Abstract
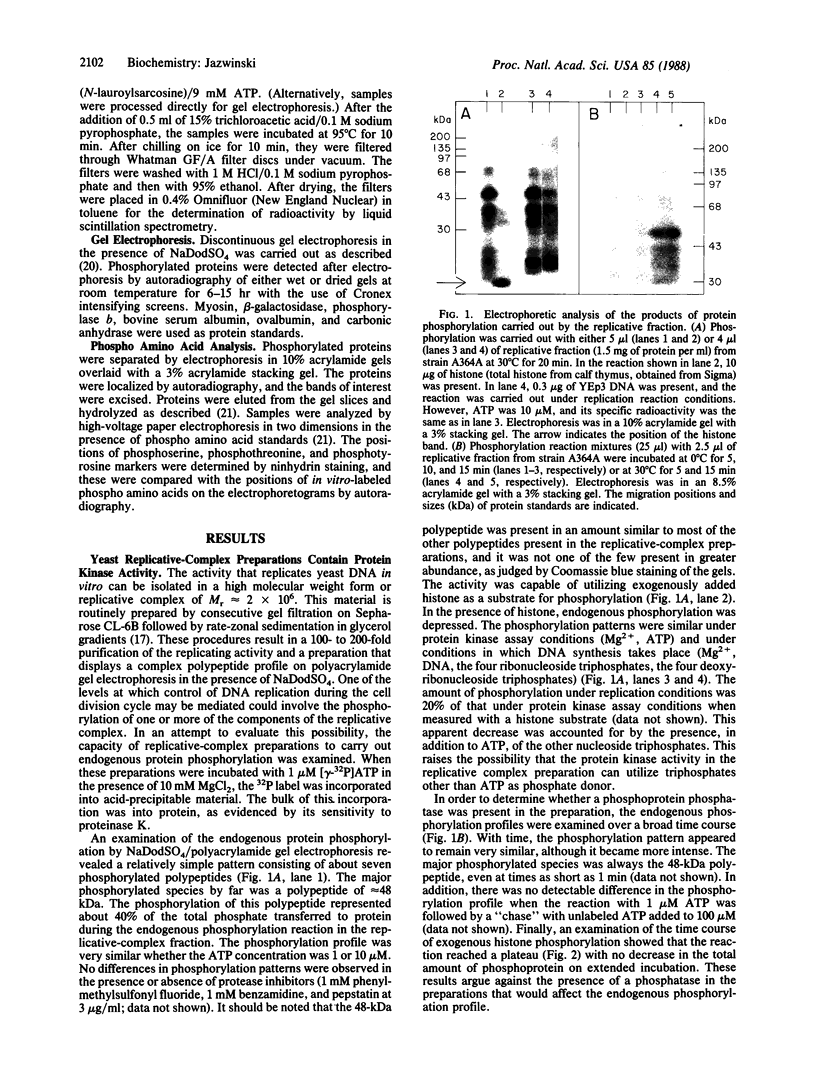
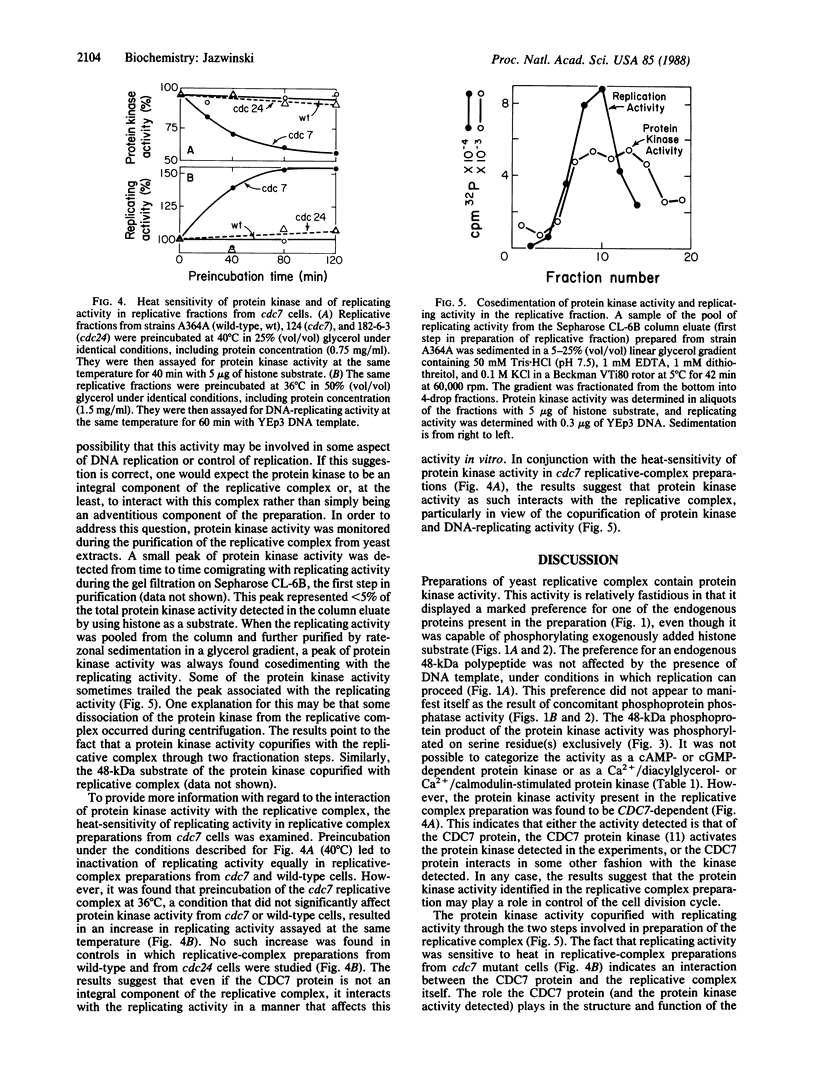
A protein kinase activity was identified in preparations of DNA-replicative complex from the budding yeast Saccharomyces cerevisiae. The activity phosphorylated only a few of the endogenous proteins in the replicative fraction, and it displayed a marked preference for a 48-kDa polypeptide. Despite this relative specificity, the protein kinase activity was capable of utilizing exogenously added histone as substrate. The 48-kDa polypeptide was phosphorylated on serine residue(s) exclusively by the endogenous activity in the replicative-complex preparation. The activity was not stimulated by cAMP, cGMP, Ca2+/phosphatidylserine/diacylglycerol, or Ca2+/calmodulin. It did not utilize Ca2+ or Zn2+ in the place of Mg2+, and Mn2+ was only 22% as effective in fulfilling the divalent-cation requirement. Most importantly, the protein kinase activity was heat-sensitive in replicative fractions from the cell division cycle 7 (cdc7) mutant, which arrests at or close to the G1/S boundary of the cell cycle at restrictive temperature. Thus, the activity is CDC7-dependent. An effect of heat treatment on replicating activity in the replicative fraction from cdc7 cells was also found. This result and the finding that the protein kinase activity copurified with replicating activity in the preparations suggest that the CDC7 gene product and the protein kinase activity, whether or not they are the same entity, interact with yeast replicative complex. All of these results raise the possibility that phosphorylation of components of the replication machinery may play a role in the control of initiation of DNA replication during the cell cycle. It is possible that the phosphorylation observed is part of a protein kinase cascade that regulates progress through the G1 phase of the cell cycle.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman P., Glover C. V., Osheroff N. Phosphorylation of DNA topoisomerase II by casein kinase II: modulation of eukaryotic topoisomerase II activity in vitro. Proc Natl Acad Sci U S A. 1985 May;82(10):3164–3168. doi: 10.1073/pnas.82.10.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Guerina N. G., Guo H. Y., Huang A. S. Host-dependent phosphorylation and kinase activity associated with vesicular stomatitis virus. J Biol Chem. 1982 Mar 25;257(6):3313–3319. [PubMed] [Google Scholar]
