Abstract
The Arabidopsis (Arabidopsis thaliana) CLAVATA2 (CLV2) gene encodes a leucine-rich repeat receptor-like protein (RLP) that is involved in controlling the stem cell population size in the shoot apical meristem. Our previous genome-wide functional analysis of 57 AtRLP genes revealed only a few phenotypes for mutant alleles, despite screening a wide range of growth and developmental stages and assaying sensitivity to various stress responses, including susceptibility toward pathogens. To gain further insight into the biological role of AtRLPs, in particular CLV2-related AtRLP genes, we tested their ability to complement the clv2 mutant phenotype. We found that out of four close CLV2 homologs tested, AtRLP2 and AtRLP12 could functionally complement the clv2 mutant when expressed under the control of the CLV2 promoter. This indicates that the functional specificity of these three genes is determined at the level of their transcriptional regulation. Single and double mutant combinations with impaired AtRLP2 and/or AtRLP12 did not show an aberrant phenotype, suggesting that other genes are redundant with these CLV2-like genes. To understand which protein domains are essential for CLV2 function and which parts are interchangeable between related CLV2-like proteins, we performed domain-deletion and domain-swap experiments. These experiments revealed that CLV2 remains functional without the island domain, whereas the C1 and C3 regions of the leucine-rich repeat domain are essential for functionality. Analysis of domain-swap constructs showed that the C3-G region of CLV2 can be replaced by that of AtRLP38, although it could not complement the clv2 mutant under control of the CLV2 promoter. This suggests that the C3-G region is conserved among related AtRLP members, whereas the C1 domain may determine the functional specificity of CLV2.
Receptor-like proteins (RLPs) are cell surface receptors that typically consist of an extracellular Leu-rich repeat (eLRR) domain, a transmembrane domain, and a short cytoplasmic tail that lacks an obvious intracellular signal transduction domain apart from the putative endocytosis motif found in some members (Jones and Jones, 1997; Shiu and Bleecker, 2001; Kruijt et al., 2005; Wang et al., 2008). The first identified RLP gene was tomato (Solanum lycopersicum) Cf-9, a disease resistance gene that mediates resistance against strains of the fungal leaf mold pathogen Cladosporium fulvum that carry the avirulence gene Avr9 (Jones et al., 1994). The RLP disease resistance gene family comprises the tomato Cf and Ve genes that provide resistance against C. fulvum and Verticillium species, respectively, the LeEIX genes that encode receptors for the ethylene-inducible xylanase produced by Trichoderma biocontrol fungi, apple (Malus domestica) HcrVf genes that confer resistance to the scab fungus Venturia inaequalis, and an Arabidopsis (Arabidopsis thaliana) RLP gene (AtRLP52) that provides resistance against the powdery mildew pathogen Erysiphe cichoracearum (Kawchuk et al., 2001; Belfanti et al., 2004; Ron and Avni, 2004; Ramonell et al., 2005; Malnoy et al., 2008; Fradin et al., 2009). Furthermore, we recently demonstrated that Arabidopsis AtRLP30, and possibly AtRLP18, mediates nonhost resistance toward Pseudomonas syringae pv phaseolicola (Wang et al., 2008).
In addition to defense against pathogens, AtRLP genes also play roles in plant development. The developmental AtRLP genes comprise Arabidopsis TOO MANY MOUTHS (TMM; AtRLP17), which regulates stomatal distribution by controlling meristemoid formation as well as initiation of stomatal precursor cells (Yang and Sack, 1995; Nadeau and Sack, 2002). However, probably the best studied developmental RLP gene is Arabidopsis CLAVATA2 (CLV2; AtRLP10), which, like its maize (Zea mays) ortholog FASCIATED EAR2 (FEA2), was found to be required for proper meristem development, stem cell specification, and organogenesis (Kayes and Clark, 1998; Jeong et al., 1999; Taguchi-Shiobara et al., 2001).
CLV2 is proposed to be part of a receptor complex containing the receptor-like kinase (RLK) CLV1 (Clark et al., 1997; Trotochaud et al., 1999). RLKs are cell surface receptors that differ from RLPs because they contain a cytoplasmic kinase domain (Shiu and Bleecker, 2001). It has been proposed that CLV1 and CLV2 undergo a physical interaction to form a heterodimer that acts as a receptor complex for the extracellular peptide ligand CLV3 (Clark et al., 1995; Trotochaud et al., 1999; Rojo et al., 2002; Ogawa et al., 2008). Upon CLV3 perception by the CLV1 ectodomain (Ogawa et al., 2008), the kinase domain of CLV1 is thought to activate a yet uncharacterized downstream signaling cascade to repress expression of the stem cell-promoting transcription factor WUSCHEL (Lenhard and Laux, 2003) and is thus required to maintain the stem cell population (Rojo et al., 2002; Diévart and Clark, 2004). Like CLV2, loss of function of CLV1 and CLV3 causes the progressive accumulation of undifferentiated stem cells, resulting in enlarged shoot apical meristems (SAMs) and increased floral organ numbers (Clark et al., 1993, 1995; Kayes and Clark, 1998). However, it has been shown that no additional organs develop in clv2 flowers under short-day conditions, whereas the SAM remains enlarged, suggesting that the regulation of the flower meristem by CLV2 is dependent on the physiological state of the plant (Kayes and Clark, 1998; Jeong and Clark, 2005). Alternatively, it is hypothesized that a yet unidentified RLP gene, specifically expressed under short days, is able to functionally compensate for the activity of CLV2. Furthermore, it was recently shown that CLV2 acts together with the receptor kinase CORYNE (CRN), in parallel with CLV1, to perceive CLV3 (Müller et al., 2008). CRN is identical to Suppressor of Overexpression of LLP1-2 (SOL2), and loss of function of SOL2 suppresses a short-root phenotype of transgenic plants constitutively overexpressing the CLV3/ESR19 (CLE19) gene (Casamitjana-Martínez et al., 2003; Miwa et al., 2008). Since the CRN protein lacks a distinct extracellular domain, it was proposed that CRN and CLV2 interact via their transmembrane domains to establish a functional receptor. Mutations in CRN cause stem cell proliferation, similar to clv1, clv2, and clv3 mutants. However, CRN also shares additional functions during plant development with CLV2, including floral organ and root development (Miwa et al., 2008; Müller et al., 2008).
Typically, the amino acid sequences of RLPs are divided into the conserved domains A through G, with a putative signal peptide (A), a Cys-rich region (B), the eLRR domain (C), which is composed of two LRR regions (C1 and C3) that are separated by a non-LRR island domain (C2), a Cys-rich spacer region (D), an acidic region (E), the transmembrane domain (F), and a short cytoplasmic tail (G; Jones and Jones, 1997). The eLRR domain, which is also found in the extracellular domain of RLKs, is proposed to play a versatile role in binding of ligands that can either be self (in development) or nonself (in pathogen resistance) molecules (Jones and Jones, 1997; Kobe and Kajava, 2001; Matsubayashi et al., 2002; Diévart and Clark, 2004; Torii, 2004). Especially for the tomato Cf resistance proteins, extensive functional analysis of subdomains has been carried out. Domain-swap experiments between the tomato resistance proteins Cf-4 and Cf-9 revealed that recognition specificity resides in a number of residues at the solvent-exposed β-sheet of several distant LRRs in the C1 domain as well as in the number of LRRs (van der Hoorn et al., 2001; Wulff et al., 2001). Furthermore, conserved Trp and Cys pairs in the N-terminal LRR-flanking domain that are exposed at the putative concave inner surface of the Cf-9 protein, where also recognition specificity resides, were found to be important for Cf-9 function. Finally, many of the 22 putative N-linked glycosylation sites, especially those in the putative α-helices of the LRR modules, were found to contribute to Cf-9 activity (van der Hoorn et al., 2005). Several domain-swap experiments in which Cf-2/Cf-5 and Cf-2/Cf-9 chimeras were generated confirmed that recognition specificity resides in the C1 LRR domain (Seear and Dixon, 2003; Rivas et al., 2004).
