Abstract
Background and purpose
We recently showed that lapatinib, an EGFR/HER2 inhibitor, radiosensitized breast cancer cells of the basal and HER2+ subtypes. The purpose of this study was to identify the downstream signaling pathways responsible for lapatinib-mediated radiosensitization in breast cancer.
Materials and methods
Response of EGFR downstream signaling pathways were assessed by western blot and clonogenic cell survival assays in breast tumor cells after irradiation (5 Gy), lapatinib, CI-1040, or combined treatment.
Results
In SUM102 cells, an EGFR+ basal breast cancer cell line, exposure to ionizing radiation elicited strong activation of ERK1/2 and JNK, which was blocked by lapatinib, and weak/no activation of p38, AKT or STAT3. Direct inhibition of MEK1 with CI-1040 resulted in 95% inhibition of surviving colonies when combined with radiation while inhibition of JNK with SP600125 had no effect. Lapatinib-mediated radiosensitization of SUM102 cells was completely abrogated with expression of constitutively active Raf. Treatment of lapatinib-resistant SUM185 cells with CI-1040 restored radiosensitization with 45% fewer surviving colonies when combined with radiation.
Conclusions
These data suggest that radiosensitization by lapatinib is mediated largely through inhibition of MEK/ERK and that direct inhibition of this pathway may provide an additional avenue of radiosensitization in EGFR+ or HER2+ breast cancers.
Keywords: EGFR, lapatinib, CI-1040, MEK/ERK, resistance, breast cancer
Introduction
The use of pharmacological compounds targeting specific intracellular signaling pathways to sensitize tumor cells to ionizing radiation can improve the radiotherapeutic index resulting in improved patient outcomes [1]. Importantly, using a monoclonal antibody against EGFR which could provide a multifactorial response including antibody dependent cytotoxicity, a landmark Phase III randomized trial reported disease-free and overall survival advantages for HNSCC patients when treated with cetuximab in combination with radiation in comparison to treatment with radiation alone [2].
Breast cancer arises from one of two epithelial cell populations resulting in breast cancers of basal- or luminal-origin with basal subtypes conferring a worse prognosis [3-6]. At least 50% of basal-like breast cancers are known to express EGFR as determined by IHC [7] and we recently showed that breast tumor cell lines of the basal-subtype (SUM102 and SUM149), which express elevated levels of EGFR and normal levels of HER2, are not only growth impaired and radiosensitized by treatment with the dual EGFR/HER2 inhibitor lapatinib and the EGFR-specific inhibitor, gefitinib, but also show relatively higher sensitivity to gefitinib than breast cancer cells derived from luminal origin [8, 9]. Given that EGFR expression correlates with radioresistance [10, 11] and that EGFR can signal to a variety of downstream signaling pathways including MEK>ERK, PI3K>AKT, STAT, p38 and JNK [12, 13], identification of the downstream signaling pathways that mediate radiosensitization by EGFR or EGFR/HER2 inhibitors could reveal novel alternative radiosensitizers. In this study, we evaluated the downstream signaling pathways responsible for lapatinib-mediated radiosensitization in breast cancer cells of the basal subtype and demonstrate that radiosensitization is mediated primarily through inhibition of the Raf>MEK>ERK mitogen-activated protein kinase cascade. In addition, we demonstrate that radiosensitization of a lapatinib-resistant breast cancer cell line of luminal B subtype can be sensitized by inhibition of the Raf>MEK>ERK pathway. These studies reveal important mechanisms of the signaling pathways that mediate radiosensitization by an EGFR/HER2 inhibitor in breast cancer and suggest that future studies are warranted to evaluate inhibitors of the Raf>MEK>ERK pathway as radiosensitizers in breast cancer.
Materials & Methods
Reagents
Lapatinib, a dual EGFR/HER2 kinase inhibitor (provided by GlaxoSmithKline), CI-1040, a MEKI inhibitor (provided by Pfizer), and SP600125, a JNK inhibitor II (purchased from Calbiochem) were dissolved with dimethyl sulfoxide (DMSO), aliquoted and stored frozen at −20°C. All cell culture reagents were from GIBCO (Carlsbad, CA).
Cell lines and constructs
SUM102 and SUM185 cells were cultured as previously described [8]. Constitutively active c-Raf is an N-terminal truncation mutant referred to as c-Raf(22W) and was provided in the retroviral vector pBabe by Channing Der (University of North Carolina). SUM102 cells stably expressing pBabepuro/c-Raf-(22W) or pBabepuro alone were generated by retroviral infection with virus produced utilizing the triple transfection method in 293T cells as described by the manufacturer (Stratagene). Forty-eight h post-infection, cells were selected with puromycin (1 μg/ml), selected and used en masse.
