Abstract
Studies using mouse models have established a critical role for resident satellite stem cells in skeletal muscle development and regeneration but little is known about this paradigm in human muscle. Here using human muscle stem cells, we address their lineage progression, differentiation, migration and self-renewal. Isolated human satellite cells expressed α7-integrin and other definitive muscle markers, were highly motile on laminin substrates, and could undergo efficient myotube differentiation and myofibrillogenesis. However, only a subpopulation of the myoblasts expressed Pax7 and displayed a variable lineage progression as measured by desmin and MyoD expression. Analysis identified a differentiation-resistant progenitor cell population that was Pax7+/desmin− and capable of self-renewal. This study extends our understanding of the role of pax7 in regulating human satellite stem cell differentiation and self renewal.
Keywords: Muscle Stem Cell, Satellite Cell, Pax7, Myogenic Differentiation, Skeletal Muscle
Introduction
The process of myogenesis is a complex series of events whereby mononucleated progenitor cells undergo expansion and then progress down the myogenic lineage pathway until they are differentiation-competent myoblasts. Following cues for migration and alignment, the myoblasts finally differentiate to form multinucleated myotubes, and eventually mature myofibers of skeletal muscle (Perry and Rudnick, 2000; Charge and Rudnicki, 2004). The ability of skeletal muscle to grow, maintain, and regenerate itself is dependent on a population of satellite progenitor cells that reside in between the muscle basal lamina and the cell membrane of myofibers; for review see (McKinnell et al., 2005; Peault et al., 2007). During development, myogenic progenitor cells are maintained as a proliferating cell population but eventually become a quiescent satellite cell population in adults (Montarras et al., 2005; Relaix et al., 2005). Following muscle injury or stress the adult quiescent satellite cells typically become activated, go through multiple rounds of proliferation before terminally differentiating to form myotubes. This well ordered process of myogenesis is tightly regulated by a group of master controllers termed myogenic regulatory factors (MRFs). The MRFs are basic helix-loop-helix transcription factors that include Myf-5, MRF4, MyoD, and myogenin (Blais et al., 2005; Sartorelli and Caretti, 2005). Recently, much attention has focused on the role of the paired box transcription factor Pax7, that appears to regulate the balance between satellite cell population maintenance and differentiation (Buckingham, 2007).
Pax7 is a transcription factor that is highly conserved between mouse and human, characterized by the presence of a paired box domain and a homeodomain (Schafer et al., 1994; Buckingham and Relaix, 2007). Both in vivo and in vitro analysis have shown that following activation the majority of muscle stem cells will turn on myogenic specific transcription factors such as Myf5 and MyoD, proliferate and then terminally differentiate (Yablonka-Reuveni and Rivera, 1994; Zammit et al., 2002). However, some of the population will retain Pax7 expression, turn off MyoD and return to a state of quiescence to maintain the muscle stem cell pool (Olguin and Olwin, 2004; Zammit et al., 2004). Adult Pax7 null mice demonstrate distinct muscle wasting and an extreme deficiency in muscle regeneration that is related to the loss of the satellite cell population (Seale et al., 2000; Kuang et al., 2006). Interestingly, satellite cells are present at birth in Pax7 mutant mice but are progressively diminished throughout postnatal development (Seale et al., 2000; Kuang et al., 2006). Evidence suggest that their postnatal loss is related to deficiencies in their ability to self renewal, possibly relating to proliferation or apoptotic events (Oustanina et al., 2004; Relaix et al., 2006).
Recent insights have been made into the molecular mechanism of Pax7. For example, Pax7 was shown to associate with a histone-methltransferase complex that can lead to transcriptional activation and was specifically shown to regulate Myf5 expression in this manner (McKinnell et al., 2008). Pax7 appears to regulate the maintenance of the muscle stem cell population by regulating both Myf5 and MyoD so that some cells can remain Pax7 positive and avoid terminal differentiation to maintain the population.
The microenvironment niche of muscle stem cells can also regulate many basic functions of muscle stem cells including proliferation, migration, differentiation and self-renewal (Sanes, 2003; Kuang et al., 2008). For example, the transplantation of an individual muscle fiber (containing only a few satellite cells but an intact extra-cellular niche) into irradiated muscle can give rise to thousands of new satellite cells capable of efficient proliferation, migration, fiber regeneration, and contribution to the satellite cell reservoir (Collins et al., 2005). In contrast, unsorted, cultured satellite cells, expanded away from their natural extracellular environment fail to effectively proliferate or migrate and make almost no contribution to the satellite cell reservoir following injection (Beauchamp et al., 1999; Montarras et al., 2005). Satellite cell self-renewal through asymmetrical division is another example of the extracellular niche regulating muscle stem cells (Kuang et al., 2007). During an asymmetrical division those satellite cells that remain in contact with the basal lamina become Pax7 positive and Myf5/MyoD negative and remain part of the muscle stem cell population (Kuang et al., 2007). Those cells that divide apically, losing direct basil lamina contact, become Pax7 negative and Myf5/MyoD positive progressing into the muscle lineage, and do not contribute to the reserve cell population (Kuang et al., 2007). Thus, the satellite cell niche, including the basement membrane, plays a critical role in regulating basic muscle stem cell function and self renewal.
While a great deal of work has been done to investigate the biology of muscle stem cells in mouse models, relatively little is known about their human counterparts. In the present study we investigate human muscle stem cells in tissue sections and in vitro, analyzing their lineage progression, differentiation, migration and self renewal. A subset of cells both in vivo and in culture expressed Pax7 with some Pax7+ cells showing co-expression with the transmembrane receptor cMet, a protein important for myoblast migration. Human muscle cell cultures, isolated by sorting for α7 integrin expression, displayed a more complex heterogeneity than mouse muscle cultures established in the same way. Specifically, only a subset of myoblasts within the human muscle cell cultures expressed Pax7, desmin and MyoD, providing evidence of variable lineage progression. We examined the human muscle stem cell niche, showing that laminin isoforms can regulate myoblast migration and are expressed on the surface of myotubes in vitro and in vivo. Lastly, differentiation of the human muscle cells into myotubes revealed a differentiation-resistant progenitor cell population that was Pax7+/desmin−. The presence of clonogenic cells from the differentiation-resistant cell population verified that the Pax7+/desmin− cells were capable of self-renewal. These studies support an important role for Pax7 in human muscle stem cell self-renewal and also show that important potential differences between mouse and human muscle stem cell lineage progression likely exist.
