prescription sleep medications, prayer, massage and pool therapy (4).
The health provider and patient relationship
Fibromyalgia patients can be very challenging, desperate individuals. Despite recent advances in fibromyalgia research, there is still a stigma attached to this ‘invisible illness’. Most patients experience years of suffering, many doctor visits, and investigative tests with no answers before the diagnosis is made. Patients need validation of their symptoms and need to know it is not ‘all in their head’. Providers need to listen closely to the patient history. Focusing on one symptom per visit, starting with the worst symptom affecting the patient’s quality of life, may be helpful.
Nonpharmacological management
The best studied and efficacious interventions are exercise and cognitive behaviour therapy (31).
EXERCISE
Busch et al (32) recently published a systematic review of 34 randomized controlled trials (RCTs) on the effect of exercise on fibromyalgia patients. A meta-analysis of six studies showed moderate-quality evidence that aerobic-only exercise had positive effects on global well-being and physical function. A possible improvement in pain, depression and tender points was seen (inconsistent or statistically insignificant results). Strength and flexibility were underevaluated. Improved adherence and larger numbers are necessary before a broad recommendation can be made. No evidence was found for flexibility-only exercise and its effects on fibromyalgia (32).
COGNITIVE BEHAVIOUR THERAPY
Emotional and cognitive factors (thoughts, memories, beliefs, expectations, fears) interact with how a patient handles sensory input, including pain perception (1). Changing a patient’s behaviour and thinking about their pain has been found to decrease pain severity and improve function in RCTs in fibromyalgia patients (33–35).
LIFESTYLE CHANGES
Beneficial approaches include maximizing sleep by improving sleep hygiene (5,6), eating a healthy diet, education on fibromyalgia and pacing activities (6). Three RCTs have shown benefits in patients who practise meditation, relaxation and stress management (36,37).
Pharmacological management
Many fibromyalgia patients are unusually sensitive to the adverse effects of medication. To minimize adverse effects, start treatment at a low initial dose and titrate slowly. Educate the patient about possible adverse effects, and stress that side effects may disappear as the body adjusts to the medication.
PAIN MANAGEMENT
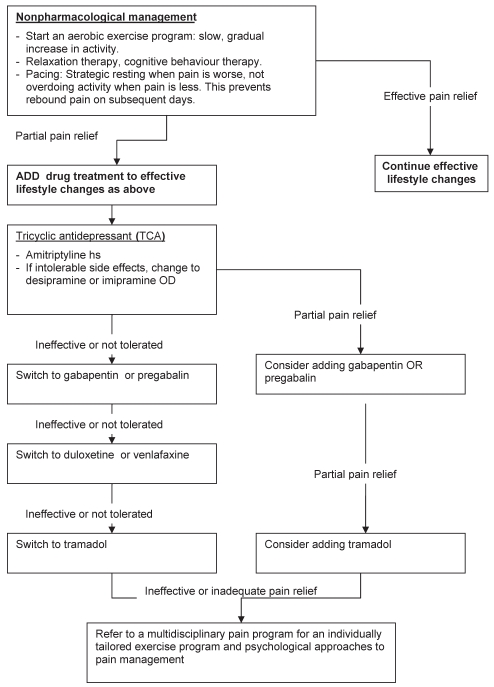
The pain experienced in fibromyalgia has similar features to neuropathic pain syndromes, such as postherpetic neuralgia and diabetic peripheral neuropathy. These pain syndromes all feature spontaneous and stimulus-dependent pain sensations including allodynia, hyperalgesia and paresthesias (38). Drugs that have been successful in the treatment of these neuropathic pain conditions have been studied in fibromyalgia. They include antidepressants, gabapentinoids and other adjuvant analgesics. Table 1 lists recommended drugs, dosages and common side effects. Figure 1 provides a flow diagram for the pain management of fibromyalgia that was extrapolated from the European League Against Rheumatism published guidelines for this condition (39). All the agents recommended for pharmacological management are evidence-based, although the order of use is simply a suggestion of the authors based on evidence of efficacy, side effect profile and cost.