Western blot analyses
Cells were plated and allowed to adhere in complete media for 24 h and treated with drug or equal amounts of vehicle control (DMSO) for 2 h before being irradiated with 5 Gy and protein lysates harvested with lysis buffer as previously described [8]. Proteins (30 μg) were separated over 12% sodium dodecyl sulphate (SDS)/poly-acrylamide gels and electrophoretically transferred to polyvinyl difluoride (PVDF), blocked, and probed with anti-phospho-ERK1/2 (T202/Y204, #9101), anti-total ERK1/2 (#9102), anti-phospho AKT (S473, #9271), anti-total AKT (#9272), anti-phospho JNK (T183/Y185, #9251), anti-total JNK (#9252), anti-phospho-p38 MAPK (T180/Y182, #9211), anti-total p38 MAPK (#9212), anti-phospho-STAT3 (Y705, #9131), anti-total STAT3 (#9132), all from Cell Signaling Technology, anti-β-actin (#A5316; Sigma), or anti-Raf1 (#sc-133; Santa Cruz Biotechnology). The secondary antibodies used were horseradish peroxidase–conjugated (Cell Signaling Technology) and visualization by enhanced chemiluminescence (Amersham). Immunoprecipitation of EGFR was with rabbit anti-EGFR (Ab22) and western blot with anti-phospho-tyrosine RC20 (BD Transduction Laboratories) as previously described [8].
Colony-forming assays
Cells were plated at low density overnight in complete media and treated for 2 h with drug or equal amounts of DMSO vehicle alone (control), irradiated or sham-irradiated with graded, single doses from a 137Cs irradiator at a dose rate of 158 cGy/min. In all conditions, the culture medium was changed 2 h post-irradiation to complete medium without drug. After 2 – 4 weeks of incubation, the samples were fixed counted as described [8]. The surviving fraction [number of colonies formed/number of cells plated x plating efficiency] was calculated from the number of colonies formed in the drug-treated, irradiated dishes compared with the number formed in the drug-treated, unirradiated control and significance as defined by p<0.001 by unpaired t-test indicated (*). The clonogenic survival curves were fitted to a linear-quadratic model (SF=e−[α * D + β * D2]) using GraphPad Prism 5.0 according to a least squares fit, weighted to minimize the relative distances squared, and compared using the extra sum-of-squares F test. Each point represents the mean surviving fraction from at least three dishes; error bars represent the standard deviation.
Results
Radiation Induced Activation of ERK1/2 and JNK are Blocked by Lapatinib
Basal-like breast cancer represents 16% of all breast cancers and patients with this subtype face a poor prognosis (reviewed in [14]). SUM102 cells are breast cancer cells of the basal-subtype that overexpress EGFR. The purpose of this study was to identify the downstream signaling pathways responsible for lapatinib-mediated radiosensitization in breast cancer using the SUM102 cells as a model system. We first verified lapatinib could block EGFR activation in SUM102 cells stimulated with EGF (Supp. Fig. 1).
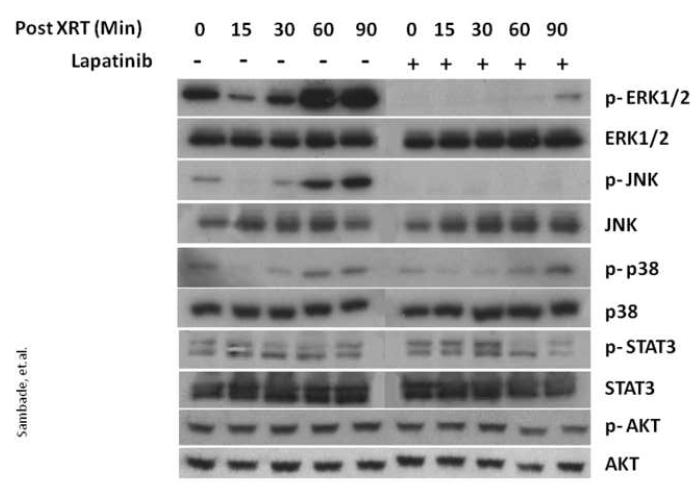
Next, to identify which pathways are activated downstream of EGFR in response to radiation, we assessed the changes in levels of activated p-ERK1/2, p-AKT, p-STAT3, p-p38, and p-JNK in SUM102 cells treated with 5Gy radiation. As shown in Fig. 1, SUM102 cells showed an initial decrease in levels of activated p-ERK1/2 and p-JNK at 15 and 30 min post-irradiation followed by a robust activation at 60 and 90 min. In contrast, while activated p-p38 was similarly inhibited at 15 post-irradiation, expression levels similar to that at baseline were restored by 60 min. In addition, no significant change in either p-STAT3 or p-AKT was seen in response to radiation.
FIG. 1. The dual EGFR/HER2 kinase inhibitor lapatinib blocks radiation-induced activation of ERK1/2 and JNK in SUM102 cells.
SUM102 cells were grown to 80% confluence in complete medium and lapatinib (2.5 μM) or DMSO vehicle control added 2 h prior to irradiation at 5 Gy. Protein lysates were collected from 0-90 min after irradiation and western blot analyses performed with the indicated antibodies.
