Abstract
Purpose
Small intestinal ulcers are frequent complications of therapy with non-steroidal anti-inflammatory drugs (NSAIDs). We present here a genetic deficiency of eicosanoid biosynthesis that illuminates the mechanism of NSAID-induced ulcers of the small intestine.
Methods
Eicosanoids and metabolites were measured by isotope-dilution with mass spectrometry. cDNA was obtained by reverse transcription and sequenced following amplification with RT-PCR.
Results
We investigated the cause of chronic recurrent small intestinal ulcers, small bowel perforations, and gastrointestinal blood loss in a 45 year old male who was not taking any cyclooxygenase inhibitor. Prostaglandin metabolites in urine were significantly depressed. Serum thromboxane B2 (TxB2) production was 4.6% of normal controls (p<0.006) and serum 12-HETE was 1.3% of controls (p<0.005). Optical platelet aggregation with simultaneous monitoring of ATP release demonstrated absent granule secretion in response to ADP and a blunted aggregation response to ADP and collagen, but normal response to arachidonic acid (AA). LTB4 biosynthesis by ionophore activated leukocytes was only 3% of controls and urinary LTE4 was undetectable. These findings suggested deficient AA release from membrane phospholipids by cytosolic phospholipase A2-α (cPLA2-α) which regulates cyclooxygenase and lipoxygenase mediated eicosanoid production by catalyzing the release of their substrate, AA. Sequencing of cPLA2-α cDNA demonstrated 2 heterozygous non-synonymous single base pair mutations: Ser111Pro (S111P) and Arg485His (R485H), as well as a known SNP: Lys651Arg (K651R).
Conclusion
Characterization of this cPLA2-α deficiency provides support for the importance of prostaglandins in protecting small intestinal integrity, and indicates that loss of prostaglandin biosynthesis is sufficient to produce small intestinal ulcers.
Introduction
Small intestinal ulcers are a well established consequence of the use of non-steroidal anti-inflammatory drugs (NSAIDS)1–5. The pharmacological property shared by all of the NSAIDs is inhibition of the cyclooxygenase enzymes that synthesize prostaglandins6. Considerable evidence indicates that inhibition of prostaglandin biosynthesis contributes to the small intestinal ulcers caused by NSAIDs. Other studies, however, have suggested that cyclooxygenase-independent effects of NSAIDS on the small intestine also are required for ulcer formation7–8. One postulated off-target mechanism has been topical injury to enterocytes by the NSAIDs during absorption of the drugs. The extent to which such topical cyclooxygenase-independent actions are required to produce small intestinal ulcers, however, has been unclear. To address whether inhibition of prostaglandin biosynthesis alone is sufficient to cause small intestinal ulcers, we present here the investigation of an inherited deficiency of prostaglandin biosynthesis that is associated with severe small intestinal ulcer disease with attendant diaphragm-like constrictions.
Methods
Study conduct
Informed consent was obtained from all participants after study approval by the Vanderbilt University Institutional Review Board. The patient was admitted to the Clinical Research Center at Vanderbilt University Medical Center (VUMC) for seven days, during which all medications were held. Consecutive 24 hour urine collections were obtained prior to phlebotomy, which was subsequently performed daily after an overnight fast. Blood specimens from family members were obtained remotely and shipped to our facility. Healthy male volunteers who reported no use of aspirin or NSAIDs in the preceding two weeks served as controls.
Urinary metabolites of PGE2, PGD2, PGI2, and TxA2
Urinary metabolites of thromboxane A2 (11-dehydrothromboxane B2; 11-dTxB2 ), prostacyclin (2,3-dinor-6-keto-prostaglandin F1α; PGI-M), prostaglandin D2 (9α,11β-dihydroxy-15-oxo-2,3,18,19- tetranorprost-5-ene-1,20-dioic acid; PGD-M), and prostaglandin E2 (11α-hydroxy-9,15- dioxo-2,3,4,5-tetranor-prostane-1,20-dioic acid; PGE-M) were assayed using mass spectrometry as described previously9–12 from separate 24 hour urine collections on different days.
Urinary LTE4
LTE4 was analyzed by a previously published LC/MS/MS method13 with minor modifications.
Platelet-derived (Serum) Thromboxane B2 (TxB2) and 12-hydroxyeicosatetraenoic acid (12-HETE)
Blood from the patient on multiple days was drawn into a glass container and allowed to clot by incubating at 37°C for 45 minutes. The serum layer was collected after centrifugation and TxB2 was assayed by GC/ECI/MS as described previously14–15. 12-HETE was assayed by LC/APCI/MS/MS using a silica HPLC column as described previously16
TxB2 production in washed platelets
Platelets were gel-filtered as described previously17. 100 μl of the platelet suspension (600,000 cells/μl) was incubated with [2H8] AA 2 μM at 37°C for 15 minutes. After stopping the reaction, TxB2 was purified, derivatized and analyzed by GC/ECI/MS.