Functional analysis of extracellular receptor domains has similarly been carried out using RLKs. The minimal hormone-binding domain for brassinosteroid hormones by Arabidopsis BRASSINOSTEROID INSENSITIVE1 (BRI1) comprises the 70-amino acid island domain (C2) and the C-terminal flanking LRR22 (Kinoshita et al., 2005). Finally, with a targeted Ala-scanning mutagenesis approach it was demonstrated that flagellin perception in Arabidopsis is dependent on a limited number of residues across LRR9 to LRR15 in the C1 domain of the RLK FLAGELLIN-SENSITIVE2 (FLS2) that binds bacterial flagellin (Dunning et al., 2007).
We have recently identified 57 RLPs in the Arabidopsis genome (AtRLPs) and reported on a genome-wide functional analysis of this gene family (Wang et al., 2008). Despite extensive analyses, few functions could be assigned to individual AtRLP members. It was suggested that the lack of phenotypes of individual T-DNA insertion mutants of AtRLP genes was caused by functional redundancy among gene family members (Wang et al., 2008). Nevertheless, an approach in which the expression of specific sets of multiple AtRLP genes was targeted by RNA interference also failed to uncover new biological functions for AtRLP genes (Ellendorff et al., 2008). To specifically investigate the role of functional redundancy among AtRLP genes, we studied the functionally characterized CLV2 gene and its closest homologs, revealing that two AtRLPs, AtRLP2 and AtRLP12, can replace CLV2 function in a clv2 mutant. Information about which domains of CLV2, and of its related homologs, are responsible for functionality and specificity is largely lacking. Therefore, a functional characterization of the CLV2 protein was performed using deletion analyses and domain swaps between CLV2 and a related RLP, AtRLP38, which was not able to complement clv2 mutants. Our data indicate that the functional specialization among CLV2, AtRLP2, and AtRLP12 can largely be attributed to differences in expression patterns. We further showed that the island domain of CLV2 is dispensable for its function and that CLV2 C3-G can be replaced by that of AtRLP38.
RESULTS
Identification of the CLV2 Subfamily
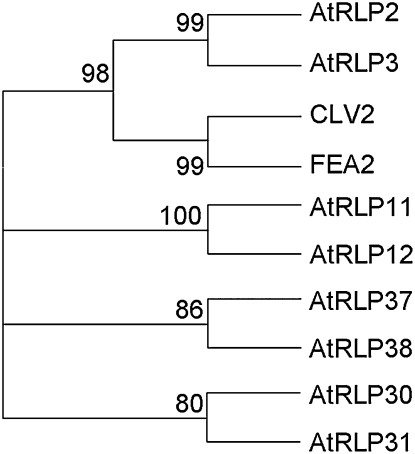
In order to identify the AtRLPs that are the most related to CLV2 (AtRLP10), the sequence similarity of the 56 remaining AtRLPs (Wang et al., 2008) with CLV2 was determined. Although the AtRLPs have a low overall sequence similarity (Fritz-Laylin et al., 2005; Wang et al., 2008), domains C3 and D are conserved. For all AtRLPs that are similar in length (700–880 amino acids) and LRR number (19–25 LRRs) to CLV2, we selected the C3-to-D region for sequence alignment and constructed a phylogenetic tree to identify CLV2-related AtRLPs (Fig. 1; Supplemental Fig. S1). In this way, eight CLV2-like RLPs, namely AtRLP2, AtRLP3, AtRLP11, AtRLP12, AtRLP30, AtRLP31, AtRLP37, and AtRLP38, were identified as the closest CLV2 homologs (Fig. 1). Four closely related protein pairs can be discriminated in the tree, namely AtRLP2 and AtRLP3, AtRLP11 and AtRLP12, AtRLP30 and AtRLP31, and AtRLP37 and AtRLP38, which, in all cases, are encoded by neighboring genes. It has previously been shown that of the 57 AtRLPs, 45 are predicted to contain a C2 island domain located between two eLRR blocks (C1 and C3; Wang et al., 2008). All eight CLV2-like AtRLPs carry an island domain that is variable in length and is followed by a C3 LRR domain of four LRRs (Supplemental Fig. S1). At the amino acid level, the eight CLV2-like RLPs exhibit a variable degree of conservation, ranging from 27% to 67% identity for the full-length proteins and 33% to 83% identity for the conserved part in the C3-to-D domain.
Figure 1.
Phylogenetic tree of putative members of the CLV2 subfamily and FEA2, the maize ortholog of CLV2. The tree was constructed for 100 bootstrap repetitions, and the numbers at the nodes indicate bootstrap support.
AtRLP2 and AtRLP3 are the closest related to the CLV2/FEA2 subclade (Fig. 1), leading to the hypothesis that especially those AtRLPs may have a biochemical function that is related to that of CLV2. One of the eight CLV2-like AtRLPs, AtRLP30, has recently been reported to play a role in nonhost resistance against P. syringae pv phaseolicola (Wang et al., 2008). No functions have been assigned to the other seven CLV2-like AtRLPs (Ellendorff et al., 2008; Wang et al., 2008).
AtRLP2 and AtRLP12 Functionally Replace CLV2 When Expressed under the Control of the CLV2 Promoter
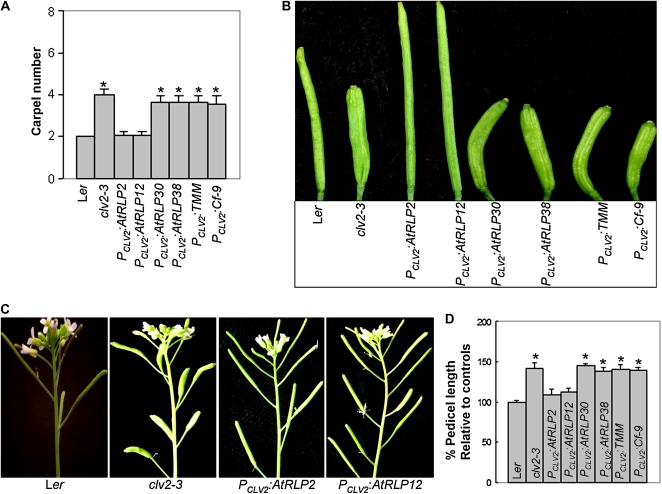
In order to test whether some of the eight CLV2-like AtRLP genes are functionally equivalent to CLV2, the ability of the CLV2-like AtRLPs to restore the phenotype of clv2 mutants was examined. To this end, four selected AtRLPs (AtRLP2, AtRLP12, AtRLP30, and AtRLP38, one member of each protein pair; Fig. 1; Supplemental Fig. S1) were expressed under the control of the CLV2 promoter (PCLV2). As a control, similar constructs were made for CLV2 and the functionally characterized TMM and tomato Cf-9 genes. All constructs were used to transform the clv2-3 mutant in the Landsberg erecta (Ler) genetic background (Kayes and Clark, 1998). A minimum of 20 T1 transformants for each construct were examined for complementation of the clv2-3 mutant phenotype by analysis of their carpel numbers. As expected, transformation of the construct containing wild-type CLV2 resulted in full complementation of clv2-3, as has been shown previously (Wang et al., 2008). Intriguingly, also AtRLP2 and AtRLP12 were able to fully rescue the clv2-3 mutant, with a mean carpel number that is comparable to the wild type (Table I; Fig. 2, A–C). In the case of PCLV2:AtRLP2, out of 30 independent transformants, 28 fully complemented clv2, with a mean carpel number 2.04 ± 0.20. In the case of AtRLP12, out of 25 PCLV2:AtRLP12 transgenic plants, 24 restored the clv2-3 mutant to the wild type (Table I; Fig. 2, A–C). However, transformants expressing AtRLP30, AtRLP38, Cf-9, and TMM driven by the CLV2 promoter showed no restoration of the carpel number phenotype (Table I; Fig. 2, A and B). In some cases, a few lines of transgenic plants expressing AtRLP30, AtRLP38, Cf-9, and TMM showed little degree of rescue by occasionally producing siliques with two or three carpels, but overall there was no statistical difference compared with clv2-3.