Results
Expression analysis of human muscle stem cell markers in tissue sections
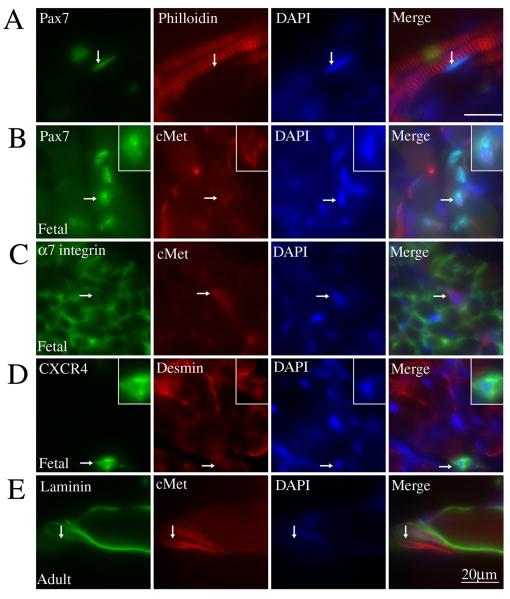
The expression of muscle lineage and muscle stem cell markers has been extensively studied using mouse models but little is known about expression of these proteins in human. To investigate the expression of human muscle lineage and muscle stem cell markers, immunohistochemistry was performed on sections of human fetal muscle (limb or tongue) and adult neck muscle (sternocleidomastoid). Pax7, a protein critical to muscle stem cell maintenance (Seale et al., 2000; Kuang et al., 2006), is expressed by cells directly adjacent to myofibers, a traditional position for satellite cells (Fig. 1A). Similarly cMet, a cell surface growth factor receptor important for myoblast migration (Bladt et al., 1995; Dietrich et al., 1999), was often co-expressed by Pax7+ cells within human fetal muscle fiber bundles (Fig. 1B). Staining for α7 integrin, the main laminin receptor in muscle, showed expected expression at the sarcolemma of myofibers and by individual myoblasts (Fig. 1C). Commonly, myoblasts positive for α7 integrin myoblasts also co-expressed cMet (Fig. 1C). Individual cells expressing desmin, an intermediate filament protein expressed by muscle, were readily identified. A subset of the desmin+ cells showed co-expression with the transmembrane protein CXCR4 when analyzed with immunostaining (Fig. 1D). Expression of CXCR4 has recently been used to isolate a subpopulation of mouse muscle stem cells that show high potential in muscle regeneration (Cerletti et al., 2008). Due to the poor integrity of the adult tissue, immunohistochemical studies were not optimal. However, good results were obtained when co-staining for cMet and laminin α2 chain was performed, showing cMet+ cells directly adjacent to the basement membrane (Fig. 1E). This cell is likely a myogenic progenitor cell given its expression of cMet and location directly adjacent to the muscle basal lamina, although definite proof will require confirmation of expression of other myogenic proteins (e.g. Pax7). Overall, these experiments show that markers of muscle stem cells established in mouse are also expressed by human muscle cells in vivo.
Figure 1. Individual human muscle cells express muscle stem cell markers including Pax7, c-Met and α7 integrin in vivo.
Human fetal (21 week) skeletal muscle (A-D) and adult skeletal muscle (E) were analyzed for expression of skeletal muscle and muscle stem cell markers. (A) Co-immunostaining for Pax7 and philloidin show a Pax7+ cell (arrow) located adjacent to individual muscle fibers. (B) Co-immunostaining for Pax7 and c-Met shows an individual cell (arrow) positive for both. Inserts in top right corner show a magnified image of the cell indicated by the arrow. (C) Immunostaining shows that α7 integrin expression is present both at the sarcolemma of fetal myofibers and in individual, cMet+ muscle cells (arrow). (D) Co-immunostaining for desmin and CXCR4 shows an individual cell within a muscle bundle co-expressing both (arrow). Inserts in top right corner show a magnified image of the cell indicated by the arrow. (E) Immunostaining of adult human myofibers shows an individual cMet+ muscle cell (arrow) located outside myofibers labeled with antibodies to laminin α2 chain. Scale Bar = 20μM
Desmin and MyoD mark subpopulations in cultured human myoblasts
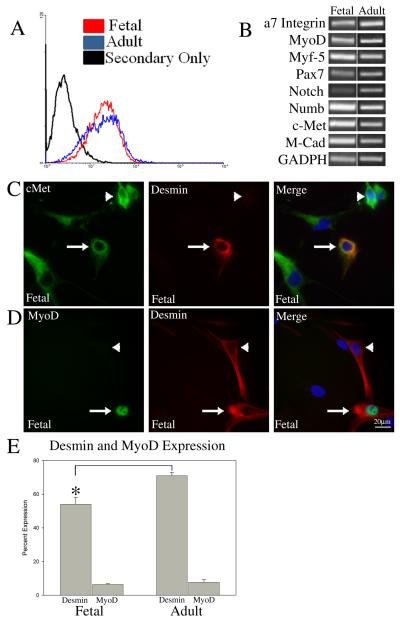
In order to further characterize the muscle stem cell population, primary myoblast cultures were derived from both human fetal and adult muscle tissue. Mononuclear cells were dissociated from fetal or adult muscle, sorted using FACS for α7 integrin expression and expanded in culture. This method (utilized by our lab and others) has been used successfully to isolate pure populations of mouse and human myoblasts and is based on the specific expression of this receptor by muscle satellite cells (Blanco-Bose et al., 2001; Ozeki et al., 2006; Kuang et al., 2007). Following expansions of the myoblasts in culture, flow cytometry confirmed that α7 integrin expression remained strong for both fetal and adult cells (Fig. 2A), suggesting minimal contamination by non-myogenic cells such as fibroblasts. For both fetal and adult isolates, we usually found a single peak of α7-expressing cells. Occasionally, for the adult preparations, some heterogeneity in labeling was found with two peaks but the reason for such variation in expression has not as yet been investigated. Semi-quantitative RT-PCR analysis showed that cultures expanded from both fetal and adult tissue consistently expressed muscle lineage markers including Myf-5, MyoD, M-cadherin, and Pax7 (Fig. 2B). The RT-PCR analysis was similar for fetal cells isolated from both tongue and limb muscle (data not shown).
Figure 2. Heterogeneous expression of MyoD and desmin by α7 integrin sorted human myoblasts in culture suggests variable lineage progression.
Primary muscle cultures from adult and fetal muscle were isolated using FACS for α7 integrin expression. (A) Flow cytometry analysis using mAb 9.1 against human α7 integrin verifies the muscle cell lineage of the established primary cultures. (B) RT-PCR analysis of adult and fetal cells demonstrates the expression of known muscle lineage markers. (C) Immunostaining for c-Met/desmin shows cells that are positive for both (arrow) and cells that express c-Met but not desmin (arrowhead). (D) Immunostaining for MyoD/desmin shows cells that are positive for both (arrow) and cells that express desmin but not MyoD (arrowhead). (E) Percentage of cells expressing desmin and MyoD as measured by immunostaining of fetal and adult myoblast cultures. Asterisk* P<0.005 denotes significant differences between desmin expression levels in fetal compared to adult. Scale Bar = 20μM
To further define the expression pattern and lineage progression of the cultured fetal and adult muscle cells immunostaining was performed using cMet, desmin and MyoD antibodies. While all cells expressed cMet, only a subset of cells expressed desmin and MyoD. Staining for CD31, a marker of endothelial cells verified that there was no contamination from cMet+ endothelial cells (data not shown). We found that approximately 50% of the fetal cells were desmin+ and approximately 70% of adult cells were desmin+ (Fig. 2C and 2E and SI Fig. 1). Immunostaining analysis of human muscle cultures using antibodies to MyoD revealed that 6% and 7% of the fetal and adult cultured cells expressed MyoD respectively (Fig. 2D and 2E and SI Fig. 1). Further, all cells that expressed MyoD also expressed desmin, but not all cells expressing desmin expressed MyoD. Thus, all of the cells in culture expressed the muscle specific protein α7 integrin and cMet, but only a subset expressed desmin and MyoD. This expression pattern differs from what is typically seen in primary mouse cultures established using FACS for α7 integrin where essentially all cells express desmin and MyoD (SI Fig. 2). The cells in our human cultures that are integrin α7 integrin+/cMet+/desmin−/MyoD− likely represent cells that have not progressed far enough into the muscle lineage to express desmin or MyoD. This suggests that the cultured fetal and adult human muscle cells sorted for α7 integrin expression contain cells at various stages of muscle lineage progression and that this progression may manifest as a more complex heterogeneity than cultured mouse muscle cells.