Immunoblotting of cPLA2 -α protein
Western blot analysis of protein was performed using rabbit anti-cPLA2 (Cell-Signaling), goat anti-COX-1 (Santa Cruz Biotechnology) or goat anti-Actin (Santa Cruz) primary antibodies and horseradish peroxidase-coupled secondary antibodies (Santa Cruz). Band intensity was quantified using image analysis software (Quantity One v4.3.1, Bio Rad).
Platelet aggregation
Citrated platelet rich plasma from peripheral venous blood from the patient and control volunteers was used to assess turbidimetric platelet aggregation induced by adenosine diphosphate (ADP), collagen, or AA. Platelet counts were adjusted to 2.6–2.8 × 108 cells/ml prior to aggregation. Simultaneous adenosine triphosphate (ATP) secretion was quantified by monitoring luminescence produced by luciferase using an optical platelet aggregometer (Chronolog).
Lymphoblast separation and culture
Lymphoblasts were separated from whole blood using Lymphosep™ lymphocyte separation medium (MP Biochemicals) and cultured after addition of cyclosporine as described by the manufacturer.
PLA2 activity
Platelets obtained on two different days from the patient and from two volunteers were frozen as PRP in citrate at −80°C. Thawed platelets or fresh cultured lymphoblasts were washed and resuspended in buffer (Hepes 10mM, Sucrose.34M Glycerol 10%, EDTA 1mM, pH 7.75) with a protease inhibitor cocktail. Whole cell lysate was obtained by sonication of cell suspension followed by brief centrifugation at 10,000 × g. Dithiothreitol 1 mM was added to prevent oxidative enzyme degradation. PLA2 activity was measured from whole cell lysate (50 μg protein) with a radiolabeled substrate, l-3- phosphatidylcholine, 1-stearoyl-2-[1-14 C] arachidonyl (14 C-SAPC; 450 pmol, 55,000 dpm), under conditions described by Leslie and Gelb18 (NaCl 150mM, BSA 1mg/ml, Hepes 42mM, CaCl2 10mM, pH 7.5). Substrate hydrolysis was measured over a time period demonstrating linear kinetics. Reactions were stopped by addition of chloroform:methanol 2:1 and the extracted lipids were separated by thin layer chromatography. Residual substrate and hydrolyzed arachidonic acid were quantified by analyzing radioactivity using a Bioscan AR-200 imaging scanner.
cPLA2 -α cDNA sequencing
Oligonucleotide primer sequences were those previously published19. cDNA was obtained by reverse transcription (Invitrogen Superscript III and Elongase Kits) using total RNA isolated with a whole blood RNA collection system (QIAGEN PAXgene) as a template and oligo dT as a primer. Following RT-PCR amplification using Primer 1 and Primer 2 oligonucleotide primers, DNA sequencing was done using Primers 1–14 with Applied Biosystems Big Dye v3.1. All of the variants detected in RT-PCR products were confirmed by direct sequencing of the corresponding segments of genomic DNA.
Statistics
Normally distributed continuous variables were compared using t-test with Welch’s correction applied when variances were different. Mann-Whitney U test was used for comparison of continuous variables without normal distribution. PLA2 activity was compared using two-way ANOVA. All analyses were performed using Graphpad Prism 4.0 software for MS Windows. Means are reported ± standard deviation unless otherwise specified.
Results
Clinical findings
A 45 year old white American male of Italian descent presented with a life-long history of occult gastrointestinal blood loss, chronic anemia, iron deficiency, and frequent bouts of abdominal pain as a child and young adult. Repeated episodes of acute gross gastrointestinal bleeding late in his fourth decade and multiple episodes of small bowel perforation required five surgical interventions between 38–45 years of age. Surgical exploration of the small intestine and intra-operative endoscopy revealed multiple recurrent small intestinal ulcerations. Histologic specimens of the ileum and jejunum revealed multiple small, well-demarcated ulcers with minimal surrounding inflammation. Stenotic ileal web formations were also described proximal to several ulcers. Misoprostol (800 mcg daily), an oral prostaglandin E1 analog, had been initiated with subsequent resolution of anemia for a period of 27 months until GI bleeding recurred again.