Table I.
Carpel numbers of various mutants and transgenic lines generated in this study
| Genotype | Construct | Daylength | Carpel No. (±se) |
|---|---|---|---|
| clv2-3 | PCLV2:CLV2 | Long days | 2.05 ± 0.09 |
| clv2-3 | PCLV2:AtRLP2 | Long days | 2.04 ± 0.20 |
| clv2-3 | PCLV2:AtRLP12 | Long days | 2.06 ± 0.20 |
| clv2-3 | PCLV2:AtRLP30 | Long days | 3.60 ± 0.36 |
| clv2-3 | PCLV2:AtRLP38 | Long days | 3.62 ± 0.32 |
| clv2-3 | PCLV2:TMM | Long days | 3.61 ± 0.32 |
| clv2-3 | PCLV2:Cf-9 | Long days | 3.59 ± 0.35 |
| clv2-3 | Deletion construct Δ1 | Long days | 2.06 ± 0.28 |
| clv2-3 | Deletion construct Δ2 | Long days | 3.86 ± 0.46 |
| clv2-3 | Deletion construct Δ3 | Long days | 3.75 ± 0.52 |
| clv2-3 | Deletion construct Δ4 | Long days | 4.03 ± 0.41 |
| clv2-3 | PCLV2:CLV2RLP38-I | Long days | 3.86 ± 0.46 |
| clv2-3 | PCLV2:CLV2RLP38-II | Long days | 2.10 ± 0.21 |
| clv2-3 | PCLV2:CLV2RLP38-III | Long days | 3.85 ± 0.40 |
| clv2-3 | PCLV2:CLV2RLP38-IV | Long days | 3.92 ± 0.32 |
| clv2-3 | PCLV2:CLV2RLP38-V | Long days | 3.95 ± 0.22 |
| clv2-3 | – | Long days | 3.98 ± 0.28 |
| clv2-7 | – | Long days | 2.65 ± 0.65 |
| atrlp2 clv2-7 | – | Long days | 2.66 ± 0.60 |
| atrlp2 atrlp12 | – | Long days | 2.00 ± 0.00 |
| atrlp12 clv2-7 | – | Long days | 2.52 ± 0.69 |
| clv2-3 | – | Short days | 2.28 ± 0.21 |
| clv2-7 | – | Short days | 2.05 ± 0.15 |
| atrlp2 clv2-7 | – | Short days | 2.02 ± 0.10 |
| atrlp2 atrlp12 | – | Short days | 2.00 ± 0.00 |
|
atrlp12 clv2-7 |
– |
Short days |
2.02 ± 0.16 |
Figure 2.
AtRLP2 and AtRLP12 complement the clv2-3 mutant. A, Average number of carpels per flower for multiple independent transgenic lines of PCLV2:AtRLP2, PCLV2:AtRLP12, PCLV2:AtRLP30, PCLV2:AtRLP38, PCLV2:TMM, PCLV2:Cf-9, wild-type Ler, and the clv2-3 mutant. For each construct, a minimum of 20 T1 transgenic plants with 30 siliques per plant were counted for each mean number. B, Representative siliques of wild-type Ler, clv2-3, PCLV2:AtRLP2, PCLV2:AtRLP12, PCLV2:AtRLP30, PCLV2:AtRLP38, PCLV2:TMM, and PCLV2:Cf-9 plants. C, Representative inflorescences of 3-week-old Ler, clv2-3, PCLV2:AtRLP2, and PCLV2:AtRLP12. D, Mean pedicel length of multiple independent transgenic lines of PCLV2:AtRLP2, PCLV2:AtRLP12, PCLV2:AtRLP30, PCLV2:AtRLP38, PCLV2:TMM, PCLV2:Cf-9, and the clv2-3 mutant relative to the wild type. At least 50 pedicels for each genotype were measured. Asterisks in A and D indicate significant differences (P < 0.01) compared with the respective wild types. [See online article for color version of this figure.]
The clv2 mutation also causes pedicel elongation (Kayes and Clark, 1998). We also assessed the pedicel length of transgenic plants carrying the different constructs. The pedicel length of clv2 mutants is about 40% longer than that of wild-type plants, while the pedicel length of transgenic plants expressing PCLV2:AtRLP2 and PCLV2:AtRLP12 is very close to the pedicel length of wild-type Arabidopsis plants (Fig. 2D). However, the pedicel length of transgenic plants carrying AtRLP30, AtRLP38, Cf-9, and TMM is comparable to that of clv2 (Fig. 2D), revealing that those genes cannot substitute the CLV2 function in pedicel development.
We subsequently overexpressed AtRLP2 and AtRLP12 under the control of the cauliflower mosaic virus 35S promoter in wild-type Arabidopsis. The overall growth and appearance of AtRLP2- and AtRLP12-overexpressing plants were indistinguishable from wild-type plants grown under normal growth conditions (data not shown).
AtRLP2 and AtRLP12 Show a Distinct Expression Level with CLV2 in the SAM but Overlapping Expression Patterns in Other Organs
Although AtRLP2 and AtRLP12 were able to rescue the phenotype of the clv2-3 mutant when expressed under the control of the CLV2 promoter, atrlp2 and atrlp12 mutants do not show enlarged meristems or multicarpel phenotypes themselves (Wang et al., 2008). Therefore, we examined the expression patterns of CLV2-like genes in the SAM by querying a newly available expression map of SAM (Supplemental Fig. S2; Yadav et al., 2009). In general, very low expression of AtRLP2, AtRLP3, AtRLP11, AtRLP12, AtRLP30, and AtRLP31 was detected in the shoot apex (Supplemental Fig. S2A; Yadav et al., 2009), while CLV2 was found to be relatively highly expressed (Supplemental Fig. S2A), despite the fact that expression is much lower than that of CLV1 (Yadav et al., 2009). Indeed, the result is also confirmed by the analysis of public microarray data from Genevestigator (Supplemental Fig. S2A; Zimmermann et al., 2004). Altogether, the distinct expression level in the SAM of CLV2 and other CLV2-like members suggested that the subfunctionalization of these CLV2-like genes has largely occurred from their promoter specificities and the resulting expression domain.
To gain a more comprehensive view of CLV2-like gene expression, a search of the Web-based microarray database Genevestigator was conducted (Zimmermann et al., 2004; Supplemental Fig. S2B). AtRLP2 and AtRLP3 showed the highest similarity in their profiles both in expression pattern and in expression level (Supplemental Fig. S2), leading to the hypothesis that these two paralogous genes possess conserved functions after duplication. AtRLP11 and AtRLP12 also share a similar overall expression profile, while AtRLP11 shows a remarkably lower expression level than AtRLP12 (Supplemental Fig. S2B). CLV2 is expressed at a constitutive level in many tissues examined (Jeong et al., 1999) and is also confirmed by microarray analysis (Supplemental Fig. S2B). Furthermore, CLV2 expression also correlated well with several other CLV2-like genes in some organs. CLV2 and AtRLP12 both exhibit a high expression levels in seedlings, roots, and later roots (Supplemental Fig. S2B). AtRLP3, AtRLP31, and CLV2 display very similar expression profiles in seedlings and leaves (Supplemental Fig. S2B). Given that genes functioning in similar processes often display similar expression patterns, we expected that CLV2 may have overlapping functions with other members in those organs. Interestingly, a survey of the SAM expression map revealed that several CLV2-like genes exhibit expression in the shoot apex (Supplemental Fig. S3), while their functions in the regulation of SAM remain unclear.