To determine whether the radiation-induced activation of ERK1/2 and JNK are mediated by upstream activation of EGFR, SUM102 cells were pretreated either with the dual EGFR/HER2 kinase inhibitor lapatinib or vehicle for 2 h prior to radiation treatment. As shown in Fig. 1, inhibition of EGFR/HER2 by lapatinib abolished radiation-induced activation of p-ERK1/2, and p-JNK while p-p38, p-STAT3 and p-AKT were unchanged. These data demonstrate that in basal breast cancer cells with overexpression of EGFR, response to ionizing radiation involves EGFR-mediated activation of ERK1/2 and JNK.
Lapatinib-Mediated Radiosensitization is Mediated Primarily Through Inhibition of MEK1/2>ERK1/2
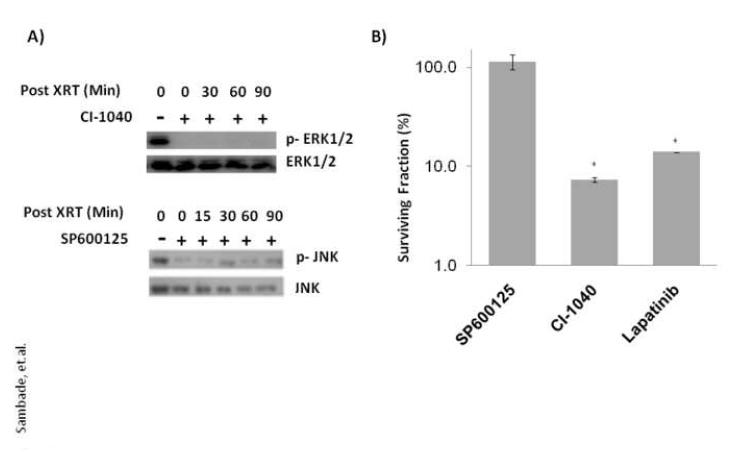
Since lapatinib can inhibit activation of ERK and JNK in response to radiation we sought to determine whether direct inhibition of ERK1/2 or JNK could likewise radiosensitize cells. To determine this, SUM102 cells were pretreated for 2 hr with vehicle (DMSO) alone or specific inhibitors against MEK1 (CI-1040) or JNK (SP600125) prior to irradiation at 5 Gy and percentage of surviving colonies compared among treatment groups. As shown in Fig. 2, inhibition of MEK1 with CI-1040 inhibited colony formation by 95% compared to control DMSO-treated cells while JNK inhibition with SP600125 showed no ability to radiosensitize using drug doses that effectively inhibited, at least in part, activation of ERK1/2 and JNK, respectively, in response to irradiation. These data suggest that EGFR-mediated radiosensitization is mediated largely through inhibition of MEK>ERK.
FIG. 2. Radiosensitization by lapatinib is mediated primarily through inhibition of MEK.
(A) SUM102 cells were grown to 80% confluence in complete medium and treated with CI1040 (1 μM), SP600125 (20 μM) or vehicle (DMSO) 2 h prior to irradiation at 5 Gy. Protein lysates were collected at 0-90 min after irradiation and western blot analyses performed with the indicated antibodies. (B) SUM102 cells were treated with SP600125 (20 μM), CI-1040 (1 μM), lapatinib (2.5 μM ) or vehicle (DMSO) 2 h prior to irradiation at 5 Gy and the media replaced without drug and colonies counted after 2 weeks. Surviving fractions of each treatment were normalized to cells treated with radiation alone (5 Gy). * indicates p<0.001 by unpaired t-test.
Constitutively Active RAF Abrogates Lapatinib-Mediated Radiosensitization
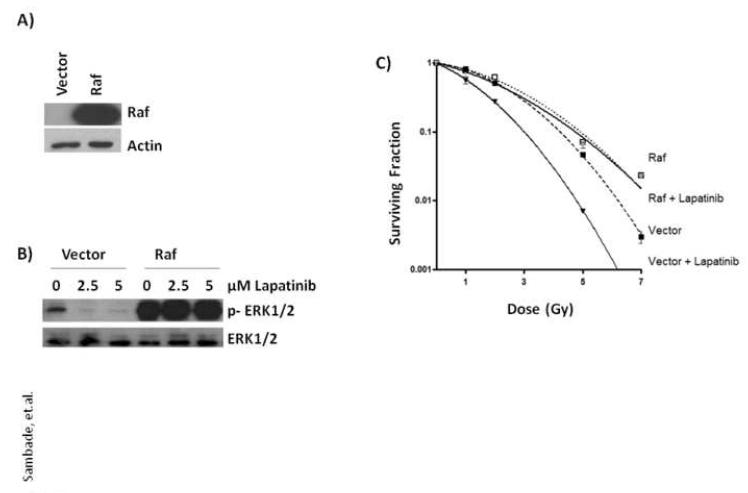
If lapatinib-mediated radiosensitization of SUM102 cells is mediated primarily by inhibition of MEK1/2>ERK1/2, then constitutive activation of the Raf>MEK>ERK pathway should block lapatinib-mediated radiosensitization. To test this, stable cell lines of SUM102 cells were first generated that express either empty vector or constitutively active Raf. As expected, the cells expressing Raf exhibited high levels of activated p-ERK1/2 and were completely resistant to lapatinib-mediated inhibition of p-ERK1/2 while cells expressing vector alone exhibited low levels of active p-ERK1/2 which could be abolished with lapatinib treatment (Fig. 3). We next compared the ability of the cells expressing Raf to block lapatinib-mediated radiosensitization of the SUM102 cells over a dose range of 0 – 7 Gy. As shown in Fig. 3, vector expressing SUM102 cells were radiosensitized when pretreated with lapatinib compared to DMSO vehicle. Interestingly, SUM102 cells expressing Raf were not only more resistant to irradiation compared to vector expressing cells with resistance reaching 7.5-fold at 7 Gy, but also completely insensitive to radiosensitization by lapatinib. That activated Raf can confer radioresistance is consistent with our previous studies showing Raf-mediated resistance to irradiation in rat intestinal epithelial cells [15]. Thus, constitutive activation of the Raf>MEK>ERK pathway alone is sufficient to block lapatinib-mediated radiosensitization suggesting that the Raf>MEK>ERK pathway plays a major role in EGFR-mediated radioresistance.
