Abstract
Na+ and K+ homeostasis are crucial for plant growth and development. Two HKT transporter/channel classes have been characterized that mediate either Na+ transport or Na+ and K+ transport when expressed in Xenopus laevis oocytes and yeast. However, the Na+/K+ selectivities of the K+-permeable HKT transporters have not yet been studied in plant cells. One study expressing 5′ untranslated region-modified HKT constructs in yeast has questioned the relevance of cation selectivities found in heterologous systems for selectivity predictions in plant cells. Therefore, here we analyze two highly homologous rice (Oryza sativa) HKT transporters in plant cells, OsHKT2;1 and OsHKT2;2, that show differential K+ permeabilities in heterologous systems. Upon stable expression in cultured tobacco (Nicotiana tabacum) Bright-Yellow 2 cells, OsHKT2;1 mediated Na+ uptake, but little Rb+ uptake, consistent with earlier studies and new findings presented here in oocytes. In contrast, OsHKT2;2 mediated Na+-K+ cotransport in plant cells such that extracellular K+ stimulated OsHKT2;2-mediated Na+ influx and vice versa. Furthermore, at millimolar Na+ concentrations, OsHKT2;2 mediated Na+ influx into plant cells without adding extracellular K+. This study shows that the Na+/K+ selectivities of these HKT transporters in plant cells coincide closely with the selectivities in oocytes and yeast. In addition, the presence of external K+ and Ca2+ down-regulated OsHKT2;1-mediated Na+ influx in two plant systems, Bright-Yellow 2 cells and intact rice roots, and also in Xenopus oocytes. Moreover, OsHKT transporter selectivities in plant cells are shown to depend on the imposed cationic conditions, supporting the model that HKT transporters are multi-ion pores.
Intracellular Na+ and K+ homeostasis play vital roles in growth and development of higher plants (Clarkson and Hanson, 1980). Low cytosolic Na+ and high K+/Na+ ratios aid in maintaining an osmotic and biochemical equilibrium in plant cells. Na+ and K+ influx and efflux across membranes require the function of transmembrane Na+ and K+ transporters/channels. Several Na+-permeable transporters have been characterized in plants (Zhu, 2001; Horie and Schroeder, 2004; Apse and Blumwald, 2007). Na+/H+ antiporters mediate sequestration of Na+ into vacuoles under salt stress conditions in plants (Blumwald and Poole, 1985, 1987; Sze et al., 1999). Na+ (cation)/H+ antiporters are encoded by six AtNHX genes in Arabidopsis (Arabidopsis thaliana; Apse et al., 1999; Gaxiola et al., 1999; Yokoi et al., 2002; Aharon et al., 2003). A distinct Na+/H+ antiporter, Salt Overly Sensitive1, mediates Na+/H+ exchange at the plasma membrane and mediates cellular Na+ extrusion (Shi et al., 2000, 2002; Zhu, 2001; Ward et al., 2003). Electrophysiological analyses reveal that voltage-independent channels, also named nonselective cation channels, mediate Na+ influx into roots under high external Na+ concentrations (Amtmann et al., 1997; Tyerman et al., 1997; Buschmann et al., 2000; Davenport and Tester, 2000); however, the underlying genes remain unknown.
Potassium is the most abundant cation in plants and an essential nutrient for plant growth. The Arabidopsis genome includes 13 genes encoding KUP/HAK/KT transporters (Quintero and Blatt, 1997; Santa-María et al., 1997; Fu and Luan, 1998; Kim et al., 1998), and 17 genes have been identified encoding this family of transporters in rice (Oryza sativa ‘Nipponbare’; Bañuelos et al., 2002). Several KUP/HAK/KT transporters have been characterized as mediating K+ uptake across the plasma membrane of plant cells (Rigas et al., 2001; Bañuelos et al., 2002; Gierth et al., 2005).