Table 1.
Medications used in fibromyalgia management
| Drug class | Drug example | Dosage | Common adverse effects | Comments |
|---|---|---|---|---|
| Tricyclic antidepressants | Amitriptyline, desipramine, imipramine | 10–50 mg qhs | Sedation, orthostatic hypotension, heart block, dry mouth, urinary retention, constipation | Much less expensive than SNRIs |
| Selective serotonin reuptake inhibitors | Fluoxetine | 10–80 mg once a day | Nausea, tremor, somnolence | Conflicting results |
| SNRIs | Venlafaxine | 37.5–225 mg twice a day | Nausea, dry mouth, ataxia, hypertension, withdrawal effects | Take with food to decrease nausea |
| Duloxetine | 30 mg once a day to 60 mg twice a day | Sedation, dizziness, dry mouth | FDA-approved indication for fibromyalgia | |
| Anticonvulsants | Gabapentin | Initial: 100–300 mg qhs, then: 600–900 mg three times a day | Fatigue, weight gain, peripheral edema, ataxia, nystagmus, diplopia | |
| Pregabalin | Initial: 50–75 mg qhs, then: 75–225 mg twice a day | Dizziness, sedation, dry mouth, weight gain | FDA-approved indication for fibromyalgia | |
| Sedatives | Zopiclone | 3.75–7.5 mg qhs | Dry mouth, bitter taste, residual sedation | Nonbenzodiazepine |
| Clonazepam | 0.125–0.5 mg qhs | Dizziness, incoordination, dependence | Helps restless legs symptoms | |
| Sodium oxybate | Initial: 625 mg qhs, ×2 doses 4 h apart; then: 2.25–3 g qhs, ×2 doses 4 h apart | Headache, nausea, dizziness, pain, somnolence, dependence | Very quick onset of action, take while lying in bed; restricted medical doctor prescribing | |
| Other | Tramadol 37.5 mg and acetaminophen 325 mg | 2–8 tablets/day, divided doses | Nausea, seizures, sedation, respiratory depression, ataxia, constipation | Maximum 8 tablets/day to avoid acetaminophen toxicity. Increased seizure risk |
| Tramadol extended release | 100–400 mg once a day | Caution with drugs that increase serotonin |
FDA United States Food and Drug Administration; hs At bedtime or in the evening; q every; SNRI Serotonin noradrenaline reuptake inhibitor
Figure 1).
Recommended flow diagram for pain management of fibromyalgia. Extrapolated from the European League Against Rheumatism published guidelines for fibromyalgia (39). hs At bedtime or in the evening; OD Once a day
Antidepressants
To date, tricyclic antidepressants (TCAs) have been the most studied class of analgesics, although no fibromyalgia trials have directly compared TCAs with the newer serotonin noradrenaline reuptake inhibitors (SNRIs) (40). Beneficial effects on pain are independent of the antidepressant effects.
Amitriptyline and doxepin are the most commonly studied TCAs in fibromyalgia. A meta-analysis of nine controlled studies of tricyclics showed significant improvement in pain, stiffness, tenderness, fatigue and sleep quality when compared with placebo (5). Unfortunately, adverse effects are common with the TCAs. Administering the dose at bedtime will minimize the anticholinergic effects and take advantage of the sedative effects. Trying a different drug within the TCA class with a modified adverse effect profile can be beneficial. A change from amitriptyline to desipramine or imipramine can minimize anticholinergic and sedative effects. Other drugs may be required in patients unable to tolerate the anticholinergic, antiadrenergic, antihistaminergic and quinidine-like effects seen with the TCAs (40).
Among the selective serotonin reuptake inhibitors, fluoxetine and paroxetine were found to be more effective than citalopram (30,41). A small trial of 19 fibromyalgia patients showed that both amitriptyline 25 mg/day and fluoxetine 20 mg/day provided significant improvement in pain, function and well-being when compared with placebo. Patients receiving both amitriptyline and fluoxetine had significant benefits compared with placebo or each drug used alone (40).