FIG. 3. Constitutively active Raf abrogates lapatinib-mediated radiosensitization.
(A) SUM102 cells with stable expression of constitutively active Raf were generated by retroviral infection with constructs expressing Raf or empty vector as control and protein lysates compared by western blot analysis with anti-Raf or anti-actin antibodies. (B) SUM102 cells expressing Raf or vector were treated with increasing amounts of lapatinib for 2 h, protein lysates collected and p-ERK1/2 levels compared by western blot analysis. (C) SUM102 cells expressing Raf or vector alone were plated at low density and allowed to adhere for 24 h. Two h prior to irradiation, cells were exposed to lapatinib (2.5 μM) or DMSO (control) and treated with single, graded doses of radiation as indicated and the media replaced without drug 2 h post-irradiation. Colonies of >25 cells were counted approximately 2 weeks after treatment and clonogenic surviving fractions generated with each point representing the mean surviving fraction calculated from three independent experiments done in triplicate for each treatment condition; error bars represent the standard deviation.
Lapatinib-Resistant SUM185 Cells are Radiosensitized by Inhibition of MEK
SUM185 cells are a breast tumor cell line of the luminal B subtype that we previously demonstrated to express elevated levels of HER2 with normal levels of EGFR which are insensitive to the antiproliferative and radiosensitizing effects of lapatinib [8, 16]. We reasoned that insensitivity of SUM185 cells to lapatinib might be due to additional mutations or other signaling aberrancies that, like EGFR, also activate the Raf>MEK>ERK pathway resulting in lapatinib resistance. We hypothesized that if radiosensitization is mediated primarily by inhibition of the Raf>MEK>ERK pathway as in the SUM102 cells, then direct inhibition of MEK in the SUM185 cells should restore radiosensitization. To test this hypothesis, SUM185 cells were pretreated with lapatinib or the MEK1 inhibitor (CI-1040) prior to irradiation at 5 Gy and percentage of surviving colonies compared to cells pretreated with DMSO alone. As shown in Fig. 4, while SUM185 cells showed no radiosensitization to lapatinib, inhibition of the MEK>ERK pathway with CI-1040, as demonstrated by diminished levels of activated p-ERK1/2, effectively restored radiosensitization. Collectively, these data demonstrate that activation of the Raf>MEK>ERK pathway by EGFR/HER2 and alternative activators plays an important role in the response to radiation such that direct inhibition of the Raf>MEK>ERK pathway could provide an additional avenue of therapeutic radiosensitization in breast cancer tumors that stain positive histochemically for p-ERK1/2.
FIG. 4. Lapatinib-resistant SUM185 cells are radiosensitized by MEK inhibition.
(A) SUM185 cells were grown to 80% confluence in complete medium and CI-1040 (1 μM) or DMSO vehicle control added 2 h prior to irradiation at 5 Gy. Protein lysates were collected from 0-90 min after irradiation and western blot analyses performed with anti-pERK1/2 or anti-ERK1/2 as a loading control. (B) SUM185 cells were treated with CI-1040 (1 μM), lapatinib (2.5 μM ) or vehicle (DMSO) 2 h prior to irradiation at 5 Gy and the media replaced without drug 2 h post-irradiation and colonies >50 cells counted after 2 weeks. Surviving fractions for each drug were normalized to cells treated with radiation alone (5 Gy). * indicates p<0.001 by unpaired t-test.
Discussion
We previously demonstrated that lapatinib-mediated inhibition of EGFR in breast cancer cell lines of the basal-subtype resulted in inhibition of proliferation and radiosensitization [8]. In the present study we show that the primary downstream signaling pathway responsible for lapatinib-mediated radiosensitization is through inhibition of the Raf>MEK>ERK mitogen-activated protein kinase cascade. We demonstrate that direct inhibition MEK alone is sufficient to radiosensitize basal breast cancer cells and luminal B breast cancer cells which are lapatinib-resistant. Thus, we hypothesize that inhibition of the Raf>MEK>ERK pathway may represent an alternative therapeutic approach to radiosensitize breast cancers with elevated activation of and “addiction” to this pathway.