Colonoscopy and esophagogastroduodenoscopy had been performed on multiple occasions and always had been normal. Mesenteric arteriograms, gastrin secretion and laboratory and histologic evaluation for celiac sprue and inflammatory bowel disease were all normal. He denied having had diarrhea, nausea, vomiting or constipation. He had no history of bruising or non-gastrointestinal bleeding symptoms or severe or recurrent infections.
A clear cell renal carcinoma (Furman Grade II) was resected at age 40. A brief period of mild wheezing in early childhood was reported without subsequent respiratory complaints. A shellfish allergy causing urticaria was reported. Medications included iron sulfate and a multivitamin in addition to misoprostol. Use of nonsteroidal anti-inflammatory and corticosteroid medications was specifically denied.
The patient’s father had died with multiple myeloma, and varying malignancies were reported in second and third degree relatives. No renal malignancy was reported in family members. There was no family history of ulcers.
Physical examination revealed a well-developed male. Blood pressure was normal. A 2/6 systolic murmur was heard at the left sternal border. His abdomen was notable for multiple well-healed surgical scars. Physical exam was otherwise unremarkable.
An electrocardiogram and an echocardiogram were normal. Ultrasonography demonstrated normal kidneys with evidence of prior partial resection. Blood cell counts, electrolytes, renal and liver function values were normal.
Urinary metabolites of PGE2, PGD2, PGI2, and TxA2
Quantification of the patient’s urinary prostanoid metabolites demonstrated reduced levels compared with reference values (Table 1). The patient’s mother and sister did not have reduced urinary prostanoid metabolite levels.
Table 1.
| Urinary Eicosanoid Metabolites | |||
|---|---|---|---|
| Patient | Reference range | patient vs. normal mean (%) | |
| 11-dTxB2 (pg/mg creatinine) | 58.7 ± 15.1A | 96–644 | 15.90% |
| PGI-M (pg/mg creatinine) | 53.7 ± 39.3A | 65.1–291.9 | 30.0% |
| PGD-M (ng/mg creatinine) | 0.39 ± 0.35A | 0.74–1.64 (males) 0.72–1.2 (females) | 32.8 |
| PGE-M (ng/mg creatinine) | 4.48 ± 1.65A | 7.4–13.4 (males) 4.6–7.4 (females) | 43.1 |
| LTE4 (pg/mg creatinine) | Undetectable | 19–60 | |
Urinary metabolites of TxA2(11-dTxB2), PGI2 (PGI-M), PGD2 (PGD-M), and PGE2 (PGE-M) were measured by mass spectroscopy from 24-hour urine collections. Normal values for urinary prostanoid metabolites were published previously (6–9).
Mean outside of reference range.
Platelet-derived (Serum) TxB2 and 12-HETE
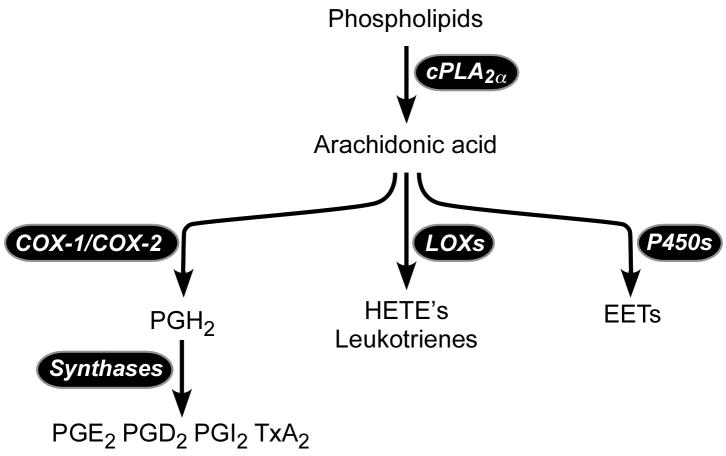
The finding that 11d-TxB2 excretion was reduced by 84.1% led to the hypothesis that TxA2 biosynthesis by the platelet was impaired. This was assessed by measurement of TxB2 released into the serum during blood clotting, which is derived almost entirely from platelets20. The patient’s mean serum TxB2 was reduced by 95.9% (Table 2). This could reflect pharmacologic inhibition of platelet COX-1, COX-1 deficiency or lack of the COX-1 substrate, AA (Figure 1). A genetic deficiency of thromboxane synthase seemed unlikely due to the depressed biosynthesis of other prostanoids. To distinguish between these possibilities, the product of the platelet 12-lipoxygenase, 12-HETE, was measured in serum; 12-HETE should be unchanged or increased with pharmacologic or genetic impairment of COX-1 catalytic activity. The level of 12-HETE in the patient’s serum was decreased by 97.8% (Table 2), a finding consistent with an almost complete absence of the release of AA during platelet activation. The patient’s mother and sister exhibited intermediate values for platelet-derived TxB2 and 12-HETE that were below or at the lower end of the normal range.