Of the SNRIs, venlafaxine, duloxetine and milnacipran have been shown to provide benefit to fibromyalgia patients (41). The adverse effect profile may be superior to that of the TCAs because SNRIs do not interact with cholinergic, adrenergic or histaminergic receptors, or sodium channels (41). However, the SNRIs are much more expensive than TCAs.
Venlafaxine is more potent at inhibiting serotonin reuptake at lower doses (100 mg/day or less) and stronger at inhibiting noradrenaline reuptake at higher doses (more than 100 mg/day) (40). A randomized, double-blind, placebo-controlled study (40) of venlafaxine 75 mg/day for six weeks in fibromyalgia patients failed to show significant improvement in pain measures. The secondary measures (total fibromyalgia impact questionnaire [FIQ], and FIQ pain and fatigue subscales) did improve significantly compared with placebo. The relatively low dose used in this trial may have blunted the analgesic benefit. Two other venlafaxine studies (42,43) showed promising results, but the impact was limited by the open-label study design.
Duloxetine is approved in Canada and the United States for the management of painful diabetic neuropathy and major depressive disorder; it has recently received United States Food and Drug Administration (FDA) approval for the treatment of fibromyalgia. Two large, randomized, double-blind, placebo-controlled trials of duloxetine in fibromyalgia patients in doses of up to 60 mg twice a day showed significant improvement in pain scores, irrespective of concomitant major depressive disorder (44,45). The latter trial by Arnold et al (45) involved 354 women and compared placebo with duloxetine 60 mg once a day or twice a day. There was no significant difference in efficacy outcome measures between the two active treatment groups relative to placebo. A recent RCT of duloxetine in 520 fibromyalgia patients with or without depression found that daily doses of duloxetine 60 mg and 120 mg improved pain symptoms and overall patient global ratings of improvement at three months, and pain symptoms at six months. Significantly more patients discontinued the study secondary to an adverse event in the 120 mg treatment group compared with placebo (46).
Milnacipran is a SNRI marketed in Europe and Asia but not presently available in North America. The FDA is currently reviewing an application for the indication of fibromyalgia. Milnacipran also has mild NMDA inhibition properties that may inhibit or attenuate central sensitization, and therefore may enhance pain control in comparison with venlafaxine and duloxetine (40,41). A phase 2 trial showed significant improvement in pain, global well-being and fatigue compared with placebo (47).
Anticonvulsants
Gabapentin and pregabalin have shown efficacy in the management of painful diabetic neuropathy and postherpetic neuralgia (1). Their mechanism of action is likely caused by binding to the voltage-dependent calcium channel in the CNS, blocking the influx of calcium into the neuron (15) and thereby reducing excitatory neurotransmitter release.
Gabapentin in doses of between 1200 mg and 2400 mg per day in fibromyalgia patients in a randomized, placebo-controlled, double-blind trial showed significant improvement in pain, FIQ score and sleep. It was generally well tolerated (48).
Pregabalin in doses of 150 mg, 300 mg and 450 mg per day showed that 450 mg/day significantly decreased pain in a double-blind, placebo-controlled trial. The 300 mg and 450 mg per day groups both realized significant improvement in quality of sleep compared with the placebo group (49). Two more recent trials of similar design also showed significant benefit (50,51). Based on this evidence, pregabalin was approved by the FDA for the management of fibromyalgia. The most frequent side effects of pregabalin in fibromyalgia patients are drowsiness, dizziness, blurred vision, weight gain, dry mouth, and hand and feet swelling. Adverse effects may be minimized by starting at low doses and increasing weekly as tolerated (52).