Laminin, a muscle stem cell niche component, promotes migration of human myoblasts
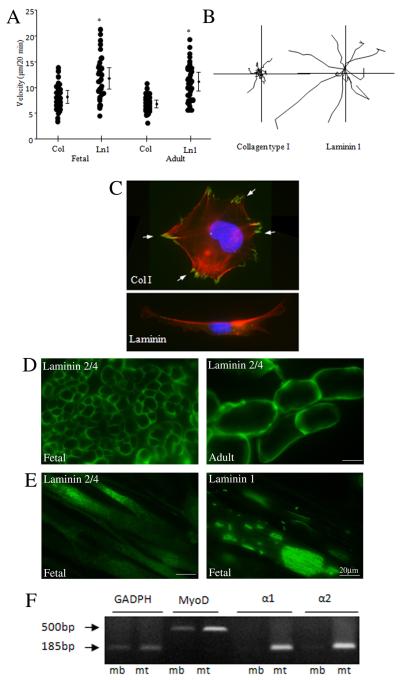
Locomotion of skeletal muscle precursor cells is an important step during muscle development and post lesion muscle regeneration. Myoblast motility needed for cell-cell contact and fusion is known to be influenced by extracellular matrix (ECM) proteins and expression of adhesion receptors. To continue our analysis of cultured α7 integrin+ human muscle cells we next investigated cell migration by plating both adult and fetal muscle cells onto either collagen type 1 or laminin-1 and measuring cell migration using video time-lapse microscopy. Both fetal and adult myoblasts showed similar enhanced motility on laminin compared to collagen-coated substrates (Fig. 3A). Analysis of cell tracts revealed that although cells attached to type 1 collagen, they migrated poorly on this substrate, barely above background (Fig. 3B). In contrast, cells plated on laminin 1 moved at a rate several fold greater than cells plated on type 1 collagen, with the motility tracts showing a mostly high persistent course of migration on laminin 1 (Fig. 3B). We next sought to further characterize the cell adhesions and their role in migration by seeding cells on to the different ECM ligands and then evaluating focal adhesion formation by immunofluorescent staining. Staining for paxillin, a focal adhesion protein, showed a much higher density of larger focal adhesions formed in human fetal myoblasts plated on collagen compared to those cells plated on laminin (Fig. 3C). Since, highly motile cells tend to form less focal adhesions than cells with lower motility, this suggests that the increased motility of human muscle cells is likely due to a decreased tendency to form focal adhesions when plated on a laminin substrate.
Figure 3. Laminin, a muscle stem cell niche component, promotes migration of human myoblasts.
(A) Cell motility of fetal and adult muscle cells was measured after seeding cells on type I collagen and laminin-1 coated substrates by video time-lapse microscopy. Asterisk* P<0.001 denotes significant differences between migration on collagen and laminin. (B) Individual cell tracks are shown for each substrate for fetal cells. (C) Focal adhesion formation on type I collagen and laminin-1 substrates. Fetal cells were plated on coverslips coated with type I collagen or laminin-1. After 2 h, cells were fixed, stained for paxillin and philloidin (polymerized actin), and examined by immunofluorescence microscopy. The localization of paxillin in focal adhesion on collagen is indicated by arrows (D) Immunostaining of fetal and adult muscle sections using antibodies to laminin α2 chain shows expression on the surface of individual muscle fibers. (E) Immunostaining for laminin α2 chain and laminin α1 chain showed expression of these isoforms by differentiated human myotubes in culture with a unique surface localization pattern for laminin α1. (F) Semiquantitative RT-PCR detected mRNA for α1 and α2 chains of laminin in fetal myotubes (mt) but not in myoblast (mb) cultures. MyoD mRNA expression, included as a positive control, was elevated in differentiated cultures.
Given laminin’s influence on myoblast migration we next sought to investigate the expression of laminin isoforms at the human muscle stem cell niche using human tissue sections and cultured cells. As expected, staining of both fetal and adult muscle sections using an antibody to the laminin α2 chain strongly labeled the basement membrane of individual myofibers (Fig. 3D). Note the larger size of individual muscle fibers in the adult tissue compared to the fetal. We next analyzed laminin expression in differentiating fetal myotubes. Immunostaining for either laminin α1 or laminin α2 showed expression of both isoforms on the surface of the myotubes (Fig. 3E). Interestingly, while the expression of laminin α2 was uniform along the length of myotubes, laminin α1 was confined to concentrated patches on the myotube surface (Fig. 3E). RT-PCR analysis further confirmed expression of laminin isoforms by fetal myotubes showing that both lamininα1 and lamininα2 chain expression were increased following myotube formation (Fig. 3F). MyoD, which is up-regulated as myoblasts differentiate, was included as a positive control in the RT-PCR analysis. These experiments show that specific laminin isoforms are expressed in the human muscle stem cell niche and that laminin can promote human myoblast migration.
Pax7 expression marks a differentiation-resistant muscle progenitor cell population capable of self-renewal
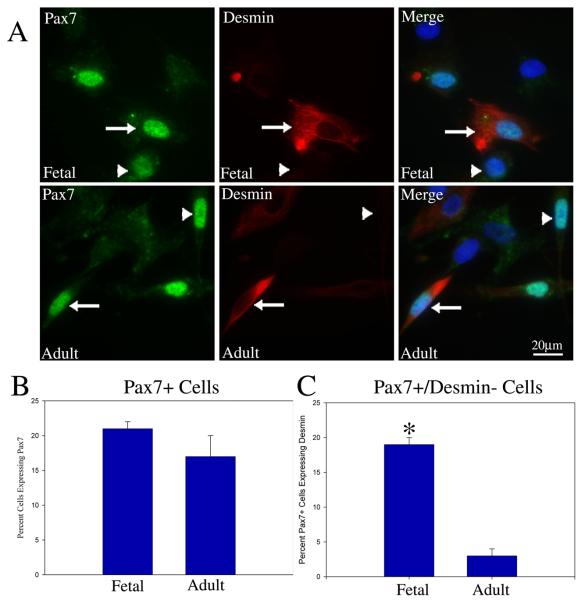
The transcription factor Pax7 is critical for muscle stem cell self-renewal in mouse but that same role in human muscle is not well established. To begin our analysis of Pax7 in human muscle stem cells, we analyzed Pax7 expression in both fetal and adult cultures and found that approximately 20% of cells expressed Pax7 (Fig. 4A and 4B). We found that most Pax7+ cells also co-expressed desmin but not all. Specifically, 20% and 4% of the Pax7+ cells did not co-express desmin in the fetal and adult cultures, respectively (Fig. 4A and 4C). This suggests that in the fetal cultures, a greater proportion of Pax7+ cells (represented by the Pax7+/desmin− cells) are less progressed into the muscle lineage compared to the adult cultures. Mouse muscle cultures established using α7 integrin sorting show uniform expression of Pax7 by all cells (SI Fig. 2). This difference in expression pattern further supports the possibility that human muscle cultures have a more complex heterogeneity than mouse counterparts.