Ionic balance, especially the Na+/K+ ratio, is a key factor of salt tolerance in plants (Niu et al., 1995; Maathuis and Amtmann, 1999; Shabala, 2000; Mäser et al., 2002a; Tester and Davenport, 2003; Horie et al., 2006; Apse and Blumwald, 2007; Chen et al., 2007; Gierth and Mäser, 2007). Salinity stress is a major problem for agricultural productivity of crops worldwide (Greenway and Munns, 1980; Zhu, 2001). The Arabidopsis AtHKT1;1 transporter plays a key role in salt tolerance of plants by mediating Na+ exclusion from leaves (Mäser et al., 2002a; Berthomieu et al., 2003; Gong et al., 2004; Sunarpi et al., 2005; Rus et al., 2006; Davenport et al., 2007; Horie et al., 2009). athkt1;1 mutations cause leaf chlorosis and elevated Na+ accumulation in leaves under salt stress conditions in Arabidopsis (Mäser et al., 2002a; Berthomieu et al., 2003; Gong et al., 2004; Sunarpi et al., 2005). AtHKT1;1 and its homolog in rice, OsHKT1;5 (SKC1), mediate leaf Na+ exclusion by removing Na+ from the xylem sap to protect plants from salinity stress (Ren et al., 2005; Sunarpi et al., 2005; Horie et al., 2006, 2009; Davenport et al., 2007).
The land plant HKT gene family is divided into two classes based on their nucleic acid sequences and protein structures (Mäser et al., 2002b; Platten et al., 2006). Class 1 HKT transporters have a Ser residue at a selectivity filter position in the first pore loop, which is replaced by a Gly in all but one known class 2 HKT transporter (Horie et al., 2001; Mäser et al., 2002b; Garciadeblás et al., 2003). While the Arabidopsis genome includes only one HKT gene, AtHKT1;1 (Uozumi et al., 2000), seven full-length OsHKT genes were found in the japonica rice cv Nipponbare genome (Garciadeblás et al., 2003). Members of class 1 HKT transporters, AtHKT1;1 and SKC1/OsHKT1;5, have a relatively higher Na+-to-K+ selectivity in Xenopus laevis oocytes and yeast than class 2 HKT transporters (Uozumi et al., 2000; Horie et al., 2001; Mäser et al., 2002b; Ren et al., 2005). The first identified plant HKT transporter, TaHKT2;1 from wheat (Triticum aestivum), is a class 2 HKT transporter (Schachtman and Schroeder, 1994). TaHKT2;1 was found to mediate Na+-K+ cotransport and Na+ influx at high Na+ concentrations in heterologous expression systems (Rubio et al., 1995, 1999; Gassmann et al., 1996; Mäser et al., 2002b). Thus, class 1 HKT transporters have been characterized as Na+-preferring transporters with a smaller K+ permeability (Fairbairn et al., 2000; Uozumi et al., 2000; Su et al., 2003; Jabnoune et al., 2009), whereas class 2 HKT transporters function as Na+-K+ cotransporters or channels (Gassmann et al., 1996; Corratgé et al., 2007). In addition, at millimolar Na+ concentrations, class 2 HKT transporters were found to mediate Na+ influx, without adding external K+ in Xenopus oocytes and yeast (Rubio et al., 1995, 1999; Gassmann et al., 1996; Horie et al., 2001). However, the differential cation transport selectivities of the two types of HKT transporters have not yet been analyzed and compared in plant cells.
A study of the barley (Hordeum vulgare) and wheat class 2 transporters has suggested that the transport properties of HvHKT2;1 and TaHKT2;1 expressed in yeast are variable, depending on the constructs from which the transporter is expressed, and have led to questioning of the K+ transport activity of HKT transporters characterized in Xenopus oocytes and yeast (Haro et al., 2005). It was further proposed that the 5′ translation initiation of HKT proteins in yeast at nonconventional (non-ATG) sites affects the transporter selectivities of HKT transporters (Haro et al., 2005), although direct evidence for this has not yet been presented. However, recent research has shown a K+ permeability of OsHKT2;1 but not of OsHKT1;1 and OsHKT1;3 in Xenopus oocytes. These three OsHKT transporters show overlapping and also distinctive expression patterns in rice (Jabnoune et al., 2009).