Preclinical studies have shown effective radiosensitization of a wide array of different cancer cell lines and xenografts with a variety of inhibitors that target either EGFR alone (e.g., cetuximab, erlotinib) or multiple EGFR-family members (e.g., lapatinib) [reviewed in ([17-20]. Responses have been impressively consistent between studies, with radiosensitization accompanied by inhibition of proliferation, cell cycle arrest, decreased angiogenesis/VEGF expression, and increased apoptosis in most studies [8, 21-26]. The improved clinical outcome demonstrated in HNSCC patients treated with cetuximab, a monoclonal antibody against EGFR, in combination with radiation in comparison to treatment with radiation alone, provided the first definitive evidence of radiosensitization by EGFR inhibitors supporting similar efforts in other cancer subtypes that exhibit activation of the EGFR family of receptors [2]. In addition, early clinical trials with inhibitors of the EGFR family of receptors as radiosensitizers has shown promise [reviewed in [27]). However, given that EGFR can signal to a variety of downstream effectors, the precise mechanism of radiosensitization by EGFR blockade has not been fully elucidated. The purpose of our study was to identify those downstream signaling pathways important in mediating radiosensitization by lapatinib in breast cancers.
Previous studies have demonstrated a clear role of EGFR/HER2 downstream signaling in response to radiation [20]. There are several studies that support a role for PI3K>AKT signaling, an important EGFR/HER2 downstream signaling effector, in radioresistance. In radioresistant lung cancer cell lines, constitutive AKT activation was frequently observed and PI3K inhibitors showed ability to radiosensitize [28]. In a radioresistant HNSCC cell line, inhibition of EGFR and direct inhibition of the PI3K>AKT pathway resulted in radiosensitization, suggesting that aberrant EGFR activation of PI3K>AKT was responsible for radioresistance [29]. Toulany et al. showed radioresistance is mediated by AKT in K-ras mutant breast and lung cancer cells through Ras-mediated autocrine signaling to EGFR [30, 31]. Our previous findings of Ras-mediated radioresistance also implicated PI3K>AKT signaling as PI3K inhibitors reversed, at least in part, Ras-mediated radioresistance which could also be abrogated with EGFR inhibitors [15, 32]. Interestingly, our studies here of SUM102 cells showed no change in levels of activated AKT either in the presence or absence of lapatinib in response to radiation suggesting that the PI3K>AKT pathway does not play an important role either in the response to radiation or mediate the radiosensitizing effects of lapatinib in basal breast cancer.
We and others previously showed a link between EGFR activation of the Raf>MEK>ERK pathway in response to radiation and the ability of constitutively active Raf to confer radioresistance in other cell types [15, 33, 34]. Consistent with these studies, our findings here in SUM102 cells expressing constitutively active Raf demonstrated a 7.5-fold increase in surviving colonies after radiation treatment compared to control cells supporting a role for the Raf>MEK>ERK pathway in conferring radioresistance in basal breast cancer. Importantly, we found that SUM102 cells elicited strong activation of ERK1/2 in response to irradiation which could be blocked by pretreatment with lapatinib. These data show that EGFR-mediated activation of the downstream Raf>MEK>ERK pathway plays an important role in response to radiation. This was supported by additional studies whereby MEK was directly inhibited with CI-1040 with a resulting 95% inhibition of surviving colonies when combined with radiation. Our findings showing the importance of Raf>MEK>ERK signaling in breast cancers of the basal subtype are consistent with those by Mirzoeva et.al. who recently compared susceptibility among breast cancer subtypes and found the basal-subtype to be the most sensitive to MEK inhibitors [35].
While JNK has previously been implicated as a promoter of apoptosis in response to irradiation and other radiosensitizers in some cancer cells [36-38], our studies do not support its role in mediating radioresistance in basal breast cancer. While SUM102 cells treated with ionizing radiation elicited activation of JNK which was blocked by lapatinib, treatment with the JNK inhibitor SP600125 resulted in no radiosensitization. However, the lack of radiosensitization observed with SP600125 may be reflective of a lack of drug potency and specificity of SP600125 rather than a lack of an important role of JNK in the radioresponse [39].
Little is known about the role, if any, of STAT signaling in response to radiation while STATs have been shown to be important regulators of breast cancer cell proliferation and survival [40]. A recent study with a hepatoma cell line showed an increase in STAT3 expression with increasing radiation dose [41]. A separate study in prostate cancer cells found an association of increased pSTAT1 levels with radioresistant cell lines [42]. Our studies here showed little change in activated p-STAT3 levels in response to irradiation suggesting that lapatinib-mediated radiosensitization is likely not mediated by inhibition of STAT3.