Tramadol
Tramadol is a weak mu-opioid receptor agonist and an inhibitor of serotonin and noradrenaline reuptake (5,41). A double-blind, placebo-controlled trial (53) in 315 fibromyalgia patients compared placebo with Tramacet (Janssen-Ortho Inc, Canada; tramadol 37.5 mg and acetaminophen 325 mg). Patients taking a mean (± SD) of 4±1.8 tablets daily were significantly more likely to continue treatment than placebo. The patients also had significantly improved pain and physical function. The most common adverse effects in the Tramacet group were nausea, dizziness, somnolence and constipation.
Dopamine agonists
Dopamine agonists may be of benefit in fibromyalgia patients especially if they also have RLS, which further inhibits deep restorative sleep. RLS is 10 times more common in fibromyalgia patients than in the general population (54). Dopamine agonists have been shown to be effective in RLS (54). A randomized, double-blind, placebo-controlled study of pramipexole 4.5 mg at bedtime or in the evening was performed with 60 fibromyalgia patients. Pramipexole patients showed significant improvement in pain, fatigue, function and global status (55). However, ropinirole was studied in another trial using a similar design with negative results (54).
Opioids
Conventional opioid analgesics have produced inconsistent results in the management of fibromyalgia. Ineffectiveness may be explained by increased endorphin release and decreased mu-opioid receptor binding in fibromyalgia patients compared with healthy controls matched for age and sex (56). Conventional opioid analgesics are generally not recommended in the management of fibromyalgia, although an Internet survey reported some benefit (6).
SEDATIVE MANAGEMENT
Nonrestorative, disrupted sleep is common in fibromyalgia. Zopiclone and zolpidem (United States product) are nonbenzodiazepines shown to improve sleep and decrease fatigue (5,41). Gamma-hydroxybutyrate (GHB; sodium oxybate) is a precursor to gamma-aminobuytric acid and is a very powerful sedative (5). It is an agonist for the gamma-aminobuytric acid-B and GHB receptor. GHB also modulates dopaminergic activity by increasing synthesis of dopamine. Two studies in fibromyalgia patients using 4.5 g or 6 g at night (divided into two doses each evening or at bedtime, and 4 h later) showed improvement in deep sleep (with a decrease in intrusive alpha-waves), pain and fatigue (15).
Complementary and alternative medicine
There is little research on herbs and supplements for fibromyalgia (6). The Internet is abound with ‘fibromyalgia cures’ that are very enticing to the desperate fibromyalgia patient. These products are not cures and are often expensive. Melatonin has been shown to promote sleep in fibromyalgia patients (5).
The effects of acupuncture in fibromyalgia are not clear and require well-designed clinical trials (10). A systematic review of five RCTs showed positive but short-lived results in three studies using electroacupuncture, and negative results in two studies. Acupuncture is not supported by these results for fibromyalgia treatment (57).
SUMMARY
Effective management of fibromyalgia symptoms is complex and requires a multidisciplinary approach evaluating pain, function and the psychosocial milieu of the fibromyalgia patient. Response and tolerance to treatment varies among patients. Nonpharmacological interventions are a necessity and complement drug therapy. A ‘strategic polypharmacy’ approach using drugs with different mechanisms of action can be helpful. An organized approach that manages the worst symptom first can facilitate easier management of the other symptoms. Effective symptom control is expanding as the underlying pathophysiology of fibromyalgia becomes clearer. The emergence of novel agents that are more effective in shutting down central sensitization may be more effective in treating this disabling condition. The provider can help by believing in the patient, and evaluating and treating the patient systematically.
REFERENCES
- 1.Bergman S. Management of musculoskeletal pain. Best Pract Res Clin Rheumatol. 2007;21:153–66. doi: 10.1016/j.berh.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 2.National Fibromyalgia Partnership. FMS Monograph. <http://www.fmpartnership.org/engmonog.htm> (Version current at November 12, 2008).