Characterization of the atrlp2, atrlp12, and clv2 Mutant Combinations
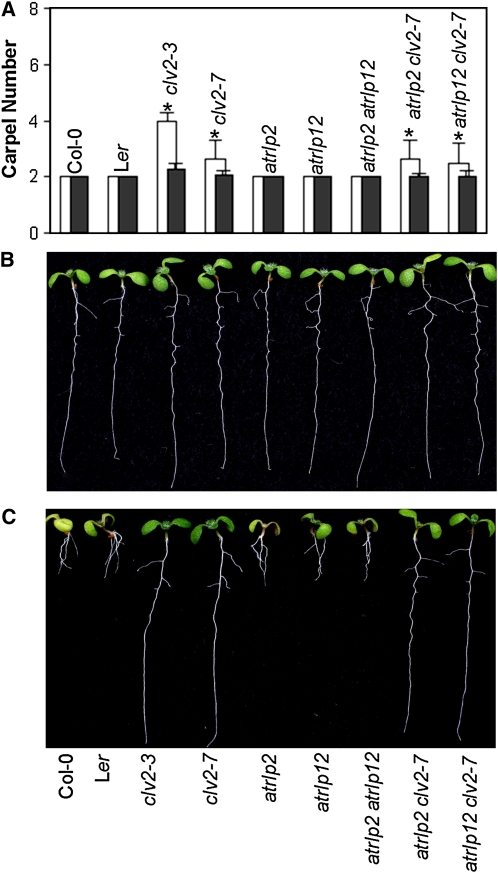
To reveal possible overlapping functions among AtRLP2, AtRLP12, and CLV2 during plant development, we generated combinations of double mutations for these genes. In a recent study, we characterized a new clv2 allele in the Columbia (Col-0) ecotype, atrlp10, which exhibited similar phenotypes as reported for previously characterized clv2 alleles that are in the Ler ecotype (Kayes and Clark, 1998; Jeong et al. 1999; Wang et al., 2008). In accordance with previous designation (Kayes and Clark, 1998; Müller et al., 2008), we renamed atrlp10 as clv2-7. To avoid background effects, we crossed the atrlp2 and atrlp12 mutants with the clv2-7 mutant (all in the Col-0 background) to generate the double mutants atrlp2 clv2-7, atrlp12 clv2-7, and atrlp2 atrlp12. The double mutants were further phenotypically characterized with respect to their overall growth and development. In none of the combinations did the double mutants exhibit any additional phenotype. Specifically, the carpel number as well as the response of the double mutants to treatment with a synthetic CLV3p peptide that corresponds to the conserved CLE motif that is present in CLV3-like peptide ligands (Fiers et al., 2005) were tested. Similar to wild-type plants and single mutant lines, atrlp2 atrlp12 double mutants did not show altered phenotypes with respect to either carpel number or CLE peptide treatment (Table I; Fig. 3). The carpel numbers of the double mutants that contained clv2-7 (atrlp2 clv2-7 and atrlp12 clv2-7) were similar to those of clv2-7 (Table I; Fig. 3A). Furthermore, atrlp2 clv2-7 and atrlp12 clv2-7 double mutants failed to respond to treatment with the CLE peptides, resulting in normal long roots (Fig. 3, B and C), as is also the case for clv2-7 single mutants. In an attempt to unravel the function of AtRLP2 and AtRLP3, we made an RNA interference construct to target the expression of AtRLP2 and AtRLP3 simultaneously (Ellendorff et al., 2008). However, no developmental anomalies were observed for the RNA interference lines (data not shown).
Figure 3.
Phenotypic analysis of double mutants. A, Mean number of carpels per flower (±se) from plants grown under long-day conditions (white bars) and short-day conditions (black bars) of the double mutants atrlp2 atrlp12, atrlp2 clv2-7, and atrlp12 clv2-7 with Col-0, Ler, clv2-3, and clv2-7. At least 100 siliques for each genotype were counted for the mean numbers. Asterisks indicate significant differences (P < 0.01) compared with the respective wild types. B and C, CLE peptide sensitivity assay, in which CLV3p peptide was added at a concentration of 10 μm. Representative 8-d-old seedlings grown in the absence (B) and in the presence (C) of CLV3p peptide are shown. [See online article for color version of this figure.]
It has been proposed that a yet unidentified RLP protein is able to functionally compensate the activity of CLV2 based on the fact that the floral meristem defects of the clv2 mutant are suppressed under short-day conditions (Kayes and Clark, 1998; Jeong and Clark, 2005). Therefore, we tested whether the atrlp2 clv2-7 and atrlp12 clv2-7 double mutants developed additional floral organs under short-day conditions. For all mutants, the carpel number decreased to values similar to those of wild-type plants (Table I; Fig. 3A).
Deletion Analysis of the CLV2 Gene
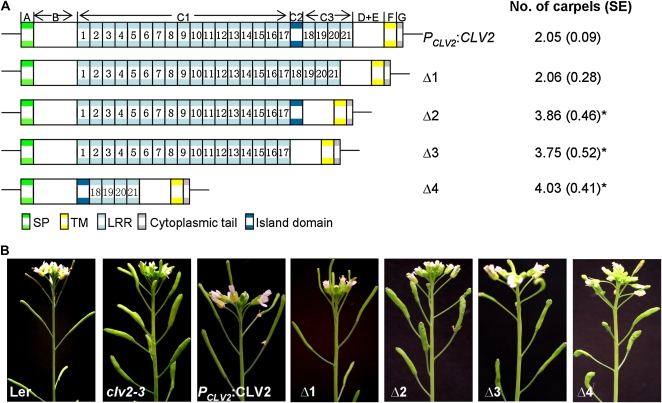
The structure of RLP proteins is composed of distinct domains A to G (Jones and Jones, 1997; Fig. 4A; Supplemental Fig. S1). To test the contributions of various domains to CLV2 function, four deletion constructs were designed in which different domains of CLV2 were removed (Fig. 4A; constructs Δ1–Δ4) based on the CLV2 protein organization. In the Δ1 construct, the C2 domain (the island domain) was removed, while in the Δ2 construct, LRR18 to LRR21, which compose the C3 domain, was removed. In the Δ3 construct, both the C2 and C3 domains were removed, while in the Δ4 construct, the C1 LRR domain (LRR1–LRR17) was removed (Fig. 4A). All mutant CLV2 genes, driven by the CLV2 promoter, were transformed to the clv2-3 mutant to test their functionality. Reverse transcription-PCR analysis of multiple transgenic plants demonstrated the expression of the corresponding transgenes (Supplemental Fig. S4). Subsequently, a minimum of 25 transformants for each construct were examined for complementation of the clv2-3 mutant phenotype by analysis of the carpel numbers. As expected, PCLV2:CLV2 resulted in a complete restoration of the clv2-3 mutant to a wild-type carpel number (Table I; Fig. 4). For deletion construct Δ1, 21 out of 22 T1 independent transgenic lines fully complemented the clv2-3 mutant, exhibiting a mean carpel number of 2.06 ± 0.28 (Table I; Fig. 4). In contrast, deletion constructs Δ2, Δ3, and Δ4 could not complement the clv2-3 mutant (Table I; Fig. 4), albeit some siliques of a few transgenic lines formed two to three carpels, thus resulting in a slightly decreased carpel number.
Figure 4.
CLV2 deletion analysis. A, Schematic representation of the PCLV2:CLV2 and four deletion constructs (Δ1–Δ4) that were generated by removing the genomic sequence of the C2 island domain (Δ1), the genomic sequence of LRR18 to LRR21 that compose the C3 domain (Δ2), the genomic sequence of both the C2 and C3 domains (Δ3), or the genomic sequence of LRR1 to LRR17 that compose the C1 domain (Δ4). Lines indicate noncoding sequence, and boxes indicate coding sequence. The mean numbers of carpels per flower (±se) of transgenic clv2-3 plants expressing the different constructs are indicated on the right. For each construct, a minimum of 20 T1 transgenic plants with 30 siliques per plant were counted for the mean numbers. Asterisks indicate significant differences (P < 0.01) compared with the respective wild types. B, Inflorescences of wild-type (Ler), clv2-3, and transgenic clv2-3 plants expressing the various constructs shown in A.