The report of Haro et al. (2005) has opened a central question addressed in this study: are the Na+/K+ transport selectivities of plant HKT transporters characterized in heterologous systems of physiological relevance in plant cells, or do they exhibit strong differences in the cation transport selectivities in these nonplant versus plant systems? To address this question, we analyzed the Na+/K+ transport selectivities of the OsHKT2;1 and OsHKT2;2 transporters expressed in cultured tobacco (Nicotiana tabacum ‘Bright-Yellow 2’ [BY2]) cells. OsHKT2;1 and OsHKT2;2 are two highly homologous HKT transporters from indica rice cv Pokkali, sharing 91% amino acid and 93% cDNA sequence identity (Horie et al., 2001). OsHKT2;1 mediates mainly Na+ uptake, which correlates with the presence of a Ser residue in the first pore loop of OsHKT2;1 (Horie et al., 2001, 2007; Mäser et al., 2002b; Garciadeblás et al., 2003). In contrast, OsHKT2;2 mediates Na+-K+ cotransport in Xenopus oocytes and yeast (Horie et al., 2001). Furthermore, at millimolar Na+ concentrations, OsHKT2;2 mediates Na+ influx in the absence of added K+ (Horie et al., 2001). Recent research on oshkt2;1 loss-of-function mutant alleles has revealed that OsHKT2;1 from japonica rice mediates a large Na+ influx component into K+-starved roots, thus compensating for lack of K+ availability (Horie et al., 2007). But the detailed Na+/K+ selectivities of Gly-containing, predicted K+-transporting class 2 HKT transporters have not yet been analyzed in plant cells.
Here, we have generated stable OsHKT2;1- and OsHKT2;2-expressing tobacco BY2 cell lines and characterized the cell lines by ion content measurements and tracer influx studies to directly analyze unidirectional fluxes (Epstein et al., 1963). These analyses showed that OsHKT2;1 exhibits Na+ uptake activity in plant BY2 cells in the absence of added K+, but little K+ (Rb+), influx activity. In contrast, OsHKT2;2 was found to function as a Na+-K+ cotransporter/channel in plant BY2 cells, showing K+-stimulated Na+ influx and Na+-stimulated K+ (Rb+) influx. The differential K+ selectivities of the two OsHKT2 transporters were consistently reproduced by voltage clamp experiments using Xenopus oocytes here, as reported previously (Horie et al., 2001). OsHKT2;2 was also found to mediate K+-independent Na+ influx at millimolar external Na+ concentrations. These findings demonstrate that the cation selectivities of OsHKT2;1 and OsHKT2;2 in plant cells are consistent with past findings obtained from heterologous expression analyses under similar ionic conditions (Horie et al., 2001; Garciadeblás et al., 2003; Tholema et al., 2005). Furthermore, the shift in OsHKT2;2 Na+-K+ selectivity depending on ionic editions is consistent with the model that HKT transporters/channels are multi-ion pores (Gassmann et al., 1996; Corratgé et al., 2007). Classical studies of ion channels have shown that ion channels, in which multiple ions can occupy the pore at the same time, can change their relative selectivities depending on the ionic conditions (Hille, 2001). Moreover, the presence of external K+ and Ca2+ was found here to down-regulate OsHKT2;1-mediated Na+ influx both in tobacco BY2 cells and in rice roots. The inhibitory effect of external K+ on OsHKT2;1-mediated Na+ influx into intact rice roots, however, showed a distinct difference in comparison with that of BY2 cells, which indicates a possible posttranslational regulation of OsHKT2;1 in K+-starved rice roots.
RESULTS
OsHKT2;1 and OsHKT2;2 Expression in Tobacco BY2 Cell Lines
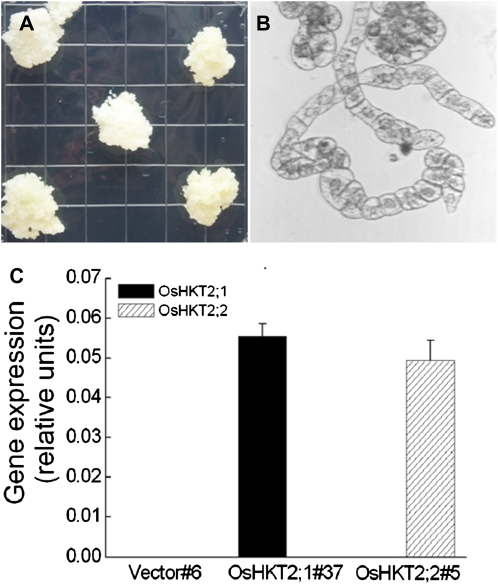
Po-OsHKT2;1 and Po-OsHKT2;2 were identified in indica rice cv Pokkali. These two highly homologous HKT transporters share 93% identical cDNA sequence and 91% identical amino acid sequence (Horie et al., 2001). The OsHKT2;1 and OsHKT2;2 transporters show differential Na+/K+ selectivities in heterologous systems. However, their Na+/K+ transport selectivities have not yet been studied in plant cells. We cloned and transformed tobacco BY2 cells with the Po-OsHKT2;1 and Po-OsHKT2;2 cDNAs driven by the cauliflower mosaic virus 35S promoter. Stable transformants were selected on solid Linsmaier and Skoog (LS) medium (Nagata et al., 1981) supplemented with 100 μg mL−1 kanamycin and 250 μg mL−1 carbenicillin. Stable kanamycin-resistant subcultures of calli were selected by several sequential rounds of subcultures on solid LS plates (Fig. 1A). Each callus was transferred to liquid LS medium to initiate suspension culture BY2 cell lines (Fig. 1B). Real-time PCR was performed to determine the expression levels of OsHKT2;1 and OsHKT2;2 in the selected BY2 cell lines. OsHKT2;1 and OsHKT2;2 were stably expressed in transformed cell lines but were not expressed in vector control cells (Fig. 1C). Two lines, OsHKT2;1#37 and OsHKT2;2#5, were chosen for further analyses.