- 3.Abeles AM, Pillinger MH, Solitar BM, Abeles M. Narrative review: The pathophysiology of fibromyalgia. Ann Inter Med. 2007;146:726–34. doi: 10.7326/0003-4819-146-10-200705150-00006. [DOI] [PubMed] [Google Scholar]
- 4.Bennett RM, Jones J, Turk D, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:27. doi: 10.1186/1471-2474-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mease P. Fibromyalgia syndrome: Review of clinical presentation, pathogenesis, outcome measures, and treatment. J Rheumatol Suppl. 2005;75:6–21. [PubMed] [Google Scholar]
- 6.Jain AK, Carruthers BM, van de Sande MI.Fibromyalgia syndrome: A clinical case definition and guidelines for medical practitionersAn overview of the Canadian consensus document. <http//sacfs.asn.au/download/consensus_overview_fms.pdf> (Version current at November 12, 2008).
- 7.Goldenberg DL.Clinical manifestations and diagnosis of fibromyalgia in adults<http://www.uptodate.com/patients/content/topic.do?topicKey=~yF_Fabo3Q5GAUd> (Version current at November 13, 2008).
- 8.Kashikar-Zuck S, Lynch AM, Graham TB, Swain NF, Mullen SM, Noll RB. Social functioning and peer relationships of adolescents with juvenile fibromyalgia syndrome. Arthritis Care Res. 2007;57:474–80. doi: 10.1002/art.22615. [DOI] [PubMed] [Google Scholar]
- 9.Sarzi-Puttini P, Buskila D, Carrabba M, Doria A, Atzeni F. Treatment strategy in fibromyalgia syndrome: Where are we now? Semin Arthritis Rheum. 2007;37:353–65. doi: 10.1016/j.semarthrit.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Rooks DS. Fibromyalgia treatment update. Curr Opin Rheumatol. 2007;19:111–7. doi: 10.1097/BOR.0b013e328040bffa. [DOI] [PubMed] [Google Scholar]
- 11.Shuer ML. Fibromyalgia symptom constellation and potential therapeutic options. Endocrine. 2003;22:67–75. doi: 10.1385/ENDO:22:1:67. [DOI] [PubMed] [Google Scholar]
- 12.Clark S, Tindall E, Bennett RM. A double-blind crossover trial of prednisone versus placebo in the treatment of fibrositis. J Rheumatol. 1985;12:980–3. [PubMed] [Google Scholar]
- 13.Rothenberg R. Fibromyalgia documentation and treatment. A guide for primary care professionals. Fibromyalgia Frontiers. 2007;15:11–6. [Google Scholar]
- 14.Goldenberg DL.Differential diagnosis of fibromyalgia<http://www.uptodate.com/patients/content/topic.do?topicKey=~IVIndOU9abHZK_> (Version current at November 13, 2008).
- 15.Wood PB, Holman AJ, Jones KD. Novel pharmacotherapy for fibromyalgia. Expert Opin Investig Drugs. 2007;16:829–41. doi: 10.1517/13543784.16.6.829. [DOI] [PubMed] [Google Scholar]
- 16.Graven-Nielsen T, Aspegren Kendall S, Henriksson KG, et al. Ketamine reduces muscle pain, temporal summation, and referred pain in fibromyalgia patients. Pain. 2000;85:483–91. doi: 10.1016/S0304-3959(99)00308-5. [DOI] [PubMed] [Google Scholar]
- 17.Staud R, Vierck CJ, Robinson ME, Price DD. Effects of the N-methyl-D-aspartate receptor antagonist dextromethorphan on temporal summation of pain are similar in fibromyalgia patients and normal control subjects. J Pain. 2005;6:323–32. doi: 10.1016/j.jpain.2005.01.357. [DOI] [PubMed] [Google Scholar]
- 18.Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: Implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–46. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace DJ, Linker-Israeli M, Hallegua D, Silverman S, Silver D, Weisman MH. Cytokines play an aetiopathogenetic role in fibromyalgia: A hypothesis and pilot study. Rheumatology (Oxford) 2001;40:743–9. doi: 10.1093/rheumatology/40.7.743. [DOI] [PubMed] [Google Scholar]