- Donaldson R. W., Gerner E. W. Phosphorylation of a high molecular weight DNA polymerase alpha. Proc Natl Acad Sci U S A. 1987 Feb;84(3):759–763. doi: 10.1073/pnas.84.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G., Brizuela L., Potashkin J., Beach D. Identification of p34 and p13, human homologs of the cell cycle regulators of fission yeast encoded by cdc2+ and suc1+. Cell. 1987 Jul 17;50(2):319–325. doi: 10.1016/0092-8674(87)90227-3. [DOI] [PubMed] [Google Scholar]
- Durban E., Goodenough M., Mills J., Busch H. Topoisomerase I phosphorylation in vitro and in rapidly growing Novikoff hepatoma cells. EMBO J. 1985 Nov;4(11):2921–2926. doi: 10.1002/j.1460-2075.1985.tb04024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Fong H. K., Hurley J. B., Hopkins R. S., Miake-Lye R., Johnson M. S., Doolittle R. F., Simon M. I. Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2162–2166. doi: 10.1073/pnas.83.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M., Edelman G. M. A DNA primase from yeast. Purification and partial characterization. J Biol Chem. 1985 Apr 25;260(8):4995–5002. [PubMed] [Google Scholar]
- Jazwinski S. M., Edelman G. M. Acitivity of yeast extracts in cell-free stimulation of DNA replication. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3933–3936. doi: 10.1073/pnas.73.11.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M., Edelman G. M. Evidence for participation of a multiprotein complex in yeast DNA replication in vitro. J Biol Chem. 1984 Jun 10;259(11):6852–6857. [PubMed] [Google Scholar]
- Jazwinski S. M., Edelman G. M. Protein complexes from active replicative fractions associate in vitro with the replication origins of yeast 2-micrometers DNA plasmid. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3428–3432. doi: 10.1073/pnas.79.11.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M. Evidence for the involvement of a single major species of replicative complex in DNA synthesis from two diverse nuclear replicons in yeast. Biochem Biophys Res Commun. 1987 Feb 13;142(3):1053–1058. doi: 10.1016/0006-291x(87)91521-x. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M., Niedzwiecka A., Edelman G. M. In vitro association of a replication complex with a yeast chromosomal replicator. J Biol Chem. 1983 Mar 10;258(5):2754–2757. [PubMed] [Google Scholar]