Figure 4. A subpopulation of the α7 integrin sorted human muscle cells express the muscle stem cell marker Pax7 and show variable expression of desmin.
(A) Immunostaining for Pax7 and desmin of fetal (top row) and adult (bottom row) muscle cells show that some cells are Pax7+/desmin+ (arrows) while others are Pax7+/desmin− (arrowheads). (B) Quantification of the immunostaining shows that in both fetal and adult muscle cultures ~20% of cells stain positive for Pax7. (C) Immunostaining for Pax7 and desmin shows that within the fetal cultures approximately 20% of the Pax7+ cells did not express desmin while in the adult approximately 4% of the Pax7+ subpopulation was desmin negative. Asterisk* P <0.002 denotes significant differences between fetal and adult. Scale Bar = 20μM
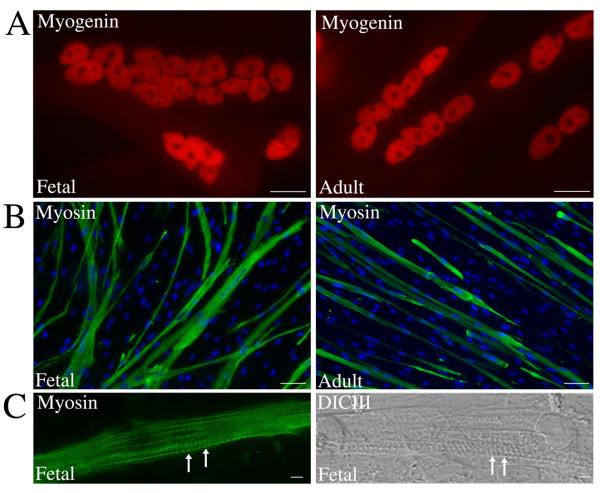
To analyze lineage progression during myotube formation and to investigate Pax7 expression under differentiation conditions, the muscle cultures were induced to differentiation following high density plating in differentiation medium. After 7-10 days, both fetal and adult human muscle cells formed numerous large, multinucleated myotubes. Immunostaining for myogenin (Fig. 5A), a transcription factor expressed by differentiated myotubes, and myosin (Fig. 5B), a contractile protein expressed by mature myotubes, confirmed appropriate myotube maturation and lineage progression. Myofibrillogenesis, the formation of the muscle contractile apparatus associated with later stages of myotube maturation, had clearly been initiated in some myotubes (Fig. 5C arrows). Thus, both human fetal and adult muscle cell cultures can readily progress through muscle lineage to form multinucleated myotubes and undergo myofibrillogenesis.
Figure 5. Myofibrillogenesis by human myotubes formed in culture.
Human fetal and adult muscle cultures were maintained in differentiation medium for 7-10 days and immunostained. Immunostaining for myogenin (A) and myosin (B) shows expression of these proteins by multinucleated myotubes in both fetal and adult cultures. (C) Image shows formation of the contractile apparatus (arrows) that was observed in some myotubes. Scale Bar = 20μM
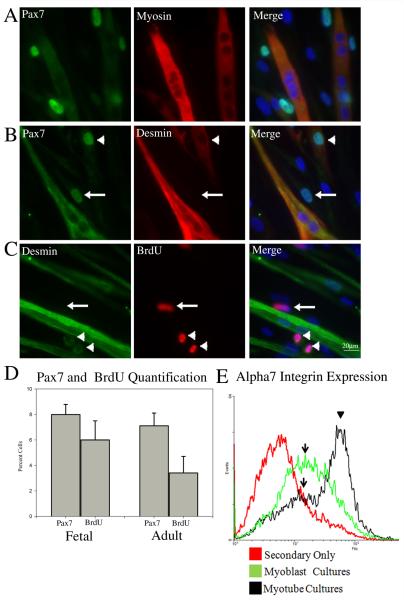
Efficient muscle generation (and regeneration) requires not only the ability to form myotubes but also the capacity of the muscle stem cell population to self-renew. Analysis of differentiated cultures revealed a distinct subset of cells, in both the fetal and adult cultures, that were differentiation-resistant despite prolonged exposure to differentiation conditions. We speculated that these cells may represent a progenitor stem cell population that expresses Pax7. Immunostaining for Pax7 and Myosin (Fig. 6A) shows that Pax7 was expressed by a portion (~8%) (Fig. 6D) of the differentiation-resistant cells but never by differentiated myotubes. Both Pax7+/desmin− and Pax7+/desmin+ cells were present in the differentiation-resistant population of both fetal and adult cells suggesting variable lineage progression among the Pax7+ differentiation-resistant cells (Fig. 6B and SI Fig 1C). Desmin was also highly expressed by differentiated myotubes (Fig. 6B and SI Fig 1C). To investigate if the differentiation-resistant cells were still actively proliferating, BrdU was added for 24 hours to the muscle cultures after 10 days in differentiation medium. Interestingly, approximately 6% of differentiation-resistant cells were able incorporate BrdU and were therefore still actively cycling, a percentage similar to that of the Pax7+ population (Fig. 6C and 6D). Both desmin+ and desmin− differentiation resistant cells incorporated BrdU while nuclei in the desmin+ differentiated myotube were never BrdU+ (Fig. 6C). Unfortunately, technical difficulties prevented co-staining to investigate BrdU incorporation and Pax7 expression.
Figure 6. Pax7 expression marks a differentiation-resistant muscle progenitor cell population.