The C3-G Region of CLV2 Can Be Replaced by That of AtRLP38
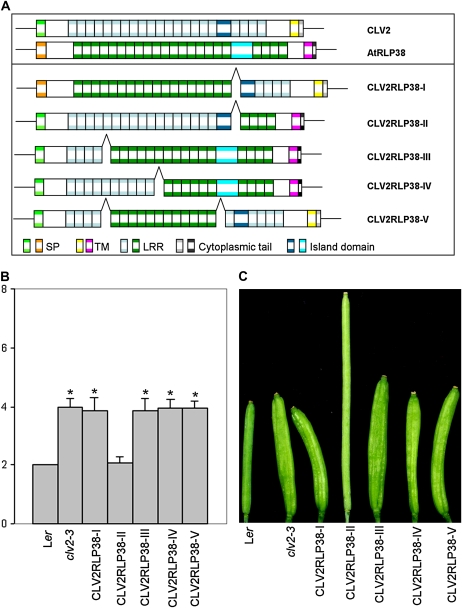
AtRLP38 encodes one of the eight CLV2-like AtRLPs that share 42% overall sequence similarity and 28% identity with CLV2 (Wang et al., 2008; Fig. 1; Supplemental Figs. S1, S5, and S6). While AtRLP38 contains 22 LRRs, CLV2 contains 21 LRRs (Fig. 1; Supplemental Figs. S1 and S6). As has been noted previously for other AtRLPs, sequence alignment of CLV2 and AtRLP38 showed that the similarity is highest in the C3 LRR domain and the C-terminal region (Wang et al., 2008; Supplemental Fig. S5), and most variation is found in the N-terminal part of the C1 LRR domain. AtRLP38 is not able to complement the clv2-3 mutant when expressed under control of the CLV2 promoter, suggesting that both AtRLPs have distinct functions (Fig. 2, A, B, and D). To further determine the requirement for individual CLV2 domains in regulating meristem development, we generated several chimeras between CLV2 and AtRLP38 (Fig. 5A), which were expressed under the control of the CLV2 promoter in clv2-3. Of these, only PCLV2:CLV2RLP38-II could complement the clv2-3 mutant phenotype, as shown by restoration of the carpel number to the wild-type level (Table I; Fig. 5, B and C). Out of 28 T1 independent transgenic lines, six lines showed complete complementation, as shown by a mean carpel number of 2.10 ± 0.21 (Table I; Fig. 5, B and C). In this chimera, domains A to C2, including the large LRR region of the C1 domain and the island domain, were derived from CLV2, while the C3 to G domains, including the cytoplasmic tail, were derived from AtRLP38 (Fig. 5A). Despite swapping the N-terminal domain of the CLV2 protein with the N-terminal domain of AtRLP38, the protein retained CLV2 biochemical activity (Fig. 5, B and C). No complementation of the clv2-3 phenotype was observed for the other constructs (PCLV2:CLV2RLP38-I, -III, -IV, and -V) in which the C1 domain of CLV2 was partially or completely exchanged with that of AtRLP38 (Table I; Fig. 5), suggesting that the C1 domain is critical for CLV2 function. Occasionally, in a very few transgenic plants expressing the PCLV2:CLV2RLP38-I, PCLV2:CLV2RLP38-III, PCLV2:CLV2RLP38-IV, and PCLV2:CLV2RLP38-V chimeras, some siliques of the plants developed two or three carpels, suggesting that these chimeric constructs could incidentally complement the clv2-3 mutation. None of the chimeric receptors showed any dominant-negative effects.
Figure 5.
Analyses of domain swaps between CLV2 and AtRLP38. A, Schematic representation of the diverse chimeric proteins CLV2RLP38-I, CLV2RLP38-II, CLV2RLP38-III, CLV2RLP38-IV, and CLV2RLP38-V. The structures of various constructs are shown by different colors representing the distinct domains of the proteins. The junction between CLV2 and RLP38 in each chimeric construct is indicated by an open triangle. All constructs are driven by the CLV2 promoter. B and C, Complementation results for expression of the CLV2-AtRLP38 chimeric receptors (CLV2RLP38) in clv2-3. B, The mean number of carpels per flower (±se) of multiple independent transgenic lines for clv2-3 transformed with PCLV2:CLV2RLP38-I to -V. The mean number of carpels for wild-type Ler and the clv2-3 mutant are shown as controls for comparison. For each construct, a minimum of 20 T1 transgenic plants with 30 siliques per plant were counted for the mean numbers, while in the case of PCLV2:CLV2RLP38-II, only the complete rescued transgenic lines were analyzed for the mean number. Asterisks indicate significant differences (P < 0.01) compared with the respective wild types. C, Comparisons of representative siliques from Ler, clv2-3, PCLV2:CLV2RLP38-I, PCLV2:CLV2RLP38-II, PCLV2:CLV2RLP38-III, PCLV2:CLV2RLP38-IV, and PCLV2:CLV2RLP38-V.
DISCUSSION
An initial genome-wide functional analysis of AtRLP gene T-DNA insertion mutants revealed only a few phenotypes, despite the screening of a wide range of conditions (Wang et al., 2008). This observation suggests a high degree of functional redundancy among the AtRLP family members. In this study, we first focused on CLV2 and its closest homologs. A subfamily consisting of CLV2 and the eight most conserved CLV2-like AtRLPs was determined, and the functional conservation among the CLV2-like genes was tested by their ability to complement the clv2-3 mutant. These experiments showed that, when expressed under control of the CLV2 promoter, AtRLP2 and AtRLP12 were able to complement the developmental phenotypes of the clv2-3 mutant, while the other tested CLV2-like AtRLP members were not. Our results suggested that the subfunctionalization of some CLV2 subfamily members, at least among CLV2, AtRLP2, and AtRLP12, was primarily determined by the evolutionary divergence of their promoter specificities and expression patterns rather than by their protein-coding regions. A functional diversity among closely related genes is, at least in part, due to diversification in gene expression.
None of the double mutant combinations showed additive phenotypic effects when compared with the clv2-7 mutant allele. The observation that AtRLP3 and AtRLP11 are duplicated genes of AtRLP2 and AtRLP12, respectively, suggests a similar function for these paralogues. In addition, the fact that AtRLP2/AtRLP3 and AtRLP11/AtRLP12 exhibit very similar overall expression profiles reinforces the hypothesis that they possess a conserved function. Therefore, the lack of additional phenotypes in the double mutants might be due to remaining activities of AtRLP3 and AtRLP11. Consequently, different higher order mutant combinations, for instance, AtRLP2/AtRLP3 and AtRLP11/AtRLP12 mutants in combination with the clv2 mutant, are needed to unravel the function of these CLV2-like genes.
We showed that AtRLP30 and AtRLP38 were unable to substitute the function of CLV2 when expressed under control of the CLV2 promoter, suggesting that they may have evolved toward distinct biological functions. Indeed, AtRLP30 has been demonstrated to be involved in nonhost resistance toward the bacterial pathogen P. syringae pv phaseolicola (Wang et al., 2008). This suggests that the function of each gene in a gene family does not have to be correlated to the bioinformatic and phylogenetic inference, despite the observation that CLV2 constitutes a subfamily with AtRLP30 and AtRLP38. Taken together, functional diversity as well as redundancy exists in the CLV2 subfamily.