Figure 1.
A, Tobacco BY2 calli expressing OsHKT2;1 on selective LS medium as a representative example. B, Tobacco BY2 suspension cells expressing OsHKT2;1 as a representative example. C, Real-time PCR analyses of OsHKT2;1 and OsHKT2;2 expression in tobacco BY2 cell lines expressing OsHKT2;1, OsHKT2;2,or empty vector DNA constructs (n = 3; ±se). [See online article for color version of this figure.]
OsHKT2;1, But Not OsHKT2;2, Exhibits Na+ Uptake Activity under Low Na+ and without Added K+
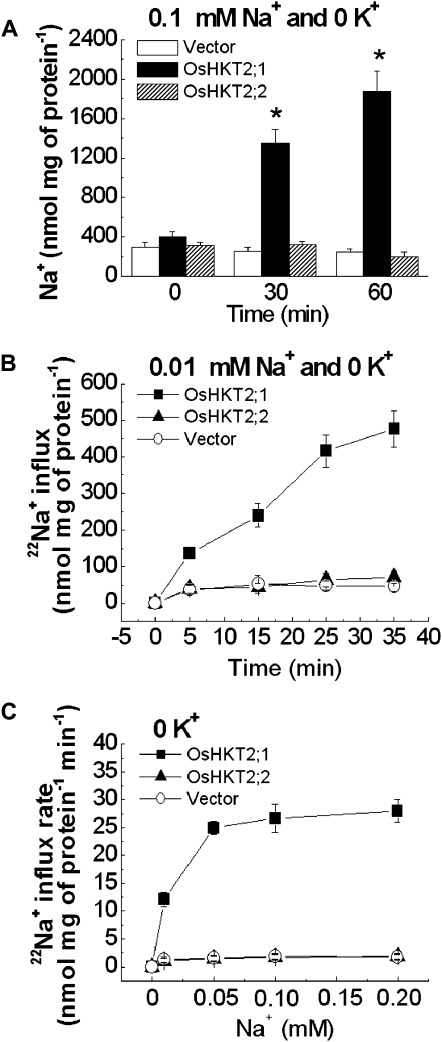
In order to study Na+/K+ transport mediated by OsHKT2;1 and OsHKT2;2 in plant cells, measurements of Na+ content and 22NaCl tracer influx experiments were performed using transgenic BY2 cell lines. The Na+ content of 4-d-old BY2 cells expressing OsHKT2;1, OsHKT2;2, or empty vector was determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES) analyses after exposing each cell line to a 0.1 mm Na+-containing solution for 30 and 60 min. Incubation in a 0.1 mm Na+-containing solution led to dramatic increases in the Na+ content only in OsHKT2;1-expressing BY2 cells (Fig. 2A). However, neither control nor OsHKT2;2-expressing BY2 cells showed a significant difference in Na+ accumulation compared with the initial Na+ content at time 0 (Fig. 2A). Time-dependent tracer influx analyses at 0.01 mm external Na+ showed OsHKT2;1-mediated Na+ influx (Fig. 2B). Interestingly, however, no significant Na+ transport activity was found in OsHKT2;2-expressing BY2 cells compared with the control cell line (Fig. 2B). Results from time-dependent tracer influx experiments are consistent with the Na+ accumulation phenotype of each BY2 cell line (Fig. 2, A and B).
Figure 2.