- 20.Russell IJ, Vaeroy H, Javors M, Nyberg F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550–6. doi: 10.1002/art.1780350509. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe F, Russell IJ, Vipraio G, Ross K, Anderson J. Serotonin levels, pain threshold, and fibromyalgic symptoms in the general population. J Rheumatol. 1997;24:555–9. [PubMed] [Google Scholar]
- 22.Russell IJ, Orr MD, Littman B, et al. Elevated cerebrospinal fluid levels of substance P in patients with fibromyalgia syndrome. Arthritis Rheum. 1994;37:1593–601. doi: 10.1002/art.1780371106. [DOI] [PubMed] [Google Scholar]
- 23.Vaerøy H, Helle R, Førre O, Kåss E, Terenius L. Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: New features for diagnosis. Pain. 1988;32:21–6. doi: 10.1016/0304-3959(88)90019-X. [DOI] [PubMed] [Google Scholar]
- 24.Giovengo SL, Russell IJ, Larson A. Increased concentration of nerve growth factor in the cerebrospinal fluid of patients with fibromyalgia. J Rheumatol. 1999;26:1564–9. [PubMed] [Google Scholar]
- 25.Peterson J. Understanding fibromyalgia and its treatment options. Nurse Pract. 2005;30:48–57. doi: 10.1097/00006205-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Park DC, Glass JM, Minear M, Crofford LJ. Cognitive function in fibromyalgia patients. Arthritis Rheum. 2001;44:2125–33. doi: 10.1002/1529-0131(200109)44:9<2125::AID-ART365>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–43. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 28.Yunus MB. Central sensitivity syndromes: Fibromyalgia and other similar conditions, the concept for unifying. Fibromyalgia Aware. 2002. May-Aug. pp. 41–45.
- 29.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: Premature aging of the brain? J Neurosci. 2007;27:4004–7. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glass JM. Cognitive dysfunction in fibromyalgia and chronic fatigue syndrome: New trends and future directions. Curr Rheumatol Rep. 2006;8:425–9. doi: 10.1007/s11926-006-0036-0. [DOI] [PubMed] [Google Scholar]
- 31.Dadabhoy D, Clauw DJ. Therapy insight: Fibromyalgia – different type of pain needing a different type of treatment. Nat Clin Pract Rheumatol. 2006;2:364–72. doi: 10.1038/ncprheum0221. [DOI] [PubMed] [Google Scholar]
- 32.Busch AJ, Schachter CL, Overend TJ, Peloso PM, Barber KA. Exercise for fibromyalgia: A systematic review. J Rheumatol. 2008;35:1130–44. [PubMed] [Google Scholar]
- 33.Nielson WR, Walker C, McCain GA. Cognitive behaviour treatment of fibromyalgia syndrome: preliminary findings. J Rheumatol. 1992;19:98–103. [PubMed] [Google Scholar]
- 34.White KP, Nielson WR. Cognitive behavioural treatment of fibromyalgia syndrome: A follow-up assessment. J Rheumatol. 1995;22:717–21. [PubMed] [Google Scholar]
- 35.Creamer P, Singh BB, Hochberg MC, Berman BM. Sustained improvement produced by nonpharmacologic intervention in fibromyalgia: Results of a pilot study. Arthritis Care Res. 2000;13:198–204. doi: 10.1002/1529-0131(200008)13:4<198::aid-anr4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan KH, Goldenberg DL, Galvin-Nadeau M. The impact of a medication-based stress reduction program on fibromyalgia. Gen Hosp Psychiatry. 1993;15:284–9. doi: 10.1016/0163-8343(93)90020-o. [DOI] [PubMed] [Google Scholar]
- 37.Wigers SH, Stiles TC, Vogal PA. Effects of aerobic exercise versus stress management treatment in fibromyalgia. Scand J Rheumatol. 1996;25:77–86. doi: 10.3109/03009749609069212. [DOI] [PubMed] [Google Scholar]
- 38.Offenbaecher M, Ackenheil M. Current trends in neuropathic pain treatments with special reference to fibromyalgia. CNS Spectr. 2005;10:285–97. doi: 10.1017/s1092852900022616. [DOI] [PubMed] [Google Scholar]
- 39.Carville SF, Arendt-Nielsen S, Bliddal H, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67:536–41. doi: 10.1136/ard.2007.071522. [DOI] [PubMed] [Google Scholar]