Lastly, the molecular underpinnings that confer resistance to EGFR/HER2 inhibitors are poorly understood. While EGFR/HER2 inhibitors remain an attractive therapy option, accurate molecular predictors of response are lacking along with an understanding of the mechanisms that support the development of resistance [43-45]. Oncogenic addiction is a proposed mechanism by which a tumor cell becomes largely reliant on a primary activated oncogene [46]. It is thought that therapeutic resistance can develop to the primary oncogene if a secondary oncogenic stimulus can activate the same downstream pathway(s) [47]. In this sense, tumor cells can respond to inhibition of an upstream activator of a pathway to which they are “addicted” by “switch-hitting” to maintain activation of the pathway to which they are “addicted”. For e.g., in NSCLC and HNSCC cells, resistance to the anti-EGFR antibody, cetuximab, is associated with increased expression of and a switching from EGFR to HER2, HER3 and cMET with resultant maintenance of addiction to activation of ERK1/2 and AKT [48]. In a separate study of NSCLC cells, lack of response to cetuximab also correlated with maintenance of pathway addiction with lack of observed cetuximab-mediated inhibition of either ERK or AKT phosphorylation [49]. In breast cancer, resistance to Trastuzumab, a monoclonal antibody directed against HER2, can be overcome by treatment with lapatinib reportedly through its ability to inhibit HER2-mediated activation of and switching to the insulin-like growth factor I receptor [50].
In our studies, we previously showed that despite effective inhibition of HER2, lapatinib was ineffective at inhibiting ERK1/2 and AKT activation in SUM185 cells which were completely resistant to growth inhibition or radiosensitization by lapatinib [8]. In our present study we demonstrated that direct inhibition of MEK with CI-1040 blocked activation of ERK1/2 and effectively radiosensitized the lapatinib-resistant SUM185 cells with 45% fewer colonies surviving radiation plus CI-1040 treatment relative to control cells treated with radiation alone. While the ability to radiosensitize SUM185 cells with CI-1040 was not as robust as that seen for SUM102 cells, the differences in the mechanisms by which MEK1/2>ERK1/2 become activated through various positive regulators including receptor and non-receptor tyrosine kinases, integrins, growth factors, cytokines, GPCRs and direct mutations in Ras or Raf combined with loss of negative regulators (e.g. MAP kinase phosphatases), along with the magnitude and duration of MEK1/2>ERK1/2 activation [reviewed in [51]], and how dependent a tumor cell becomes to an oncogenic signaling pathway [reviewed in [52]] could account for the differences in CI-1040 sensitivity observed in these cell lines. Here, SUM102 cells have relatively higher ERK1/2 activation than SUM185 cells and might be more “addicted” to MEK1/2>ERK1/2 signaling and thus more sensitive to inhibitors of this pathway. While we have not identified the alternative upstream oncogenic signal responsible for continued activation of ERK1/2 in the presence of lapatinib in the SUM185 cells, these cells represent a model system in which to study resistance and demonstrate that identification of the pathway(s) to which a tumor cell has become addicted, in this case the MEK1/2>ERK1/2 MAPK pathway, can provide additional therapeutic options as demonstrated here with CI-1040.
Collectively, our study supports a model in EGFR+ basal breast cancers whereby EGFR-mediated activation of the Raf>MEK>ERK pathway plays a vital role in response to radiation with lapatinib or CI-1040-mediated blockade of this response providing radiosensitization. In addition, inactivation of the Raf>MEK>ERK pathway with MEK inhibitors could provide an alternative radiosensitizing treatment option in other breast cancers that are “addicted” to this pathway. Our data support further studies to evaluate the ability of inhibitors of the Raf>MEK>ERK pathway to radiosensitize breast cancers. A major challenge in EGFR-targeted therapies, as in most cancer therapies, is to find new ways to predict and overcome the development of resistance. The clinical evaluation of Raf>MEK>ERK “addiction” and the therapeutic use of efficacious MEK inhibitors could provide a solution to that challenge.
Supplementary Material
SUPP. FIG. 1. Lapatinib blocks EGFR activation in EGFR+ SUM102 cells. SUM102 cells were growth factor starved overnight and lapatinib or DMSO vehicle control added 2 h prior to stimulation with EGF (10 ng/ml) and protein lysates collected at 90 min and EGFR immunoprecipitated and western blot analyses performed with the indicated antibodies.
Acknowledgements
Supported by CA83753 (C.I.S.), CA115888 (C.I.S., J.M.S.), National Institutes of Health, Pfizer, and GlaxoSmithKline.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement The authors declare no actual or potential conflicts of interest exist. The involvement of Pfizer and GlaxoSmithKline in this study was only to provide study drug (CI-1040 from Pfizer and lapatinib from GSK) and had no involvement in the study design, data collection, data analysis or interpretation or any role in the writing of the manuscript or the decision to submit the manuscript for publication.