OsHKT2;1 mediates Na+ influx, whereas OsHKT2;2 does not, in the absence of external K+. A, OsHKT2;1 increases Na+ accumulation in cultured tobacco BY2 cells. Na+ contents are shown for transgenic BY2 cell lines exposed to a buffer containing 0.1 mm Na+ and no added K+ for 0, 30, or 60 min. Na+ contents were determined by ICP-OES (n = 6; ±sd; * P < 0.001 compared with vector controls). B, OsHKT2;1 mediates Na+ influx from a 0.01 mm Na+ and 0 mm K+ buffer, whereas OsHKT2;2 does not, compared with vector controls. Na+ influx data are from time-dependent short-term 22Na+ influx experiments using transgenic BY2 cell lines in influx buffer containing 0.01 mm Na+ and no added K+ (n = 3; ±sd). C, OsHKT2;1 mediates enhanced Na+ influx as a function of the external Na+ concentration with no added K+, whereas OsHKT2;2 does not, compared with vector controls. Concentration-dependent short-term 22Na+ influx rates of transgenic BY2 cell lines are analyzed at 0 to 0.2 mm external Na+ without K+ (n = 3; ±sd; time = 15 min).
Concentration-dependent short-term unidirectional 22Na+ influx experiments showed that OsHKT2;1 mediated Na+ influx in BY2 cells at 0 to 0.2 mm external Na+ with no added K+ (Fig. 2C). In contrast, OsHKT2;2 did not show Na+ influx activity compared with vector-transformed control cells (Fig. 2C). A kinetic analysis of OsHKT2;1-mediated Na+ influx in BY2 cells in the concentration range tested in this study showed an apparent Na+ affinity of approximately 0.014 mm Na+ and a Vmax of approximately 31 nmol mg−1 protein min−1 (Table I). Note that the apparent affinity of a transporter depends on multiple parameters, including membrane potential, electrical coupling to other cells, and intracellular ion concentrations (Schroeder et al., 1994). Taken together, these results suggest that OsHKT2;1 functions as a Na+ transporter/channel, while OsHKT2;2 does not, in the presence of low external Na+ and no added extracellular K+ in plant cells.
Table I.
Michaelis-Menten curve-fitting results for 22Na+ and 86Rb+ influx kinetics of OsHKT2;1- and OsHKT2;2-expressing tobacco BY2 cells (n = 3)
Curve fitting was performed with Microcal origin 6.0 software (http://microcal-origin.software.informer.com/6.0/).
| Sample | r2 | Km | Vmax |
|---|---|---|---|
| mm | nmol mg−1 protein min−1 | ||
| OsHKT2;1a (Na+) | 0.996 | 0.014 ± 0.002 | 31 ± 0.9 |
| OsHKT2;2b (Na+) | 0.980 | 0.077 ± 0.03 | 26 ± 4.0 |
| OsHKT2;2c (Rb+) |
0.986 |
0.035 ± 0.01 |
19 ± 1.6 |
Results of fits to data from Figure 2C, showing 22Na+ influx kinetics of OsHKT2;1 transporter with no added K+.
Results of fits to data from Figure 3B, showing 22Na+ influx kinetics of OsHKT2;2 transporter with 0.1 mm extracellular K+.
Results of fits to data from Figure 4C, showing 86Rb+ influx kinetics of OsHKT2;2 transporter with 0.01 mm extracellular Na+.