- 40.Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;8(Suppl 2):S63–74. doi: 10.1111/j.1526-4637.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 41.Arnold LM. Biology and therapy of fibromyalgia. New therapies in fibromyalgia. Arthritis Res Ther. 2006;8:212. doi: 10.1186/ar1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dwight MM, Arnold LM, O’Brien H, et al. An open clinical trial of venlafaxine in fibromyalgia. Psychosomatics. 1998;39:14–7. doi: 10.1016/S0033-3182(98)71375-1. [DOI] [PubMed] [Google Scholar]
- 43.Sayar K, Aksu G, Ak I, Tosun M. Venlafaxine treatment in fibromyalgia. Ann Pharmacother. 2003;37:1561–5. doi: 10.1345/aph.1D112. [DOI] [PubMed] [Google Scholar]
- 44.Arnold LM, Lu Y, Crofford LJ, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50:2974–84. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- 45.Arnold LM, Rosen A, Pritchett YL, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain. 2005;119:5–15. doi: 10.1016/j.pain.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 46.Russell IJ, Mease PJ, Smith TR, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: Results from a 6-month, randomized, double-blind, placebo-controlled fixed dose trial. Pain. 2008;136:432–44. doi: 10.1016/j.pain.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 47.Gendreau RM, Thorn MD, Gendreau JF, et al. Efficacy of milnacipran in patients with fibromyalgia. J Rheumatol. 2005;32:1975–85. [PubMed] [Google Scholar]
- 48.Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: A randomized, double-blind, placebo-controlled multicenter trial. Arthritis Rheum. 2007;56:1336–44. doi: 10.1002/art.22457. [DOI] [PubMed] [Google Scholar]
- 49.Crofford LJ, Rowbotham MC, Mease PJ, et al. Pregabalin 1008-105 Study Group Pregabalin for the treatment of fibromyalgia syndrome. Results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52:1264–73. doi: 10.1002/art.20983. [DOI] [PubMed] [Google Scholar]
- 50.Arnold L, Russell I, Duan W, et al. A 14-week, randomized, double-blind, placebo-controlled, monotherapy trial of pregabalin (BID) in patients with fibromyalgia syndrome (FMS) J Pain. 2007;8(Suppl):S24. doi: 10.1016/j.jpain.2008.03.013. (Abst) [DOI] [PubMed] [Google Scholar]
- 51.Crofford LJ, Mease PJ, Simpson SL, et al. Fibromyalgia relapse evaluation and efficacy for durability of meaningful relief (FREEDOM) trial: A 6-month, double-blind, placebo-controlled trial of treatment with pregabalin. Pain. 2008;136:419–31. doi: 10.1016/j.pain.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 52.Wood PB. Pregabalin for the treatment of fibromyalgia. Practical Pain Management. 2007. Jul-Aug. pp. 55–6.
- 53.Bennett RM, Kamin M, Karim R, Rosenthal N. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: A double-blind, randomized, placebo-controlled study. Am J Med. 2003;114:537–45. doi: 10.1016/s0002-9343(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 54.Holman AJ. Treatment of fibromyalgia: A changing of the guard. Womens Health. 2005;1:409–20. doi: 10.2217/17455057.1.3.409. [DOI] [PubMed] [Google Scholar]
- 55.Holman AJ, Myers RR. A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthritis Rheum. 2005;52:2495–505. doi: 10.1002/art.21191. [DOI] [PubMed] [Google Scholar]
- 56.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10, 000–6. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayhew E, Ernst E. Acupuncture for fibromyalgia – a systematic review of randomized clinical trials. Rheumatology (Oxford) 2007;46:801–4. doi: 10.1093/rheumatology/kel406. [DOI] [PubMed] [Google Scholar]