Table 2.
Eicosanoids Derived From Platelets and Leukocytes
| Platelet-derived Eicosanoids | Patient | Reference Range † | Patient vs. Normal Mean (%) |
|---|---|---|---|
| TxB2 (ng/ml) | 11.0 ± 6.9* | 40.2 – 415 | 4.82 % |
| 12-HETE (ng/ml) | 9.5 ± 9.6* | 44.2 – 1279 | 1.44 % |
| Leukocyte-derived Eicosanoids | |||
| LTB4 (ng/ml) | 7.2 ± 3.3* | 223 – 271 | 2.96% |
Mean outside of reference range.
Values reported are from male volunteers.
Figure 1. Arachidonic acid cascade initiated by cPLA2α.
P450, cytochrome P450; LOX, lipoxygenase; COX, cyclooxygenase; HETE, hydroxyeicosatetraenoic acid; EET, epoxyeicosatrienoic acid.
Urinary Leukotriene E4 (LTE4)
LTE4 was below the limit of detection of 1 pg/ml in the patient’s urine whereas the range of normal values was 19–60 pg/ml.
Whole Blood Leukotriene B4 (LTB4)
LTB4 produced by whole blood activated by calcium ionophore from the patient measured 7.2 ± 3.3 ng/ml whereas whole blood from healthy volunteers produced 243.7 ± 22.6 ng/ml LTB4 (Table 2).
TxA2 Biosynthesis
COX-1 activity was assessed by measuring production of thromboxane A2 by washed platelets after incubation with [2H8] AA. Both deuterated and non-deuterated TxB2 were quantified, reflecting conversion by COX-1 of exogenous and endogenous substrate, respectively. [2H8] TxB2 levels were similar between the patient and controls, reflecting normal COX-1 activity in the patient’s platelets. Platelet activation by exogenous agonists, including arachidonic acid, induces release of endogenous arachidonic acid from cellular storage pools. Notably, non-deuterated TxB2 levels from the patient’s platelets were much lower than controls (0.04 vs. 0.51 ng/103 platelets; n=1), indicating an impaired release of endogenous AA.
Platelet aggregation
Platelet function was assessed by monitoring platelet aggregation and dense granule secretion in response to stimulation by ADP or collagen. ADP 5μM and collagen 2 μg/ml were the lowest doses of these reagents inducing granule secretion in all controls. ADP 5 μM induced a diminished aggregation of the patient’s platelets compared with controls (n=6), mean 73.0 ± 8.29 vs. 92.67 ± 11.91 maximum % aggregation (p<0.005). Collagen 2 μg/ml also induced less aggregation in the patient, inducing a mean % maximal aggregation of 61.0 ± 14.8 vs. 95.33 ± 15.2 in controls (p<0.005). Notably, ATP release, which is a measure of dense granule secretion, in response to ADP was completely absent in the patient’s platelets as opposed to controls which released a mean 0.88 ± 0.33 nmol ATP (p=0.001). ATP release in response to collagen 2μg/ml also was decreased significantly in the patient vs. controls, mean 0.16 ± 0.032 vs. 1.1 ± 0.34 nmol (p=0.002). Normal aggregation and ATP release were observed in the patient’s PRP with addition of AA 250 μM.
cPLA2α protein in platelets
Immunobloting for cPLA2α in the patient’s platelets detected protein of expected molecular weight but in diminished quantity (approximately 38% of control). COX-1 was detected in equal amounts in the patient and controls.
PLA2 activity
The patient’s mean total PLA2 activity in sonicated platelets represented 27.2 % of the activity in controls, with mean activity 0.77 ± 0.32 vs. 2.83 ± 0.47 pmol/min/50μg protein (p<0.0005) in controls.
cPLA2α DNA sequencing
Three transitions encoding non-synonymous codons in the patient’s cPLA2α alleles were found by sequencing cPLA2α cDNA. These included a T to C transition (c.[331T>C]) resulting in a TCT to CCT substitution encoding a Ser to Pro change at residue 111 (p.[S111P]), a G to A transition (c.[ 1454G>A]) resulting in a CGT to CAT substitution encoding an Arg to His change at residue 485 (p.[R485H]) and an A to G transition (c.[ 1952A>G]) resulting in an AAG to AGG substitution encoding a Lys to Arg change at residue 651 (p.[K651R]). The patient was heterozygous for all three transitions and no changes were found in the 3′ or 5′ untranslated regions. Sequencing of cPLA2α cDNA from the patient’s mother and sister showed that the mother was heterozygous for the p.[S111P] variant but was homozygous for the p.[R485] and p.[K651] alleles. The sister was heterozygous for both the p.[R485H] and p.[K651R] variants but was homozygous for p.[S111] alleles. This inheritance pattern demonstrates that the patient was compound heterozygous with the p.[S111P] transition on one allele and both the p.[R4885H] and p.[K651R] transitions on the other allele. All of the variants detected in cDNA products were confirmed by direct sequencing of the corresponding segments of genomic DNA.