OsHKT2;2 Mediates K+-Stimulated Na+ Uptake into Tobacco BY2 Cells
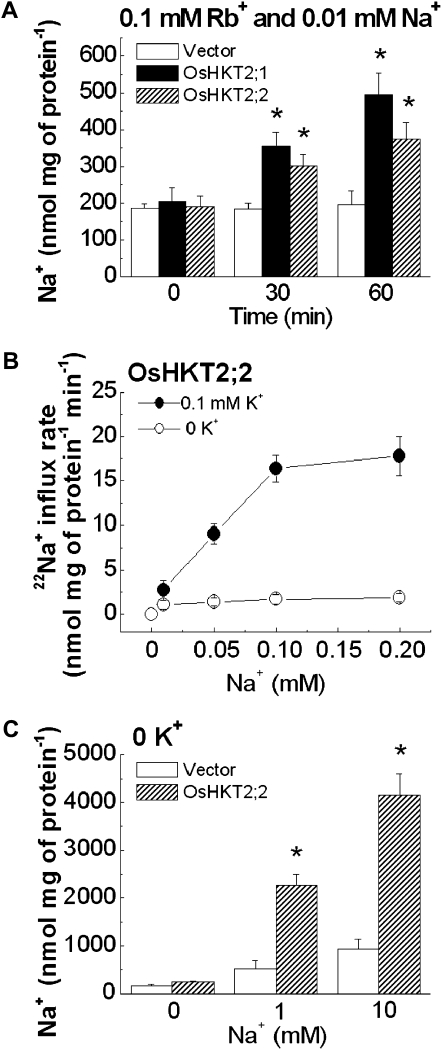
As OsHKT2;2 showed clear Na+ transport activity in the presence of extracellular K+ when expressed in Xenopus oocytes (Horie et al., 2001), we next characterized Na+ and K+ transport properties of each transgenic BY2 cell line in the presence of both Na+ and K+ (Rb+). ICP-OES analyses were initially performed. Four-day-old BY2 cells expressing OsHKT2;1, OsHKT2;2, or empty vector were treated with a 0.1 mm Rb+ and 0.01 mm Na+ solution for 30 and 60 min. Interestingly, Rb+ stimulated significant Na+ accumulation in OsHKT2;2-expressing BY2 cells (Fig. 3A), which was not the case when Na+ was added as a sole alkali cation source in the buffer solution (Fig. 2A).
Figure 3.
Extracellular K+ (Rb+) stimulated OsHKT2;2-mediated Na+ influx. A, OsHKT2;2-expressing tobacco BY2 cells exhibited increased Na+ accumulation in the presence of 0.1 mm Rb+. Na+ contents are shown for transgenic BY2 cell lines exposed to a buffer containing 0.1 mm Rb+ and 0.01 mm Na+ for 0, 30, or 60 min, as determined by ICP-OES (n = 6; ±sd; * P < 0.001 compared with vector controls). B, OsHKT2;2 mediated K+-dependent Na+ influx. Na+ influx kinetics are shown for concentration-dependent short-term 22Na+ influx analyses using transgenic BY2 cell lines in influx buffer containing 0 to 0.2 mm external Na+ with or without 0.1 mm K+ (n = 3; ±sd; time = 15 min). C, OsHKT2;2-expressing tobacco BY2 cells show enhanced Na+ accumulation at millimolar external Na+ concentrations without adding extracellular K+. Na+ contents are shown for OsHKT2;2-expressing BY2 cell lines exposed to a buffer containing either 1 mm Na+ or 10 mm Na+ for 60 min, as determined by ICP-OES (n = 6; ±sd; * P < 0.001 compared with vector controls).
To more directly analyze K+-stimulated Na+ influx activity of OsHKT2;2, concentration-dependent short-term unidirectional 22Na+ influx experiments were performed in the presence or absence of 0.1 mm K+. OsHKT2;2 mediated Na+ influx into BY2 cells at 0 to 0.2 mm external Na+ and 0.1 mm external K+ conditions, but not in the absence of added external K+ (Fig. 3B). A kinetic analysis showed an apparent Na+ affinity for OsHKT2;2-mediated Na+ influx into BY2 cells of approximately 0.077 mm and a Vmax of approximately 26 nmol mg−1 protein min−1 when 0.1 mm K+ was added (Table I). Taken together, these results strongly suggest that OsHKT2;2 mediates K+-stimulated Na+ uptake into plant BY2 cells. Furthermore, at millimolar Na+ concentrations of 1 and 10 mm Na+, OsHKT2;2 showed Na+ accumulation in tobacco BY2 cells without adding extracellular K+ (Fig. 3C), consistent with previous findings on class 2 HKT transporters in Xenopus oocytes and yeast (Rubio et al., 1995; Horie et al., 2001).