Human muscle cultures were grown in differentiation medium for 7-10 days to induce myotube formation and then immunostained. (A) Staining with anti-myosin and anti-Pax7 antibodies showed that Pax7 is expressed by some differentiation-resistant cells but never by myotubes. (B) Immunostaining for Pax7 and desmin demonstrated that some cells in the differentiation-resistant population are Pax7+/desmin+ (arrowhead) while others are Pax7+/desmin− (arrow). (C) Cultures were pulsed with BrdU for 24 hours and processed to assess BrdU incorporation and desmin expression. Frequently, mononuclear cells were identified that were BrdU+/desmin+ (arrowhead) cells or BrdU+/desmin− (arrow). (D) Percentage of differentiation-resistant cells expressing Pax7 and BrdU after 10 days in differentiation medium for fetal and adult cultures. (E) Flow cytometry was used to analyze α7 integrin expression among cells in the differentiation-resistant cell population. The data indicates that within the myotube cultures, some cells express a high level of α7 integrin (arrowhead) (presumably differentiated myotubes) whereas a second distinct population of cells retain a low level of α7 integrin expression (arrow) (presumably differentiation-resistant progenitor cells). Scale Bar = 20μM
As myoblasts differentiate there is an increase in α7 integrin expression (George-Weinstein et al., 1993; Xiao et al., 2003). Consequently, a differentiation-resistant progenitor cell population should maintain a low level of α7 integrin expression compared to a differentiated cell population. To investigate α7 integrin expression in the differentiation-resistant population, differentiating fetal cultures containing both myotubes and differentiation-resistant cells were detached and filtered through a 40 μm mesh filter. This process removed large multinucleated myotubes but left small myotubes as well as the differentiation-resistant cells. The filtered cells were then subjected to flow cytometry to measure α7 integrin expression. Flow cytometry identified two distinct cell populations (Fig. 6E): (1) a population expressing very high levels of α7 integrin (arrowhead) typical of differentiating myoblasts (and myotubes) and (2) a population expressing a lower level of α7 integrin (arrow), an expression profile expected for a differentiation-resistant progenitor cell population. Interestingly, the cell population in the differentiated cultures with low α7 integrin levels shows a very similar expression level to myoblasts not exposed to differentiation conditions (Fig. 6E arrows).
To further confirm the progenitor cell nature of the differentiation-resistant cells two approaches were utilized: (1) we tested whether the differentiation-resistant cells could be expanded and induced to form myotubes a second time and (2) a clonal analysis of the fetal differentiation-resistant cells was performed. Differentiated cultures containing both myotubes and differentiation-resistant cells were filtered through a 40 μm filter to remove large myotubes and the remaining cells were plated in high serum media for expansion. The differentiation-resistant cells readily expanded when cultured in high serum medium and were then induced to differentiate. As with the original cultures, large myotubes were formed by day 7 although there was a slight decrease in the fusion index with the second round of differentiation at 88% (p< 0.05) of the first round of differentiation (a 12% decrease) (Fig 7A). Interestingly, unlike the first round of differentiation when Pax7+ cells were readily present, very few Pax7+ cells (arrow) were present after the second round of differentiation (Fig. 7A). Less than 1% of differentiation resistant cells expressed Pax7 following the second induction of differentiation compared to ~8% with the first differentiation. Also, these Pax7+ cells (arrow) showed a very weak staining intensity compared to the Pax7+ cells present after the first round of myotube formation (Fig. 7A). Thus the differentiation-resistant cell population could be re-expanded and induced to form multinucleated myotubes a second time but demonstrated a large reduction in the number of residual Pax7+ cells following the second cycle of differentiation.
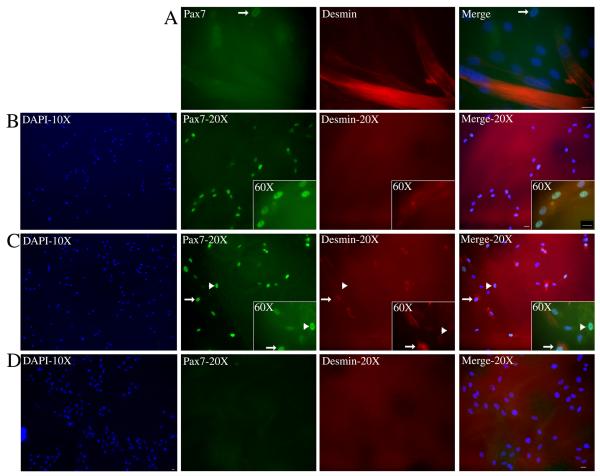
Figure 7. The differentiation-resistant cells demonstrate characteristics of a progenitor cell population.
Differentiation-resistant cells were isolated from fetal multinucleated myotubes as detailed in the material and methods section and then re-expanded to form myotubes or plated at low density for clonal analysis. (A) Differentiation-resistant cells that were expanded and induced to differentiate a second time were immunostained for Pax7 and desmin. The staining showed that differentiation-resistant cells could form myotubes but had a coordinated loss of Pax7+ cells (arrow). (B,C,D) Clonal analysis of the differentiation-resistant cells revealed three types of colonies that formed with respect to Pax7 and desmin expression. The first image of each row shows a 10X view of the colony using DAPI exposure. (B) Example of a colony where all cells within the colony were Pax7+/desmin−. (C) Example of a rare colony showing cells that were Pax7+/desmin− (arrowhead) as well as cells that were Pax7+/desmin+ (arrow). (D) Example of a colony where all cells were Pax7−/desmin−. Scale Bar = 20μM
We next subjected the fetal differentiation-resistant cells to clonal analysis. We found that three types of colonies formed with respect to Pax7 and desmin expression. Approximately half of the colonies (18/35) that formed were made up uniformly of Pax7+ cells that did not express desmin (Fig. 7B). The other half of the colonies (15/35) contained cells that were negative for both Pax7 and desmin (Fig. 7D). These negative staining colonies contained a similar number of cells per colony (all colonies contained an average of ~100 cells) but could be easily distinguished from the Pax7+ as these cells had larger nuclei and formed more densely packed colonies. Lastly, rare colonies were sometimes observed in which all the cells expressed Pax7 but approximately half of the cells in the colony also co-expressed desmin (Fig. 7C). These results suggest the existence of a Pax7+/desmin− progenitor cell population in human muscle cultures that can resist differentiation and has the capacity for self-renewal.
Discussion
In this paper we sought to analyze the lineage progression, differentiation, migration and self-renewal of human muscle stems cells, a group of cells that are well described in mouse but have not been extensively characterized in human tissues. We found that fetal and adult human muscle cultures, sorted for integrin alpha7 expression, display a variable lineage progression (as measured by Pax7, desmin and MyoD expression) and show a more complex heterogeneity than mouse cultures established in a similar fashion. We show that the transcription factor Pax7 is expressed by individual muscle cells both in vivo and in vitro and further show that Pax7 expression marks a differentiation-resistant muscle progenitor cell population capable of self-renewal.
Expression of muscle stem cell markers in human
Tissue staining experiments using fetal muscle revealed individual muscle cells located within muscle fiber bundles that co-expressed Pax7 and cMet. Since cMet is associated with myoblast migration and Pax7 is expressed by muscle stem cells, these double-labeled cells likely represent muscle stem cells capable of migrating. Consistent with this idea, individual cMet+ muscle cells also co-expressed α7 integrin, the primary laminin receptor in myoblasts and myotubes. This is interesting in light of our observation that laminin, a protein we confirm is highly enriched in the human muscle stem cell niche, enhanced myoblast migration. Thus it is possible that the α7 integrin+/cMet+ cells are, at least in part, stimulated to migrate through a mechanism involving laminin activation of α7 integrin. In adult muscle tissue individual cMet+ cells were seen in close association with myotubes, an expected location for muscle progenitor cells. Unfortunately, the poor tissue integrity of the adult muscle samples precluded further staining with other progenitor markers like Pax7.