Discussion
We have characterized a novel functional deficiency of cPLA2-α resulting from compound heterozygosity for 2 rare variants(p.[S111P] and p.[R485H]) in a patient with ulcers and diaphragm-like strictures of the small intestine, platelet dysfunction, and globally decreased eicosanoid production. Our findings indicate that cPLA2-α provides substrate for virtually all eicosanoid biosynthesis by human platelets, leukocytes and the cells from which cysteinyl leukotrienes are derived, and also indicate that products of this phospholipase contribute importantly to maintaining the integrity of the small intestine.
Concerted evidence supports a causal relationship between the observed mutations and the phenotype. Both the c.[331T>C] and the c.[1454 G>A] are missense mutations. Each of these mutations is rare; neither was found in the population of 418 DNA multiethnic samples that we analyzed, and neither was reported in the NCBI SNP database. These rare mutations are associated with a rare biochemical phenotype; no evidence for a block in eicosanoid biosynthesis at the level of cPLA2-α has previously been reported in humans. The clinical phenotype of early onset of iron-deficiency anemia due to non–drug-related small bowel ulcers also is very uncommon. The biochemical phenotype of impaired biosynthesis of both TxB2 and 12-HETE in platelets cosegregates with the mutations in the family, with the compound heterozygosity for the mutations in the patient yielding virtually complete absence of their biosynthesis and with the expected intermediate reduction in biosynthesis in the family members who are heterozygous for a single mutant allele. Each of the amino acids affected by the mutations is conserved across species, and molecular modeling implies that conformational changes should result from the mutations19.
The mechanism of NSAID-induced small intestinal ulcers is informed by the finding that severe ulcers and diaphragmatic constrictions of the small intestine result from a genetic deficiency of prostaglandin biosynthesis. This supports a conclusion that loss of cyclooxygenase products is sufficient to cause complicated small intestinal ulcers. As the loss of prostaglandin biosynthesis is genetic, it is not possible to attribute this enteropathy to off-target effects of drugs.
The fact that reduced prostaglandin biosynthesis alone can cause small intestinal ulcers of course does not preclude modifying factors that could influence the development of small intestinal ulcers in a particular patient. Indeed, there is considerable inter-individual variation in the extent of small intestinal pathology produced by an NSAID. It is known, for example, that bile and intestinal microflora can modify the small intestinal response to NSAIDs. Pharmacokinetic differences would be predicted to affect the response to an NSAID, as would genetic differences in the prostaglandin biosynthetic enzymes and signaling molecules. Indeed, it could be hypothesized that heterozygous loss of a functional cPLA2-α allele would exaggerate the response to an NSAID.
Although small intestinal ulcers unrelated to use of NSAIDs or enteric-coated drugs are uncommon, a number of such patients have been reported2. CPLA2-α deficiency may be considered in the molecular differential diagnosis of such ulcers, particularly those with onset early in life. The finding of jejunoileal ulcers as a phenotype of cPLA2-α deficiency identifies a biochemical pathway linked to this disease and affords a hypothesis that loss of function at other steps in the biosynthetic or signaling pathways for the relevant prostaglandin could yield a similar phenotype. The small intestinal sequelae of cPLA2-α deficiency also become relevant in consideration of the consequences of employing selective cPLA2-α inhibitors as therapeutic agents.