OsHKT2;2 Mediates Na+-Stimulated K+ Uptake into Tobacco BY2 Cells
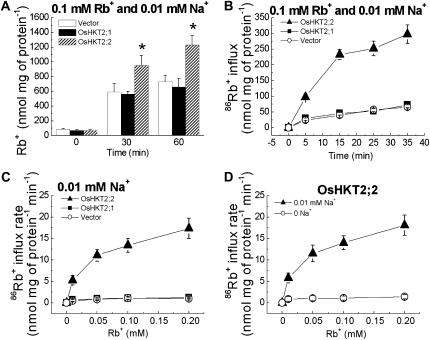
Experiments were pursued to investigate whether OsHKT2;1 and OsHKT2;2 have the ability to mediate K+ influx. As K+ is fairly abundant in plant cells and endogenous K+ could mask the effect of the OsHKT2;2 protein-mediated K+ accumulation, external K+ was replaced with the K+ analog Rb+ (Epstein et al., 1963; Kochian and Lucas, 1988) in measurements of ion contents to monitor K+ transport properties of OsHKT2;1 and OsHKT2;2 in BY2 cells. Four-day-old BY2 cells expressing OsHKT2;1, OsHKT2;2, or empty vector were exposed to a 0.1 mm Rb+ and 0.01 mm Na+ buffer solution for 30 and 60 min, and the Rb+ content was determined by ICP-OES. Only OsHKT2;2-expressing BY2 cells exhibited significantly increased Rb+ accumulation compared with that of control BY2 cells (Fig. 4A).
Figure 4.
OsHKT2;2 but not OsHKT2;1 mediates Rb+ (K+) accumulation and influx in BY2 cells. A, OsHKT2;2-expressing tobacco BY2 cells exhibit increased Rb+ accumulation. Rb+ contents are shown for transgenic BY2 cell lines exposed to a buffer containing 0.1 mm Rb+ and 0.01 mm Na+ for 0, 30, or 60 min, as determined by ICP-OES (n = 6; ±sd; * P < 0.001 compared with vector controls). B, OsHKT2;2 mediates Rb+ (K+) influx, whereas OsHKT2;1 does not, compared with vector controls. Rb+ influx kinetics are shown for time-dependent short-term 86Rb+ influx assays using transgenic BY2 cell lines in influx buffer solution containing 0.1 mm Rb+ and 0.01 mm Na+ (n = 3; ±sd). C, OsHKT2;2 mediates enhanced Rb+ (K+) influx as a function of the external Rb+ concentration, whereas OsHKT2;1 does not, compared with vector controls. Concentration-dependent short-term 86Rb+ influx rates of transgenic BY2 cell lines were analyzed at 0 to 0.2 mm external Rb+ with 0.01 mm Na+ (n = 3; ±sd; time = 15 min). D, OsHKT2;2 mediates Na+-stimulated K+ (Rb+) influx. Concentration-dependent short-term 86Rb+ influx rates of transgenic BY2 cell lines were analyzed at 0 to 0.2 mm external Rb+ with or without 0.01 mm Na+ (n = 3; ±sd; time = 15 min). Samples in C and D were measured in parallel.
More direct time-dependent tracer influx analyses using 86Rb+ showed that OsHKT2;2 mediated Rb+ influx, whereas OsHKT2;1 did not, compared with control BY2 cells (Fig. 4B), consistent with ICP data in Figure 4A. Concentration-dependent short-term unidirectional 86Rb+ influx experiments at 0 to 0.2 mm external Rb+ and 0.01 mm Na+ revealed that only OsHKT2;2 exhibited significant Rb+ (K+) influx activity, showing increases in the influx rate at increasing external Rb+ concentrations (Fig. 4C). A kinetic analysis of OsHKT2;2-mediated K+ (Rb+) influx into BY2 cells showed an apparent affinity of approximately 0.035 mm Rb+ and Vmax of approximately 19 nmol mg−1 protein min−1 (Table I; Fig. 4C).
OsHKT2;2-mediated Na+ uptake into BY2 cells was found to be external K+ dependent (Figs. 2 and 3, A and B). Therefore, we compared Rb+ influx rates of OsHKT2;2-expressiing BY2 cells at external 0 to 0.2 mm Rb+ with or without 0.01 mm Na+. OsHKT2;2 mediates Na+-stimulated Rb+ influx into BY2 cells (Fig. 4D). Taken together, the findings presented in Figures 3 and 4 showed that OsHKT2;2 mediates Na+-stimulated K+ uptake as well as K+-stimulated Na+ influx in BY2 cells, suggesting that OsHKT2;2 functions as a Na+-K+ cotransporter/channel in plant cells.