- Jazwinski S. M. Participation of ATP in the binding of a yeast replicative complex to DNA. Biochem J. 1987 Aug 15;246(1):213–219. doi: 10.1042/bj2460213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Powers S., Cameron S., Fasano O., Goldfarb M., Broach J., Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985 Jan;40(1):19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lucchini G., Francesconi S., Foiani M., Badaracco G., Plevani P. Yeast DNA polymerase--DNA primase complex; cloning of PRI 1, a single essential gene related to DNA primase activity. EMBO J. 1987 Mar;6(3):737–742. doi: 10.1002/j.1460-2075.1987.tb04815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Genetic analysis of the role of cAMP in yeast. Yeast. 1985 Sep;1(1):15–24. doi: 10.1002/yea.320010103. [DOI] [PubMed] [Google Scholar]
- Mohr I. J., Stillman B., Gluzman Y. Regulation of SV40 DNA replication by phosphorylation of T antigen. EMBO J. 1987 Jan;6(1):153–160. doi: 10.1002/j.1460-2075.1987.tb04733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M., Sclafani R. A., Fangman W. L., Rosamond J. Molecular characterization of cell cycle gene CDC7 from Saccharomyces cerevisiae. Mol Cell Biol. 1986 May;6(5):1590–1598. doi: 10.1128/mcb.6.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson T. A., Yochem J., Byers B., Nunn M. F., Duesberg P. H., Doolittle R. F., Reed S. I. A relationship between the yeast cell cycle genes CDC4 and CDC36 and the ets sequence of oncogenic virus E26. Nature. 1984 Jun 7;309(5968):556–558. doi: 10.1038/309556a0. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Hadwiger J. A., Lörincz A. T. Protein kinase activity associated with the product of the yeast cell division cycle gene CDC28. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4055–4059. doi: 10.1073/pnas.82.12.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun N., Wolf M., Besterman J., Hsieh T., Sander M., LeVine H., 3rd, Chang K. J., Cuatrecasas P. Protein kinase C phosphorylates topoisomerase II: topoisomerase activation and its possible role in phorbol ester-induced differentiation of HL-60 cells. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1603–1607. doi: 10.1073/pnas.83.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studzinski G. P., Brelvi Z. S., Feldman S. C., Watt R. A. Participation of c-myc protein in DNA synthesis of human cells. Science. 1986 Oct 24;234(4775):467–470. doi: 10.1126/science.3532322. [DOI] [PubMed] [Google Scholar]
- Tatchell K., Chaleff D. T., DeFeo-Jones D., Scolnick E. M. Requirement of either of a pair of ras-related genes of Saccharomyces cerevisiae for spore viability. Nature. 1984 Jun 7;309(5968):523–527. doi: 10.1038/309523a0. [DOI] [PubMed] [Google Scholar]