Nonselective COX inhibition by NSAIDs causes gastroduodenal ulceration with high morbidity and mortality21. In contrast, this patient with cPLA2-α deficiency suffered from severe ulcer disease exclusively of the ileum and jejunum; no gastroduodenal ulcers had been detected on repeated upper intestinal endoscopies. This restriction of ulcer disease to the jejunum and ileum has been characteristic of other reported patients with NSAID-independent small intestinal ulcers2,4. The selectivity for the jejunum and ileum could result from cPLA2-α serving as the key phospholipase providing AA substrate in these portions of the small intestine, in contrast with a different phospholipase releasing AA for biosynthesis of the PGs that protect the stomach22–23 and duodenum from ulceration. Alternatively, lipoxygenase derived products of AA may be more important contributors to ulcer formation in the stomach and duodenum than in the ileum and jejunum. LTB4 concentrations were markedly increased in the fundic mucosa of rats that received indomethacin compared with controls, and 5-lipoxygenase inhibitors have been shown to mitigate NSAID-induced gastric and intestinal lesions24. Notably, this attenuation was more profound in gastric mucosa, which demonstrated 95.4% and 98.8% reductions in lesion number and size, respectively, compared with 72.5% and 86.9% reductions in the small intestine. No such increase in gastric leukotriene products would be expected in the absence of cPLA2-α. Moreover, the findings in this patient with cPLA2-α deficiency indicate that NSAID-induced increases in leukotrienes can not be implicated in the causality of the small intestinal ulcers produced by these drugs.
A concerted body of evidence indicates that cPLA2-α is the principal and rate-limiting phospholipase responsible for initiating eicosanoid biosynthesis in the platelet25–28. Our findings strongly support the concept that in the intact platelet, cPLA2-α initiates the signaling-induced release of virtually all of the AA that is substrate for both the COX-1 and 12-lipoxygenase–derived pathways. Analysis of eicosanoid biosynthesis in activated platelets, therefore, provides an optimal biochemical phenotype with which to detect heterozygosity. LTB4 biosynthesis by blood leukocytes also is derived almost entirely via the cPLA2-α pathway.
cPLA2-α (−/−) mice28–33 manifest platelet dysfunction and have small bowel ulcers that are much less severe than in the patient lacking this enzyme. These knockout mice have cardiac hypertrophy, which is not evident in the patient.
In conclusion, we have characterized compound heterozygous CPLA2-α variants that cause platelet dysfunction and are associated with multiple recurrent small bowel ulcers. Characterization of this deficiency informs the mechanism of NSAID-induced small intestinal ulcers and has the potential for elucidating the biology and pathophysiology of cPLA2-α. These findings also have significant implications for the safety and effectiveness of pharmacologic inhibition of the cPLA2-α enzyme.
Acknowledgments
We would like to thank our patient and his family for their ongoing cooperation with this investigation, Taneem Amin for his technical assistance, Jeffrey A. Canter for his assistance, and Ornella Semino (University of Pavia, Pavia, Italy) for the generous donation of DNA samples. The assistance of Frank Harrell and Qingxia Chen with statistical analysis is greatly appreciated. This work was supported in part by NIH grants HL81009, GM15431, HL65962, and RR00095.
Contributor Information
David H. Adler, Departments of Medicine and Pharmacology, Division of Clinical Pharmacology, Vanderbilt University. 536 RRB, Vanderbilt Medical Center, Nashville, Tennessee 37232, USA, Phone: (615) 343-4847, Fax: (615) 322-5303, David.adler@vanderbilt.edu
John A. Phillips, III, Department of Pediatrics, Division of Medical Genetics, DD 2205 MCN, Vanderbilt Medical Center, Nashville, Tennessee 37232, USA, Phone: (615) 322-7601, Fax: (615) 343-9951, John.a.Phillips@Vanderbilt.edu.
Joy D. Cogan, Department of Pediatrics, Division of Medical Genetics, DD 2205 MCN, Vanderbilt Medical Center, Nashville, Tennessee 37232, USA, Phone: (615) 322-7601, Fax: (615) 343-9951, Joy.cogan@Vanderbilt.edu
Tina M. Iverson, Department of Pharmacology, Vanderbilt University, 461 PRB, Vanderbilt Medical Center, Nashville, Tennessee 37232, USA, Phone: (615) 322-7817, Fax: (615) 343-6532, Tina.iverson@Vanderbilt.edu
Jeffrey A. Stein, Department of Medicine, Division of Digestive and Liver Diseases, Columbia University, Irving Pavillion, Rm 521, 161 Fort Washington Ave, New York, New York 10032, USA, Phone: (212) 305-5444, Fax: (212) 305-3542, Jas21@columbia.edu
David A. Brenner, University of California, San Diego, Room 1318A, Biomedical Sciences Building, School of Medicine, 9500 Gilman Drive, La Jolla, California 92093, Phone: (858) 534-1501, Fax: (858) 822-0084, dbrenner@ucsd.edu
Jason D. Morrow, Departments of Medicine and Pharmacology, Division of Clinical Pharmacology, Vanderbilt University. 536 RRB, Vanderbilt Medical Center, Nashville, Tennessee 37232, USA, Phone: (615) 322-4785, Fax: (615)343-9659, Jason.morrow@Vanderbilt.edu
Olivier Boutaud, Departments of Medicine and Pharmacology, Division of Clinical Pharmacology, Vanderbilt University. 536 RRB, Vanderbilt Medical Center, Nashville, Tennessee 37232, USA, Phone: (615) 343-7398, Fax: (615) 322-5303, Olivier.boutaud@Vanderbilt.edu.