As myoblasts progress through the muscle lineage to form myotubes, Pax7 expression diminishes while expression of others markers such as MyoD and desmin increase (Zammit et al., 2006). Within our cultured human muscle cells, Pax7 was expressed by only a subpopulation of cells (~20%). As our data shows, this finding differs from what is seen in cultured mouse myoblasts established using the same α7 integrin FACS method where essentially all cells express Pax7. Also, mouse myoblast cultures established using FACS for α7 integrin uniformly express desmin (Blanco-Bose et al., 2001) which differs from the human cultures where only 50% and 70% of human cultured myoblasts express desmin in fetal and adult cultures, respectively. These findings suggest that expression of muscle stem cell and lineage markers in human muscle cultures is more complex than in mouse cultures.
While both human fetal and adult cultures contained a similar percentage of cells expressing Pax7, a much larger percentage of the Pax7+ cells in the fetal cultures did not co-express desmin (20% in fetal vs. 4% in adult). Since desmin is expressed by muscle cells as they progress into the muscle lineage (Kaufman and Foster, 1988; Conboy and Rando, 2002) it is possible that Pax7+ muscle progenitors in adult cultures are more advanced into the muscle lineage than their fetal, Pax7+ counterparts. This is supported by studies in aged mouse muscle where the progenitor cell population decreases as the balance between muscle lineage progression and progenitor cell maintenance favors lineage progression (Shefer et al., 2006; Collins et al., 2007). A possible mechanism contributing to this divergent desmin expression in Pax7+ progenitors comes from studies of asymmetrical division in muscle progenitors and its role in muscle stem cells maintenance (Conboy et al., 2007; Kuang et al., 2007). The authors found that when muscle stem cells divide asymmetrically, the daughter cell inheriting the older, original template DNA is always desmin negative whereas the other daughter cell, inheriting the immature DNA, expresses desmin, whenever desmin in expressed among the pair (Conboy et al., 2007). In this model, the desmin negative cell that retains the template DNA maintains a stem cell state while the desmin+ daughter cell becomes a transient amplifying muscle progenitor critical to muscle growth. Applying this model to our observations, it is apparent that human fetal muscle cells in culture may contain more muscle stem cells than adult counterparts.
Differentiation-resistant progenitor cells
We observed that, like muscle cultures derived from mouse, human myoblasts differentiate to form large, multinucleated myotubes. Unlike primary mouse cultures, many myoblasts were differentiation-resistant and a significant percentage of these cells expressed Pax7. Among the Pax7+ differentiation-resistant cells, we observed both desmin-expressing and desmin-negative cells, suggesting variable lineage progression among the differentiation-resistant population. Further, some of the differentiation-resistant cells were actively proliferating (as evidenced by BrdU incorporation) and maintained a basal level of α7 integrin expression even after prolonged exposure to differentiation medium. Once isolated from the myotubes and placed back into high-serum media, the differentiation-resistant population could be readily re-expanded and induced to form myotubes a second time. These observations indicate that the differentiation-resistant population contains muscle progenitors that, under the right conditions, can expand and form myotubes.
Our clonal analysis provides additional evidence for a progenitor stem cell population among the differentiation-resistant cells and further supports a role for Pax7 in their maintenance. Approximately half of the colonies formed following clonal plating of the differentiation-resistant cells were uniformly Pax7+/desmin−. Thus, clonal plating of the differentiation resistant cells allowed for the isolation of a Pax7+/ desmin− population of cells that is capable of self-renewing divisions and differentiation-resistance, properties critical to a true muscle stem cells population. A very small percentage of colonies had cells that were both Pax7+/desmin− and Pax7+/desmin+; these colonies may have arisen from a single Pax7+/desmin− progenitor that divided asymmetrical to produce Pax7+/desmin+ cells. In mouse models, it is well established that Pax7 expression is critical for a muscle progenitor cell to remain undifferentiated and thus maintain the progenitor cell population. Our data suggest a similar mechanism may be true for human muscle progenitor cells as well, where expression of Pax7 regulates progenitor cell self-renewal and differentiation. We also observed colonies containing cells that were neither Pax7+ nor desmin+ following clonal analysis of the differentiation resistant cells. Since flow cytometry confirmed that both the pre-fusion and fusion-resistant myoblasts were strongly expressing the muscle-specific α7 integrin, this suggests the fusion-resistant population may represent myoblasts that have neither effectively differentiated nor successfully maintained a progenitor cell status. Further since they express α7 integrin they are unlikely to represent contaminating fibroblasts, although it may be possible they represent dedifferentiated cells. Future experiments are planned to further characterize the status of these cells.
In mouse, Pax7 expression and progenitor cell maintenance is influenced by signals from the extracellular niche (Kuang et al., 2007; Kuang et al., 2008). It is interesting to note that most of the differentiation-resistant cells that were Pax7 positive or incorporated BrdU were in direct contact with a multinucleated myotube. It is possible that signals derived from the myotube or associated with it are required for cells to maintain Pax7 expression and preserve a progenitor cell state.
Collectively, our data shows that lessons from mouse models concerning muscle stem cell maintenance and lineage progression can also be applied to human although some differences do exist. This in vitro human system provides an extremely useful model through which to analyze the specific cellular and molecular mechanism involved in regulating the behavior of human muscle stem cells.
Experimental Procedure
Immunostaining of human muscle tissue
Human fetal muscle was obtained from the Department of Obstetrics and Gynecology, University of California, San Francisco. The study was approved by the UCSF Committee on Human Research. Fetal samples ranged in age from 21 to 24 weeks and consisted of limb and tongue tissue. Adult samples, obtained from the Department of Oral and Maxillofacial Surgery, consisted of tissues removed during repair of craniofacial anomalies. Muscle tissue obtained for immunostaining was flash frozen in Tissue-Tek OCT Compound (Sakura Fineteld, Torrance, CA) and cryosectioned in 10-μm increments. The tissue sections were then fixed and permeabilized in acetone at −20°C for 20 min. Sections were blocked in 1.0% BSA for 45 min and then immunostained.
For laminin α2 and desmin detection, sections were incubated with mouse anti-laminin2/4 (Merosin) mab1922Z (Chemicon, Temecula, CA), at 1:500 or mouse anti-human desmin clone D33 (DakoCytomation, Denmark) at 1:100 for 1hr at RT. After washing in PBS, FITC-conjugated anti-mouse secondary antibody (Jackson Labs, Bar Harbor, ME) was applied for 1 h at RT. cMet immunostaining utilized rabbit anti-cMet sc-161 at 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA) for 1hr at RT. After washing in PBS, Rhodamine-conjugated anti-rabbit secondary antibody (Jackson Labs) was applied for 1 hr at RT.
For Pax7 and α7 integrin immunostaining sections were incubated with mouse anti-Pax7 mab1675 at 1:400 (R&D Systems, Minneapolis, MN) or mouse anti-α7 integrin mAb 9.1(Vizirianakis et al., 2001) at 1:400 for 1 hr at RT. After washing in PBS, horseradish peroxidase-conjugated anti-mouse secondary antibody (1:100; Invitrogen, Carlsbad, CA) was applied for 1 hr at RT followed by a short incubation in Alexa Fluor 488-linked tyramide (1:300; Invitrogen, Carlsbad, CA). For double immunolabeling, the Pax7 and α7 integrin labeled sections were incubated with rabbit anti-cMet sc-161 at 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA) for 1hr at RT, followed by incubation in Rhodamine conjugated anti-rabbit secondary (Jackson Labs, Bar Harbor, ME). All sections were mounted with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame CA). Note: The fetal muscle images shown in figure 1 and 7 are from tongue muscle tissue but staining of muscle tissue from fetal limb was identical (data not shown).