The process of subfunctionalization, where the coding regions from closely related family members are still conserved while the cis-regulatory sequences have diverged, is a common phenomenon and has been documented for many gene families (Mazet and Shimeld, 2002; Prince and Pickett, 2002). CLV1 and the related Barely Any Meristem (BAM1–BAM3) proteins have similar biochemical functions but play opposite roles within the meristem, where BAMs act to promote and CLV1 to restrict the meristem size. The BAMs are broadly expressed in many Arabidopsis organs, which is consistent with their multiple developmental roles (DeYoung et al., 2006), while CLV1 expression is restricted to the shoot apex (Clark et al., 1997). CLV1 can fully replace BAM1 and BAM2 in developing organs, while BAM1 and BAM2 can partially replace CLV1 function within the meristem when expressed under the ERECTA (ER) promoter. These results indicate that the distinct functions for these genes are caused by differences in their transcription patterns (DeYoung et al., 2006). Similarly, two ER paralogues, ERL1 and ERL2, that display overlapping and distinct expression patterns with the ER gene, are capable of substituting the ER when expressed under the control of the ER promoter (Shpak et al., 2004). While the er null allele exhibits compact inflorescences with short lateral organs and internodes (Torii et al., 1996), erl1 and erl2 single mutants display no detectable phenotype and each of them significantly enhances the er phenotype (Shpak et al., 2004). Loss of the entire ER family conferred many severe defects (Torii et al., 1996; Shpak et al., 2004, 2005; Pillitteri et al., 2007).
To understand which protein domains are essential for CLV2 function and responsible for its functional specificity, we employed a deletion strategy and exchanged domains between different AtRLP proteins. First, we determined which domains are essential for the function of CLV2. We generated four deletion constructs, mainly focusing on the C domain, which demonstrated that the CLV2 island domain (C2) is dispensable for its function. In addition, the results indicate that the C1 and C3 regions are required for proper CLV2 functioning. The variable C2 region, which splits the variable C1 LRRs and the conserved C3 LRRs, has been suggested to be a flexible hinge-like region forming a loop between the two LRR blocks for folding into the regular LRR structure (Jones and Jones, 1997). A similar island region is also found in particular RLPs and RLKs, for instance Cfs, FEA2, and BRI1 (Jones et al., 1994; Li and Chory, 1997; Thomas et al., 1997; Taguchi-Shiobara et al., 2001), although the island domain does not share any primary sequence similarity between the family members (Torii, 2004). In contrast to our results with CLV2, previous studies have highlighted the importance of the island domain of BRI1 in brassinolide perception by RLK BRI1 (Li and Chory, 1997; Friedrichsen et al., 2000; He et al., 2000). Furthermore, the island domain as well as a single LRR motif (LRR22) has been shown to bind directly to brassinolide (Kinoshita et al., 2005), demonstrating the importance of this domain for brassinolide perception. However, the C2 domains of the tomato resistance proteins Cf-4 and Cf-9 are identical, although both proteins recognize different C. fulvum avirulence molecules, implying that the C2 domain of these proteins is unlikely to be a determinant for ligand recognition. Consistent with our results, the C2 domains of the functional orthologs FEA2 and CLV2 are poorly conserved (Jeong et al., 1999; Taguchi-Shiobara et al., 2001), sharing only 15% identity, indicating that this region is not involved in perception of a common ligand. Although in the case of BRI1 the functionality of the C2 domain has been proven, the fact that many functionally characterized RLPs and RLKs lack an island domain, such as Xa21 (Song et al., 1995), ER and ERL1-2 (Torii et al., 1996; Shpak et al., 2004, 2005), CLV1 (Clark et al., 1997), FLS2 (Gómez-Gómez and Boller, 2000), HAESA (Jinn et al., 2000), TMM (Nadeau and Sack, 2002), and BAM1 to BAM3 (DeYoung et al., 2006), argues against an important role for this region. Moreover, of the 57 RLPs found in Arabidopsis, 12 lack a C2 island domain (Wang et al., 2008).
Chimeric constructs were designed between CLV2 and AtRLP38 to unravel which protein domains determine the functional specificity of CLV2. Complementation analyses revealed that the C3-G domain of CLV2 could be replaced by that of AtRLP38. The C3-G region is highly conserved among all RLPs (Fritz-Laylin et al., 2005; Wang et al., 2008) and many functionally characterized RLKs (Jones et al., 1994; Song et al., 1995; Li and Chory, 1997; Gómez-Gómez and Boller, 2000), indicating that this region is required for a generic conserved function either in complex formation or in proper structural folding. In contrast, the complementation tests with the chimeric constructs CLV2RLP38-I, -III, -IV, and -V, in which we replaced a part of or the entire C1 domain of CLV2 by those of AtRLP38, showed that the AtRLP38 C1 region could not substitute the function of the CLV2 C1 domain. The replacement of six C1 LRRs in the CLV2LP38-IV construct already inactivated the function of CLV2. It has been reported that paired Cys residues, located in the C terminus, are probably important residues for RLP and RLK structure and thus functioning (Jeong et al., 1999; Kolade et al., 2006). Although CLV2RLP38-I, -III, -IV, and -V still have these residues, the CLV2 function was lost, indicating that other residues are also important for the function of CLV2. In previous studies, the C1 region has been demonstrated to provide the recognition specificity of Cfs (van der Hoorn et al., 2001, 2005; Wulff et al., 2001; Seear and Dixon, 2003; Rivas et al., 2004). Recently, site-directed mutagenesis of FLS2 also revealed that flagellin perception relies on limited residues in the LRR9 to LRR15 region of FLS2 (Dunning et al., 2007). Furthermore, the C1 domain is highly variable, particularly in the number of LRRs (Shiu and Bleecker, 2001; Fritz-Laylin et al., 2005; Wang et al., 2008). These findings highlight the potential role of the N-terminal LRRs of the RLPs and RLKs in the determination of functional specificity. Furthermore, polymorphisms of the CLV2 gene in different ecotypes suggest that LRR1 to LRR4 may functionally not be as important as the remaining parts of the protein (Jeong et al., 1999). This observation, as well as our domain-deletion and domain-swap results, strongly suggests that LRR5 to LRR17 of CLV2 play a critical role either in the ligand-binding specificity or in dimerization with partner proteins that are required for the CLV signaling pathway. Taken together, our studies provide valuable information on the functions of CLV2 domains and how these domains are conserved among related AtRLP family members.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The Arabidopsis (Arabidopsis thaliana) ecotypes Col-0 and Ler were used as wild types. The atrlp10 (clv2-7), atrlp2, atrlp12, and clv2-3 mutants were described previously (Kayes and Clark, 1998; Wang et al., 2008). The genotypes of the double mutants were determined by PCR using gene-specific primer pairs and a combination of T-DNA and gene-specific primers as described previously (Wang et al., 2008). Seeds were gas sterilized for 1 h by mixing 100 mL of bleach (containing 4% NaClO) and 3 mL of HCl. Subsequently, the seeds were germinated on half-strength Murashige and Skoog medium containing 1% Suc, 0.5 g/L MES, and 0.8% (w/v) agar at pH 5.8. Plants were grown on soil at 22°C either under short-day (8-h-day/16-h-night) or long-day (16-h-day/8-h-night) conditions.