John A. Oates, Departments of Medicine and Pharmacology, Division of Clinical Pharmacology, Vanderbilt University. 536 RRB, Vanderbilt Medical Center, Nashville, Tennessee 37232, USA, Phone: (615) 343-4847, Fax: (615) 322-5303, John.oates@Vanderbilt.edu
References
- 1.Maiden L, Thjodleifsson B, Seigal A, Bjarnason II, Scott D, Birgisson S, et al. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: a cross-sectional capsule enteroscopy study. Clin Gastroenterol Hepatol. 2007;5:1040–5. doi: 10.1016/j.cgh.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto T, Kudo T, Esaki M, Yano T, Yamamoto H, Sakamoto C, et al. Prevalence of non-steroidal anti-inflammatory drug-induced enteropathy determined by double-balloon endoscopy: a Japanese multicenter study. Scandinavian Journal of gastroenterology. 2008;43:490–6. doi: 10.1080/00365520701794121. [DOI] [PubMed] [Google Scholar]
- 3.Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–9. doi: 10.1016/s1542-3565(04)00603-2. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Video capsule endoscopy tp prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–41. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi Y, Yamamoto H, Kita H, Sunada K, Sato H, Yano T, et al. Non-steroidal anti-inflammatory drug-induced small bowel injuries identified by double-balloon endoscopy. World J Gastroenterol. 2005;11:4861–4. doi: 10.3748/wjg.v11.i31.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature: New Biology. 1971;231:232–5. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 7.Somasundaram S, Hayllar H, Rafi S, Wrigglesworth JM, Macpherson AJ, Bjarnason I. The biochemical basis of non-steroidal anti-inflammatory drug-induced damage to the gastrointestinal tract: a review and a hypothesis. Scandinavian Journal of Gastroenterology. 1995;30:289–99. doi: 10.3109/00365529509093280. [DOI] [PubMed] [Google Scholar]
- 8.Whittle BJ. Temporal relationship between cyclooxygenase inhibition, as measured by prostacyclin biosynthesis, and the gastrointestinal damage induced by indomethacin in the rat. Gastroenterology. 1981;80:94–8. [PubMed] [Google Scholar]
- 9.Morrow JD, Minton TA. Improved assay for the quantification of 11-dehydrothromboxane B2 by gas chromatography-mass spectrometry. Journal of Chromatography. 1993;612:179–85. doi: 10.1016/0378-4347(93)80161-v. [DOI] [PubMed] [Google Scholar]
- 10.Morrow JD, Prakash C, Awad JA, Duckworth TA, Zackert WE, Blair IA, et al. Quantification of the major urinary metabolite of prostaglandin D2 by a stable isotope dilution mass spectrometric assay. Analytical Biochemistry. 1991;193:142–8. doi: 10.1016/0003-2697(91)90054-w. [DOI] [PubMed] [Google Scholar]
- 11.Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, et al. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Analytical Biochemistry. 2004;334:266–75. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama T, Soma M, Watanabe Y, Hasimu B, Sato M, Aoi N, et al. Splicing mutation of the prostacyclin synthase gene in a family associated with hypertension. Biochemical and Biophysical Research Communications. 2002;297:1135–9. doi: 10.1016/s0006-291x(02)02341-0. [DOI] [PubMed] [Google Scholar]
- 13.Mizugaki M, Hishinuma T, Suzuki N. Determination of leukotriene E4 in human urine using liquid chromatography-tandem mass spectrometry. J Chromatogr B Biomed Sci Appl. 1999;729:279–85. doi: 10.1016/s0378-4347(99)00174-7. [DOI] [PubMed] [Google Scholar]
- 14.Daniel VC, Minton TA, Brown NJ, Nadeau JH, Morrow JD. Simplified assay for the quantification of 2,3-dinor-6-keto-prostaglandin F1 alpha by gas chromatography-mass-spectrometry. J Chromatogr B Biomed Appl. 1994;653:117–22. doi: 10.1016/0378-4347(93)e0432-p. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald GA, Healy C, Daugherty J. Thromboxane A2 biosynthesis in human disease. Federation Proceedings. 1987;46:154–8. [PubMed] [Google Scholar]