Primary and secondary muscle cell culture
The human adult and fetal tissue was processed for cell isolation by first subjecting the tissue to dissociation followed by FACS for α7 integrin expression and cultured as all described previously (Ozeki et al., 2006). Fetal myoblasts were carried in Ham’s F-10 (Mediatech, Manassas, VA), 20% fetal bovine serum (FBS) (Invitrogen), 50 U/ml penicillin, 100 μg/ml streptomycin. Adult myoblasts were carried in Ham’s F-10 (Mediatech), 20% FBS, 2.5ng/ml bFGF (Invitrogen), 50 U/ml penicillin, 100 μg/ml streptomycin. All cells used in experiments were between passage 2 and passage 9. To induce myotube formation myoblasts were plated at 80% confluency and switched to differentiation medium (DM) for 7-10 days. DM for fetal cells consisted of DMEM (Mediatech), without FBS but supplemented with Insulin-Transferrin-Selenium (Invitrogen), plus antibiotics. DM for adult cells consisted of DMEM, 2%FBS (Invitrogen), supplemented with Insulin-Transferrin-Selenium, plus antibiotics.
Flow cytometry and immunostaining
Standard flow cytometry protocols were used as previously described (Yao et al., 1996; Ozeki et al., 2006). Briefly, 1×106cells were incubated with the mouse anti-α7 integrin antibody mAb 9.1 washed, incubated with an anti-mouse FITC-conjugated secondary antibody (Jackson Labs) and subjected to flow cytometry using Becton Dickinson FACS-Star Plus flow cytometer.
For immunostaining, myoblasts or myotubes on glass coverslips were fixed in 2% paraformaldehyde for 10 min at RT and permeabilized using 0.1% TritonX-100 for 30 min and blocked 30 min in 1% BSA. For desmin/cMet double immunostaining, cells were first incubated with mouse anti-desmin (DAKO) at 1:1000 for 1 hr at RT. After washing in PBS, horseradish peroxidase-conjugated anti-mouse secondary antibody (1:100; Invitrogen, Carlsbad, CA) was applied for 1 hr at RT followed by a short incubation in Alexa Fluor 488-linked tyramide at 1:300. Cells were then incubated with rabbit anti-cMet sc-161 at 1:100 (Santa Cruz Biotechnology) for 1hr at RT, followed by incubation with Rhodamine conjugated anti-rabbit secondary (Jackson Labs, Bar Harbor, Maine).
For Pax7/desmin and MyoD/desmin double immunostaining, cells were first incubated with mouse anti-Pax7 (R&D Systems) at 1:400 or mouse anti-MyoD (BD Bioscience) for 1 hr at RT. After washing in PBS, horseradish peroxidase-conjugated anti-mouse secondary antibody (1:100; Invitrogen) was applied for 1 h at RT followed by a brief exposure to Alexa Fluor 488-linked tyramide at 1:300. Cells were then incubated with rabbit monoclonal anti-desmin cloneY66 at 1:200 (Epitomics, Burlingame CA) for 1hr at RT, followed by incubation with Rhodamine conjugated anti-rabbit secondary (Jackson Labs, Bar Harbor, Maine).
For laminin 2/4 (Merosin), laminin1, myogenin, myosin, and paxillin immunostaining cells were incubated with mouse anti-laminin 2/4 mab1922Z (Chemicon), at 1:500 or goat anti-laminin 1 sc6016 (Santa Cruz) at 1:50 or mouse anti-myogenin clone F5D (DAKO) at 1:100 or mouse anti-myosin mAb MF20 (Hybridoma Bank Iowa City, Iowa) at 1:100 or mouse anti-paxillin (BD Bioscience, San Jose, CA) at 1:400 for 1 hr at RT followed by the appropriate FITC or Rhodamine conjugated secondary antibody.
For BrdU/desmin double immunostaining, cells plated on cover slips were exposed to 10μM BrdU (Sigma St Louis, MO) for 24 hours followed by fixation in 2% paraformaldehyde for 10 min at RT. Cells were then steamed in 10mM citric acid (pH 6.0) for 15 min and cooled to room temperature in PBS. Cells were blocked in 1% BSA for 30 min at RT followed by incubation with monoclonal rat anti-BrdU at 1:250 (Novus Biological, Littleton CO) and rabbit mAb anti-desmin at 1:200 (Epitomics, Burlingame, CA) for 1hr at RT. Cells were then incubated with the appropriate FITC or Rhodamine conjugated secondary antibody for detection. All cells were mounted with Vectashield mounting medium with DAPI (Vector Laboratories).
Microscopy
Microscopy was done using a Zeiss Axiovert 200M microscope with images captured using a AxioCam MRm camera and Axiovision software.
Semi-quantitative RT-PCR
RNA was isolated using a RNeasy Mini Kit (Qiagen). cDNA was then generated using Superscript 1st Strand Synthesis System (Invitrogen) followed by PCR amplification using Taq PCR Master Mix (Qiagen) performed in an Eppendorf Mastercycler thermal cycler following manufactures recommended protocol for 30 cycles using the appropriate primers as listed 5′ to 3′: Integrin alpha7 (aatcaagttcgggaagaaagagg, gggaggtcaggttccgact), MyoD(aagcgccatctcttgaggta, gcgcctttattttgatcacc), Myf5(gtcccccgagggtgaatttg, gcccacatgaggcagtgac), Pax7 (aatcaagttcgggaagaaagagg, gggaggtcaggttccgact), Notch1 (gctggactggtgaggactg, agccctcgttacaggggtt), Numb (agaggaagactgatttccccat, actcggtcccttggaaggtag), cMet(tggtgcagaggagcaatgg, cccagtcttgtactcagcaac), M-Cad(accacaagcgtctccccta, gcctgaagcgatcagtcttct), GADPH (tgcaccaccaactgcttag, gacgcagggatgatgttc).
Migration assay
Time-lapse video microscopy was performed as described with slight modifications (Zhou and Kramer, 2005). Briefly, cells were seeded onto 6-well plates (Falcon, Becton Dickinson Labware) coated with different substrates (collagen I (10 μg/ml), laminin-1 (10 μg/ml), for 30 min. Plates were then examined in a Zeiss Axiovert inverted microscope with an X-Y scanning motorized stage (Carl Zeiss Micro Imaging, Inc., Thornwood, NY) and maintained at 37°C and 5% CO2. Images were collected at the indicated time intervals using a AxioCam MRm camera and analyzed with Axiovision software. The positions of individual nuclei were tracked to determine the relative migration rates.
Quantification and statistics
To quantify expression profiles of various proteins, random fields were chosen under bright field microscopy and then quantified for three separate experiments for each staining condition. To determine the fusion index, the percentage of nuclei located in multinucleated myotubes was counted and measured as a percentage of total nuclei from random fields. For paired analyses, t-tests were used. All error bars reported represent standard deviation.