Complementation of the clv2 Mutant by CLV2 Homologs
Full-length CLV2 (PCLV2:CLV2) including the native promoter and terminator was amplified using the primers GD1 and GD2 including the Gateway cloning sites. PCR was performed using Col-0 genomic DNA as template. The CLV2 promoter and AtRLP gene fragments for different constructs were generated using GD1/GD3 and GD4/GD5 for PCLV2:AtRLP2, GD1/GD6 and GD7/GD8 for PCLV2:AtRLP12, GD1/GD9 and GD10/GD11 for PCLV2:AtRLP30, GD1/GD12 and GD13/GD14 for PCLV2:AtRLP38, GD1/GD15 and GD16/GD17 for PCLV2:TMM, and GD1/GD18 and GD19/GD20 for PCLV2:Cf-9 (Supplemental Table S1). The PCLV2 and PCR fragments were combined using overlap extension PCR, after which the complete constructs were generated via PCR using primers GD1 and GD5 for PCLV2:AtRLP2, GD1 and GD8 for PCLV2:AtRLP12, GD1 and GD11 for PCLV2:AtRLP30, GD1 and GD14 for PCLV2:AtRLP38, GD1 and GD17 for PCLV2:TMM, and GD1and GD20 for PCLV2:Cf-9 (Supplemental Table S1). The PCR fragments were recombined into pDONR207 (Invitrogen) by a BP reaction, and subsequently all fragments were cloned into the binary vector with an LR reaction using the plasmids from the BP reaction mixed with the pKGW vector (Karimi et al., 2002) following the supplier's protocol. The plasmids were introduced into Agrobacterium tumefaciens strain C58pmp90 by electroporation and transformed into the clv2-3 mutant plants by floral dip transformation (Clough and Bent, 1998).
Generation of Deletion and Chimeric Constructs and Transformation
As for the deletion constructs, the two fragments of the deletion constructs (Δ1–Δ4) were generated using GD1/GD21 and GD2/GD22 for construct Δ1, GD1/GD23 and GD2/GD24 for construct Δ2, GD1/GD25 and GD2/GD26 for construct Δ3, and GD1/GD27 and GD2/GD28 for construct Δ4 (Supplemental Table S1). The two fragments were combined with overlap extension PCR, after which the four deletion constructs were generated via PCR using primers GD1 and GD2 (Supplemental Table S1). For the chimeric constructs between CLV2 and AtRLP38, the fragments for each construct were amplified using GD1/GD29 and GD2/GD30 for PCLV2:CLV2RLP38-I, GD1/GD31 and GD32/GD33 for PCLV2:CLV2RLP38-II, GD1/GD34 and GD33/GD35 for PCLV2:CLV2RLP38-III, GD1/GD36 and GD33/GD37 for PCLV2:CLV2RLP38-IV, and GD1/GD38 and GD2/GD39 for PCLV2:CLV2RLP38-V (Supplemental Table S1). Subsequently, the two fragments were combined with overlap extension PCR by primers GD1 and GD2 for PCLV2:CLV2RLP38-I and PCLV2:CLV2RLP38-V and primers GD1 and GD33 for PCLV2:CLV2RLP38-II, PCLV2:CLV2RLP38-III, and PCLV2:CLV2RLP38-IV (Supplemental Table S1). All resulting PCR fragments were recombined into pDONR207 and subsequently cloned into the pKGW vector (Karimi et al., 2002). The binary vectors, containing the different fragments, were sequenced before transformation to the clv2-3 mutants. Transgenic plants were created by floral dip transformation (Clough and Bent, 1998).
RNA Isolation and Reverse Transcription-PCR
Total RNA was isolated from inflorescences using the Qiagen RNAeasy Kit according to the manufacturer's instructions. Residual genomic DNA contamination was removed with RNase-free DNase I and further purified. First-strand cDNA was synthesized from 1 μg of DNA-free RNA with Moloney murine leukemia virus reverse transcriptase at 37°C for 50 min. The primers used for Δ1, Δ2, and Δ3 are GD40 and GD41, while GD41 and GD42 were used for Δ4 (Supplemental Table S1). The primer pairs GD43 and GD44 (Supplemental Table S1) were used to amplify the ACTIN gene as a control.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Domain organization of CLV2-like AtRLPs.
Supplemental Figure S2. Comparison of expression profiles of CLV2 subfamily genes.
Supplemental Figure S3. Several CLV2-like genes display an expression in SAM.
Supplemental Figure S4. Expression of deletion constructs.
Supplemental Figure S5. Sequence alignment of full-length CLV2 and AtRLP38 proteins.
Supplemental Figure S6. Domain composition comparison between CLV2 and AtRLP38 proteins.
Supplemental Table S1. Primer sequences used in this study.
Supplementary Material
Acknowledgments
We thank Dr. Renier van der Hoorn (Max Planck Institute for Plant Breeding Research) for helpful discussion.
This work was supported by the Dutch Graduate School of Experimental Plant Sciences, by the Centre for BioSystems Genomics (to G.W. and M.F.), which is part of the Netherlands Genomics Initiative/Netherlands Organization for Scientific Research, and by the Research Council for Earth and Life Sciences of the Netherlands Organization for Scientific Research (VIDI grant to B.P.H.J.T.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Martijn Fiers (martijn.fiers@wur.nl).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
References
- Belfanti E, Silfverberg-Dilworth E, Tartarini S, Patocchi A, Barbieri M, Zhu J, Vinatzer BA, Gianfranceschi L, Gessler C, Sansavini S (2004) The HcrVf2 gene from a wild apple confers scab resistance to a transgenic cultivated variety. Proc Natl Acad Sci USA 101 886–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamitjana-Martínez E, Hofhuis HF, Xu J, Liu CM, Heidstra R, Scheres B (2003) Root-specific CLE19 overexpression and the sol1/2 suppressors implicate a CLV-like pathway in the control of Arabidopsis root meristem maintenance. Curr Biol 13 1435–1441 [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM (1993) CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119 397–418 [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM (1995) CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121 2057–2067 [Google Scholar]
- Clark SE, Williams RW, Meyerowitz EM (1997) The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89 575–585 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- DeYoung BJ, Bickle KL, Schrage KJ, Muskett P, Patel K, Clark SE (2006) The Clavata1-related BAM1, BAM2 and BAM3 receptor kinase-like proteins are required for meristem function in Arabidopsis. Plant J 45 1–16 [DOI] [PubMed] [Google Scholar]
- Diévart A, Clark SE (2004) LRR-containing receptors regulating plant development and defense. Development 131 251–261 [DOI] [PubMed] [Google Scholar]
- Dunning FM, Sun W, Jansen KL, Helft L, Bent AF (2007) Identification and mutational analysis of Arabidopsis FLS2 leucine-rich repeat domain residues that contribute to flagellin perception. Plant Cell 19 3297–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellendorff U, Zhang Z, Thomma BPHJ (2008) Gene silencing to investigate the roles of receptor-like proteins in Arabidopsis. Plant Signal Behav 3 893–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers M, Golemiec E, Xu J, van der Geest L, Heidstra R, Stiekema W, Liu CM (2005) The 14-amino acid CLV3, CLE19 and CLE40 peptides trigger consumption of the root meristem in Arabidopsis through a CLAVATA2-dependent pathway. Plant Cell 17 2542–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin EF, Zhang Z, Juarez Ayala JC, Castroverde CCM, Nazar RN, Robb J, Liu CM, Thomma BPHJ (2009) Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol 150 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichsen DM, Joazeiro CA, Li J, Hunter T, Chory J (2000) Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase. Plant Physiol 123 1247–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz-Laylin LK, Krishnamurthy N, Tör M, Sjölander KV, Jones JDG (2005) Phylogenomic analysis of the receptor-like proteins of rice and Arabidopsis. Plant Physiol 138 611–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5 1003–1011 [DOI] [PubMed] [Google Scholar]
- He Z, Wang ZY, Li J, Zhu Q, Lamb C, Ronald P, Chory J (2000) Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science 288 2360–2363 [DOI] [PubMed] [Google Scholar]
- Jeong S, Clark SE (2005) Photoperiod regulates flower meristem development in Arabidopsis thaliana. Genetics 169 907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Trotochaud AE, Clark SE (1999) The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell 11 1925–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinn TL, Stone JM, Walker JC (2000) HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes Dev 14 108–117 [PMC free article] [PubMed] [Google Scholar]