References
- [1].Kvols LK. Radiation sensitizers: a selective review of molecules targeting DNA and non-DNA targets. J Nucl Med. 2005;46(Suppl 1):187S–190S. [PubMed] [Google Scholar]
- [2].Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- [3].Fadare O, Tavassoli FA. The phenotypic spectrum of basal-like breast cancers: a critical appraisal. Adv Anat Pathol. 2007;14:358–373. doi: 10.1097/PAP.0b013e31814b26fe. [DOI] [PubMed] [Google Scholar]
- [4].Fadare O, Tavassoli FA. Clinical and pathologic aspects of basal-like breast cancers. Nat Clin Pract Oncol. 2008;5:149–159. doi: 10.1038/ncponc1038. [DOI] [PubMed] [Google Scholar]
- [5].Finnegan TJ, Carey LA. Gene-expression analysis and the basal-like breast cancer subtype. Future Oncol. 2007;3:55–63. doi: 10.2217/14796694.3.1.55. [DOI] [PubMed] [Google Scholar]
- [6].Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- [7].Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- [8].Zhou H, Kim YS, Peletier A, McCall W, Earp HS, Sartor CI. Effects of the EGFR/HER2 kinase inhibitor GW572016 on EGFR- and HER2-overexpressing breast cancer cell line proliferation, radiosensitization, and resistance. Int J Radiat Oncol Biol Phys. 2004;58:344–352. doi: 10.1016/j.ijrobp.2003.09.046. [DOI] [PubMed] [Google Scholar]
- [9].Hoadley KA, Weigman VJ, Fan C, et al. EGFR associated expression profiles vary with breast tumor subtype. BMC Genomics. 2007;8:258. doi: 10.1186/1471-2164-8-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Temam S, Kawaguchi H, El-Naggar AK, et al. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J Clin Oncol. 2007;25:2164–2170. doi: 10.1200/JCO.2006.06.6605. [DOI] [PubMed] [Google Scholar]
- [11].Gee JM, Nicholson RI. Expanding the therapeutic repertoire of epidermal growth factor receptor blockade: radiosensitization. Breast Cancer Res. 2003;5:126–129. doi: 10.1186/bcr584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177–184. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- [13].Gusterson BA, Hunter KD. Should we be surprised at the paucity of response to EGFR inhibitors? Lancet Oncol. 2009;10:522–527. doi: 10.1016/S1470-2045(09)70034-8. [DOI] [PubMed] [Google Scholar]
- [14].Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26:2568–2581. doi: 10.1200/JCO.2007.13.1748. [DOI] [PubMed] [Google Scholar]
- [15].Grana TM, Rusyn EV, Zhou H, Sartor CI, Cox AD. Ras mediates radioresistance through both phosphatidylinositol 3-kinase-dependent and Raf-dependent but mitogen-activated protein kinase/extracellular signal-regulated kinase kinase-independent signaling pathways. Cancer Res. 2002;62:4142–4150. [PubMed] [Google Scholar]
- [16].Sartor CI, Zhou H, Kozlowska E, et al. Her4 mediates ligand-dependent antiproliferative and differentiation responses in human breast cancer cells. Mol Cell Biol. 2001;21:4265–4275. doi: 10.1128/MCB.21.13.4265-4275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ho C, Laskin J. EGFR-directed therapies to treat non-small-cell lung cancer. Expert Opin Investig Drugs. 2009;18:1133–1145. doi: 10.1517/13543780903066772. [DOI] [PubMed] [Google Scholar]
- [18].Baumann M, Krause M, Dikomey E, et al. EGFR-targeted anti-cancer drugs in radiotherapy: preclinical evaluation of mechanisms. Radiother Oncol. 2007;83:238–248. doi: 10.1016/j.radonc.2007.04.006. [DOI] [PubMed] [Google Scholar]
- [19].Milano G, Magne N. Anti-EGFR and radiotherapy. Cancer Radiother. 2004;8:380–382. doi: 10.1016/j.canrad.2004.09.002. [DOI] [PubMed] [Google Scholar]
- [20].Sartor CI. Mechanisms of disease: Radiosensitization by epidermal growth factor receptor inhibitors. Nat Clin Pract Oncol. 2004;1:80–87. doi: 10.1038/ncponc0048. [DOI] [PubMed] [Google Scholar]
- [21].Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000;6:2166–2174. [PubMed] [Google Scholar]
- [22].Milas L, Mason K, Hunter N, et al. In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res. 2000;6:701–708. [PubMed] [Google Scholar]
- [23].Buchsbaum DJ, Bonner JA, Grizzle WE, et al. Treatment of pancreatic cancer xenografts with Erbitux (IMC-C225) anti-EGFR antibody, gemcitabine, and radiation. Int J Radiat Oncol Biol Phys. 2002;54:1180–1193. doi: 10.1016/s0360-3016(02)03788-4. [DOI] [PubMed] [Google Scholar]
- [24].Huang SM, Li J, Armstrong EA, Harari PM. Modulation of radiation response and tumor-induced angiogenesis after epidermal growth factor receptor inhibition by ZD1839 (Iressa) Cancer Res. 2002;62:4300–4306. [PubMed] [Google Scholar]
- [25].Shintani S, Li C, Mihara M, et al. Enhancement of tumor radioresponse by combined treatment with gefitinib (Iressa, ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, is accompanied by inhibition of DNA damage repair and cell growth in oral cancer. Int J Cancer. 2003;107:1030–1037. doi: 10.1002/ijc.11437. [DOI] [PubMed] [Google Scholar]
- [26].Nyati MK, Maheshwari D, Hanasoge S, et al. Radiosensitization by pan ErbB inhibitor CI-1033 in vitro and in vivo. Clin Cancer Res. 2004;10:691–700. doi: 10.1158/1078-0432.ccr-1041-03. [DOI] [PubMed] [Google Scholar]