- 16.Yin H, Porter NA, Morrow JD. Separation and identification of F2-isoprostane regioisomers and diastereomers by novel liquid chromatographic/mass spectrometric methods. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:157–64. doi: 10.1016/j.jchromb.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 17.Boutaud O, Aronoff DM, Richardson JH, Marnett LJ, Oates JA. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H(2) synthases. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7130–5. doi: 10.1073/pnas.102588199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leslie CC, Gelb MH. Assaying phospholipase A2 activity. Methods in Molecular Biology. 2004;284:229–42. doi: 10.1385/1-59259-816-1:229. [DOI] [PubMed] [Google Scholar]
- 19.Adler DH, Cogan JD, Phillips JA, Schnetz-Boutaud N, Milne GL, Iverson T, et al. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. The Journal of Clinical Investigation. 2008;118:2121–31. doi: 10.1172/JCI30473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. The New England Journal of Medicine. 2005;353:2373–83. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. The New England Journal of Medicine. 1999;340:1888–99. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 22.Masuda S, Murakami M, Ishikawa Y, Ishii T, Kudo I. Diverse cellular localizations of secretory phospholipase A2 enzymes in several human tissues. Biochimica et Biophysica Acta. 2005;1736:200–10. doi: 10.1016/j.bbalip.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Ni Z, Okeley NM, Smart BP, Gelb MH. Intracellular actions of group IIA secreted phospholipase A2 and group IVA cytosolic phospholipase A2 contribute to arachidonic acid release and prostaglandin production in rat gastric mucosal cells and transfected human embryonic kidney cells. The Journal of Biological Chemistry. 2006;281:16245–55. doi: 10.1074/jbc.M513874200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rainsford KD. Inhibition by leukotriene inhibitors, and calcium and platelet-activating factor antagonists, of acute gastric and intestinal damage in arthritic rats and in cholinomimetic-treated mice. The Journal of Pharmacy and Pharmacology. 1999;51:331–9. doi: 10.1211/0022357991772330. [DOI] [PubMed] [Google Scholar]
- 25.Kramer RM, Roberts EF, Manetta JV, Hyslop PA, Jakubowski JA. Thrombin-induced phosphorylation and activation of Ca(2+)-sensitive cytosolic phospholipase A2 in human platelets. The Journal of Biological Chemistry. 1993;268:26796–804. [PubMed] [Google Scholar]
- 26.Bartoli F, Lin HK, Ghomashchi F, Gelb MG, Jain MK, Apitz-Castro R. Tight bindings inhibitors of 85-kDa phospholipase A2 but not 14-kDa phospholipase A2 inhibit release of free arachidonate in thrombin – stimulated human platelets. The Journal of Biological Chemistry. 1994;269:15625–30. [PubMed] [Google Scholar]
- 27.Coffey MJ, Coles B, Locke M, Bermudez-Fajardo A, Williams PC, Jarvis GE, et al. Interactions of 12-lipoxygenase with phospholipase A2 isoforms following platelet activation through the glycoprotein VI collagen receptor. FEBS letters. 2004;576:165–8. doi: 10.1016/j.febslet.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Wong DA, Kita Y, Uozumi N, Shimizu T. Discrete role for cytosolic phospholipase A(2) alpha in platelets: studies using single and double mutant mice of cytosolic and group IIA secretory phospholipase A(2) The Journal of Experimental Medicine. 2002;196:349–57. doi: 10.1084/jem.20011443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonventre JV, Huang Z, Taheri MR, O’Leary E, Li E, Moskowitz MA, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–5. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 30.Bonventre JV. The 85-kD cytosolic phospholipase A2 knockout mouse: a new tool for physiology and cell biology. J Am Soc Nephrol. 1999;10:404–12. doi: 10.1681/ASN.V102404. [DOI] [PubMed] [Google Scholar]
- 31.Sapirstein A, Bonventre JV. Specific physiological roles of cytosolic phospholipase A(2) as defined by gene knockouts. Biochimica et Biophysica Acta. 2000;1488:139–48. doi: 10.1016/s1388-1981(00)00116-5. [DOI] [PubMed] [Google Scholar]
- 32.Bonventre J. Cytosolic phospholipase A2alpha reigns supreme in arthritis and bone resorption. Trends in Immunology. 2004;25:116–9. doi: 10.1016/j.it.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Haq S, Kilter H, Michael A, Tao J, O’Leary E, Sun XM, et al. Deletion of cytosolic phospholipase A2 promotes striated muscle growth. Nature Medicine. 2003;9:944–51. doi: 10.1038/nm891. [DOI] [PubMed] [Google Scholar]