- Jones DA, Jones JDG (1997) The role of leucine-rich repeat proteins in plant defences. Adv Bot Res 24 89–167 [Google Scholar]
- Jones DA, Thomas CM, Hammond-Kosack KE, Balintkurti PJ, Jones JDG (1994) Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science 266 789–793 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A (2002) Gateway vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7 193–195 [DOI] [PubMed] [Google Scholar]
- Kawchuk LM, Hachey J, Lynch DR, Kulcsar F, Van Rooijen G, Waterer DR, Robertson A, Kokko E, Byers R, Howard RJ, et al (2001) Tomato Ve disease resistance genes encode cell surface-like receptors. Proc Natl Acad Sci USA 98 6511–6515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayes JM, Clark SE (1998) CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Development 125 3843–3851 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Caño-Delgado A, Seto H, Hiranuma S, Fujioka S, Yoshida S, Chory J (2005) Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 433 167–171 [DOI] [PubMed] [Google Scholar]
- Kobe B, Kajava AV (2001) The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol 11 725–732 [DOI] [PubMed] [Google Scholar]
- Kolade OO, Bamford VA, Ancillo G, Vera P, Jones JD, Hemmings AM (2006) In vitro characterization of the cysteine-rich capping domains in a plant leucine rich repeat protein. Biochim Biophys Acta 1764 1043–1053 [DOI] [PubMed] [Google Scholar]
- Kruijt M, De Kock MJD, de Wit PJGM (2005) Receptor-like proteins involved in plant disease resistance. Mol Plant Pathol 6 85–97 [DOI] [PubMed] [Google Scholar]
- Lenhard M, Laux T (2003) Stem cell homeostasis in the Arabidopsis shoot meristem is regulated by intercellular movement of CLAVATA3 and its sequestration by CLAVATA1. Development 130 3163–3173 [DOI] [PubMed] [Google Scholar]
- Li J, Chory J (1997) A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90 929–938 [DOI] [PubMed] [Google Scholar]
- Malnoy M, Xu M, Borejsza-Wysocka E, Korban SS, Aldwinckle HS (2008) Two receptor-like genes, Vfa1 and Vfa2, confer resistance to the fungal pathogen Venturia inaequalis inciting apple scab disease. Mol Plant Microbe Interact 21 448–458 [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y, Ogawa M, Morita A, Sakagami Y (2002) An LRR receptor-like kinase involved in perception of a peptide plant hormone, phytosulfokine. Science 296 1470–1472 [DOI] [PubMed] [Google Scholar]
- Mazet F, Shimeld SM (2002) Gene duplication and divergence in the early evolution of vertebrates. Curr Opin Genet Dev 12 393–396 [DOI] [PubMed] [Google Scholar]
- Miwa H, Betsuyaku S, Iwamoto K, Kinoshita A, Fukuda H, Sawa S (2008) The receptor-like kinase SOL2 mediates CLE signaling in Arabidopsis. Plant Cell Physiol 49 1752–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R, Bleckmann A, Simon R (2008) The receptor kinase CORYNE of Arabidopsis transmits the stem cell-limiting signal CLAVATA3 independently of CLAVATA1. Plant Cell 20 934–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD (2002) Control of stomatal distribution on the Arabidopsis leaf surface. Science 296 1697–1700 [DOI] [PubMed] [Google Scholar]
- Ogawa M, Shinohara H, Sakagami Y, Matsubayashi Y (2008) Arabidopsis CLV3 peptide directly binds CLV1 ectodomain. Science 319 294. [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Bemis SM, Shpak ED, Torii KU (2007) Haploinsufficiency after successive loss of signaling reveals novel role for ERECTA-family genes in ovule development. Development 134 3099–3109 [DOI] [PubMed] [Google Scholar]
- Prince VE, Pickett FB (2002) Splitting pairs: diverging fates of duplicated genes. Nat Rev Genet 3 827–837 [DOI] [PubMed] [Google Scholar]
- Ramonell K, Berrocal-Lobo M, Koh S, Wan J, Edwards H, Stacey G, Somerville S (2005) Loss-of-function mutations in chitin responsive genes show increased susceptibility to the powdery mildew pathogen Erysiphe cichoracearum. Plant Physiol 138 1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas S, Rougon-Cardoso A, Smoker M, Schauser L, Yoshioka H, Jones JDG (2004) CITRX thioredoxin interacts with the tomato Cf-9 resistance protein and negatively regulates defence. EMBO J 23 2156–2165 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rojo E, Sharma VK, Kovaleva V, Raikhel NV, Fletcher JC (2002) CLV3 is localized to the extracellular space, where it activates the Arabidopsis CLAVATA stem cell signaling pathway. Plant Cell 14 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron M, Avni A (2004) The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell 16 1604–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seear PJ, Dixon MS (2003) Variable leucine-rich repeats of tomato disease resistance genes Cf-2 and Cf-5 determine specificity. Mol Plant Pathol 4 199–202 [DOI] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB (2001) Plant receptor-like kinase gene family: diversity, function, and signaling. Sci STKE 113 1–13 [DOI] [PubMed] [Google Scholar]
- Shpak ED, Berthiaume CT, Hill EJ, Torii KU (2004) Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development 131 1491–1501 [DOI] [PubMed] [Google Scholar]
- Shpak ED, McAbee JM, Pillitteri LJ, Torii KU (2005) Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 309 290–293 [DOI] [PubMed] [Google Scholar]
- Song WY, Wang GL, Chen LL, Kim HS, Pi LY, Holsten T, Gardner J, Wang B, Zhai WX, Zhu LH, et al (1995) A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 270 1804–1806 [DOI] [PubMed] [Google Scholar]
- Taguchi-Shiobara F, Yuan Z, Hake S, Jackson D (2001) The fasciated ear2 gene encodes a leucine-rich repeat receptor-like protein that regulates shoot meristem proliferation in maize. Genes Dev 15 2755–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CM, Jones DA, Parniske M, Harrison K, Balint-Kurti PJ, Hatzixanthis K, Jones JDG (1997) Characterization of the tomato Cf-4 gene for resistance to Cladosporium fulvum identifies sequences that determine recognitional specificity in Cf-4 and Cf-9. Plant Cell 9 2209–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii KU (2004) Leucine-rich repeat receptor kinases in plants: structure, function, and signal transduction pathways. Int Rev Cytol 234 1–46 [DOI] [PubMed] [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, Komeda Y (1996) The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell 8 735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotochaud AE, Hao T, Wu G, Yang Z, Clark SE (1999) The CLAVATA1 receptor-like kinase requires CLAVATA3 for its assembly into a signaling complex that includes KAPP and a Rho-related protein. Plant Cell 11 393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoorn RA, Roth R, de Wit PJGM (2001) Identification of distinct specificity determinants in resistance protein Cf-4 allows construction of a Cf-9 mutant that confers recognition of avirulence protein Avr4. Plant Cell 13 273–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoorn RA, Wulff BB, Rivas S, Durrant MC, van der Ploeg A, de Wit PJGM, Jones JDG (2005) Structure-function analysis of Cf-9, a receptor-like protein with extracytoplasmic leucine-rich repeats. Plant Cell 17 1000–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Ellendorff U, Kemp B, Mansfield JW, Forsyth A, Mitchell K, Bastas K, Liu CM, Woods-Tor E, Zipfel C, et al (2008) A genome-wide functional investigation into the roles of receptor-like proteins in Arabidopsis. Plant Physiol 147 503–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff BB, Thomas CM, Smoker M, Grant M, Jones JDG (2001) Domain swapping and gene shuffling identify sequences required for induction of an Avr-dependent hypersensitive response by the tomato Cf-4 and Cf-9 proteins. Plant Cell 13 255–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RK, Girke T, Pasala S, Xie M, Reddy GV (2009) Gene expression map of the Arabidopsis shoot apical meristem stem cell niche. Proc Natl Acad Sci USA 106 4941–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Sack FD (1995) The too many mouths and four lips mutations affect stomatal production in Arabidopsis. Plant Cell 7 2227–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.