- [27].Magne N, Chargari C, Castadot P, et al. The efficacy and toxicity of EGFR in the settings of radiotherapy: Focus on published clinical trials. European Journal of Cancer. 2008;44:2133–2143. doi: 10.1016/j.ejca.2008.06.029. [DOI] [PubMed] [Google Scholar]
- [28].Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- [29].Gupta AK, McKenna WG, Weber CN, et al. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res. 2002;8:885–892. [PubMed] [Google Scholar]
- [30].Toulany M, Baumann M, Rodemann HP. Stimulated PI3K-AKT signaling mediated through ligand or radiation-induced EGFR depends indirectly, but not directly, on constitutive K-Ras activity. Mol Cancer Res. 2007;5:863–872. doi: 10.1158/1541-7786.MCR-06-0297. [DOI] [PubMed] [Google Scholar]
- [31].Toulany M, Dittmann K, Kruger M, Baumann M, Rodemann HP. Radioresistance of K-Ras mutated human tumor cells is mediated through EGFR-dependent activation of PI3K-AKT pathway. Radiother Oncol. 2005;76:143–150. doi: 10.1016/j.radonc.2005.06.024. [DOI] [PubMed] [Google Scholar]
- [32].Grana TM, Sartor CI, Cox AD. Epidermal growth factor receptor autocrine signaling in RIE-1 cells transformed by the Ras oncogene enhances radiation resistance. Cancer Res. 2003;63:7807–7814. [PubMed] [Google Scholar]
- [33].Dent P, Reardon DB, Park JS, et al. Radiation-induced release of transforming growth factor alpha activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell death. Mol Biol Cell. 1999;10:2493–2506. doi: 10.1091/mbc.10.8.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pirollo KF, Tong YA, Villegas Z, Chen Y, Chang EH. Oncogene- transformed NIH 3T3 cells display radiation resistance levels indicative of a signal transduction pathway leading to the radiation-resistant phenotype. Radiat Res. 1993;135:234–243. [PubMed] [Google Scholar]
- [35].Mirzoeva OK, Das D, Heiser LM, et al. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009;69:565–572. doi: 10.1158/0008-5472.CAN-08-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dent P, Yacoub A, Contessa J, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [37].Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ, Verheij M. Alkyl-lysophospholipids activate the SAPK/JNK pathway and enhance radiation-induced apoptosis. Cancer Res. 1999;59:2457–2463. [PubMed] [Google Scholar]
- [38].Verheij M, Bose R, Lin XH, et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress- induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- [39].Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Garcia R, Bowman TL, Niu G, et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–2513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- [41].Ma J, Ye L, Da M, Wang X. Heavy ion irradiation increases apoptosis and STAT-3 expression, led to the cells arrested at G2/M phase in human hepatoma SMMC-7721 cells. Mol Cell Biochem. 2009 doi: 10.1007/s11010-009-0069-6. [DOI] [PubMed] [Google Scholar]
- [42].Skvortsova I, Skvortsov S, Stasyk T, et al. Intracellular signaling pathways regulating radioresistance of human prostate carcinoma cells. Proteomics. 2008;8:4521–4533. doi: 10.1002/pmic.200800113. [DOI] [PubMed] [Google Scholar]
- [43].Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–2459. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- [44].Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8:215. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ryan Q, Ibrahim A, Cohen MH, et al. FDA drug approval summary: lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. Oncologist. 2008;13:1114–1119. doi: 10.1634/theoncologist.2008-0816. [DOI] [PubMed] [Google Scholar]
- [46].Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- [47].Pillay V, Allaf L, Wilding AL, et al. The plasticity of oncogene addiction: implications for targeted therapies directed to receptor tyrosine kinases. Neoplasia. 2009;11:448–458. 442. doi: 10.1593/neo.09230. following 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wheeler DL, Huang S, Kruser TJ, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Janmaat ML, Kruyt FA, Rodriguez JA, Giaccone G. Response to epidermal growth factor receptor inhibitors in non-small cell lung cancer cells: limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin Cancer Res. 2003;9:2316–2326. [PubMed] [Google Scholar]
- [50].Nahta R, Yuan LX, Du Y, Esteva FJ. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Mol Cancer Ther. 2007;6:667–674. doi: 10.1158/1535-7163.MCT-06-0423. [DOI] [PubMed] [Google Scholar]
- [51].Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- [52].Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPP. FIG. 1. Lapatinib blocks EGFR activation in EGFR+ SUM102 cells. SUM102 cells were growth factor starved overnight and lapatinib or DMSO vehicle control added 2 h prior to stimulation with EGF (10 ng/ml) and protein lysates collected at 90 min and EGFR immunoprecipitated and western blot analyses performed with the indicated antibodies.