Supplementary Material
Supplemental Figure 1. Heterogeneous expression of Pax7, MyoD and desmin by adult α7 integrin sorted human cultured cells. (A) Immunostaining for c-Met/desmin shows cells that are positive for both (arrow) and cells that express c-Met but not desmin (arrowhead). (B) Immunostaining for MyoD/desmin shows cells that are positive for both (arrow) and cells that express desmin but not MyoD (arrowhead). (C) Immunostaining for Pax7 and desmin on adult muscle cultures grown in DM for 7-10 days to induce myotube formation demonstrated that some cells in the differentiation-resistant population are Pax7+/desmin+ (arrow) while others are Pax7+/desmin− (arrowhead). Scale Bar = 20μM
Supplemental Figure 2. Homogeneous expression of Pax7, MyoD and desmin by cultured mouse myoblasts. Immunostaining of mouse primary myoblasts for (A) MyoD (B) Desmin (C) Pax7 show uniform expression by all cells for these proteins.
Acknowledgments
We would like to thank Dr. Julie Siegenthaler for assistance in tissue sectioning and critical reading of the manuscript. We also thank Dr. J. Oren Humtsoe for technical advice.
This work was supported by a grant to R.K. from the National Institutes of Health (R01DE015404).
Grant Sponsor: NIH; Grant number R01 DE 015404
References
- Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Blais A, Tsikitis M, Acosta-Alvear D, Sharan R, Kluger Y, Dynlacht BD. An initial blueprint for myogenic differentiation. Genes Dev. 2005;19:553–569. doi: 10.1101/gad.1281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Bose WE, Yao CC, Kramer RH, Blau HM. Purification of mouse primary myoblasts based on alpha 7 integrin expression. Exp Cell Res. 2001;265:212–220. doi: 10.1006/excr.2001.5191. [DOI] [PubMed] [Google Scholar]
- Buckingham M. Skeletal muscle progenitor cells and the role of Pax genes. C R Biol. 2007;330:530–533. doi: 10.1016/j.crvi.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol. 2007;23:645–673. doi: 10.1146/annurev.cellbio.23.090506.123438. [DOI] [PubMed] [Google Scholar]
- Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell acti vation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Conboy MJ, Karasov AO, Rando TA. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol. 2007;5:e102. doi: 10.1371/journal.pbio.0050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich S, Abou-Rebyeh F, Brohmann H, Bladt F, Sonnenberg-Riethmacher E, Yamaai T, Lumsden A, Brand-Saberi B, Birchmeier C. The role of SF/HGF and c-Met in the development of skeletal muscle. Development. 1999;126:1621–1629. doi: 10.1242/dev.126.8.1621. [DOI] [PubMed] [Google Scholar]
- George-Weinstein M, Foster RF, Gerhart JV, Kaufman SJ. In vitro and in vivo expression of alpha 7 integrin and desmin define the primary and secondary myogenic lineages. Dev Biol. 1993;156:209–229. doi: 10.1006/dbio.1993.1071. [DOI] [PubMed] [Google Scholar]
- Kaufman SJ, Foster RF. Replicating myoblasts express a muscle-specific phenotype. Proc Natl Acad Sci U S A. 1988;85:9606–9610. doi: 10.1073/pnas.85.24.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell IW, Ishibashi J, Le Grand F, Punch VG, Addicks GC, Greenblatt JF, Dilworth FJ, Rudnicki MA. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol. 2008;10:77–84. doi: 10.1038/ncb1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell IW, Parise G, Rudnicki MA. Muscle stem cells and regenerative myogenesis. Curr Top Dev Biol. 2005;71:113–130. doi: 10.1016/S0070-2153(05)71004-8. [DOI] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. Embo J. 2004;23:3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki N, Lim M, Yao CC, Tolar M, Kramer RH. alpha7 integrin expressing human fetal myogenic progenitors have stem cell-like properties and are capable of osteogenic differentiation. Exp Cell Res. 2006;312:4162–4180. doi: 10.1016/j.yexcr.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, Gussoni E, Kunkel LM, Huard J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- Perry RL, Rudnick MA. Molecular mechanisms regulating myogenic determination and differentiation. Front Biosci. 2000;5:D750–767. doi: 10.2741/perry. [DOI] [PubMed] [Google Scholar]
- Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem. 2003;278:12601–12604. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- Sartorelli V, Caretti G. Mechanisms underlying the transcriptional regulation of skeletal myogenesis. Curr Opin Genet Dev. 2005;15:528–535. doi: 10.1016/j.gde.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer BW, Czerny T, Bernasconi M, Genini M, Busslinger M. Molecular cloning and characterization of a human PAX-7 cDNA expressed in normal and neoplastic myocytes. Nucleic Acids Res. 1994;22:4574–4582. doi: 10.1093/nar/22.22.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizirianakis IS, Yao CC, Chen Y, Ziober BL, Tsiftsoglou AS, Kramer RH. Transfection of MCF-7 carcinoma cells with human integrin alpha7 cDNA promotes adhesion to laminin. Arch Biochem Biophys. 2001;385:108–116. doi: 10.1006/abbi.2000.2134. [DOI] [PubMed] [Google Scholar]
- Xiao J, Jethanandani P, Ziober BL, Kramer RH. Regulation of alpha7 integrin expression during muscle differentiation. J Biol Chem. 2003;278:49780–49788. doi: 10.1074/jbc.M308542200. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol. 1994;164:588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CC, Ziober BL, Squillace RM, Kramer RH. Alpha7 integrin mediates cell adhesion and migration on specific laminin isoforms. J Biol Chem. 1996;271:25598–25603. doi: 10.1074/jbc.271.41.25598. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Heslop L, Hudon V, Rosenblatt JD, Tajbakhsh S, Buckingham ME, Beauchamp JR, Partridge TA. Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp Cell Res. 2002;281:39–49. doi: 10.1006/excr.2002.5653. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, Partridge TA, Beauchamp JR. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- Zhou H, Kramer RH. Integrin engagement differentially modulates epithelial cell motility by RhoA/ROCK and PAK1. J Biol Chem. 2005;280:10624–10635. doi: 10.1074/jbc.M411900200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Heterogeneous expression of Pax7, MyoD and desmin by adult α7 integrin sorted human cultured cells. (A) Immunostaining for c-Met/desmin shows cells that are positive for both (arrow) and cells that express c-Met but not desmin (arrowhead). (B) Immunostaining for MyoD/desmin shows cells that are positive for both (arrow) and cells that express desmin but not MyoD (arrowhead). (C) Immunostaining for Pax7 and desmin on adult muscle cultures grown in DM for 7-10 days to induce myotube formation demonstrated that some cells in the differentiation-resistant population are Pax7+/desmin+ (arrow) while others are Pax7+/desmin− (arrowhead). Scale Bar = 20μM
Supplemental Figure 2. Homogeneous expression of Pax7, MyoD and desmin by cultured mouse myoblasts. Immunostaining of mouse primary myoblasts for (A) MyoD (B) Desmin (C) Pax7 show uniform expression by all cells for these proteins.