OsHKT2;2 Shows a Large K+ Permeability in Xenopus Oocytes, Whereas OsHKT2;1 Shows Little K+ Permeability Depending on Conditions
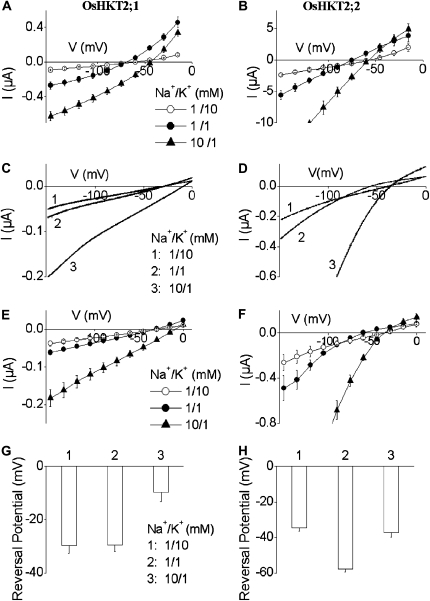
Results from other independent laboratories that have analyzed the K+ selectivity of OsHKT2;1 expressed in yeast and Xenopus oocytes are controversial (Horie et al., 2001; Golldack et al., 2002; Garciadeblás et al., 2003; Jabnoune et al., 2009). Voltage clamp experiments using Xenopus oocytes showed a low K+ permeability of OsHKT2;1 (Horie et al., 2001). However, similar electrophysiological analyses showed either relatively nonselective alkali cation selectivity (Golldack et al., 2002) or strong K+ permeability of OsHKT2;1 (Jabnoune et al., 2009). Therefore, we recorded OsHKT2;1- and OsHKT2;2-mediated currents in Xenopus oocytes. Increasing external K+ concentration from 1 to 10 mm led to small positive shifts in the reversal potential of OsHKT2;1-expressing oocytes, consistent with the results reported by Horie et al. (2001; Fig. 5A), while OsHKT2;2-expressing oocytes exhibited larger positive shifts in the reversal potential in response to an identical 10-fold increase in the K+ concentration (Fig. 5B). These results were independently found in two laboratories (see “Materials and Methods”) and were consistent with tobacco BY2 cell analyses.
Figure 5.
OsHKT2;1 and OsHKT2;2 display different Na+ and K+ selectivities in Xenopus oocytes. A and B, Average current-voltage recordings in oocytes expressing OsHKT2;1 (A) and OsHKT2;2 (B), preincubated in a 96 mm Na+-containing solution. C and D, Representative electrical recordings of OsHKT2;1-mediated (C) and OsHKT2;2-mediated (D) currents in response to voltage ramps from 0 to –150 mV in oocytes preincubated in 0.5 mm Na+-containing solution. E and F, Average current-voltage relationships of OsHKT2;1-expressing (E) and OsHKT2;2-expressing (F) oocytes as recorded in C and D. G and H, Average reversal potentials of OsHKT2;1-mediated (G) and OsHKT2;2-mediated (H) currents at the indicated bath Na+ and K+ solutions preincubated as in C and D. In C, D, G, and H, number 1 = 1 Na+/10 K+, number 2 = 1 Na+/1 K+, and number 3 = 10 Na+/1 K+ (in mm). In C to H, oocytes were preincubated in a modified medium with 0.5 mm Na+ after cRNA injection. Data are means ± sd (n = 8 in A and B, n = 5 in E, G, and H, n = 4 in F).
Further experiments were pursued with modified experimental procedures in voltage clamp experiments. OsHKT2 complementary RNA (cRNA)-injected oocytes were incubated in a modified extracellular incubation solution including 0.5 mm Na+ (instead of 96 mm Na+; for details, see “Materials and Methods”). Interestingly, OsHKT2;1-expressing oocytes incubated in the low-Na+ buffer exhibited only very small reversal potential shifts in response to a 10-fold increase in the K+ concentration that were not statistically significant (Fig. 5, C, E, and G). In contrast, OsHKT2;2-expressing oocytes continued to exhibit significant positive shifts in the reversal potential when the external K+ concentration was increased (Fig. 5, D, F, and H). Average positive shifts in the reversal potential of OsHKT2;1-expressing oocytes incubated in the low-Na+ buffer were of −0.2 ± 1.7 mV, and those in OsHKT2;2-expressing oocytes were 20.4 ± 1.3 mV, when the external K+ concentration was increased from 1 to 10 mm in the presence of 1 mm Na+ (Fig. 5, C–H).
A 10-fold increase in the Na+ concentration in the presence of 1 mm K+ led to significant positive shifts in the reversal potential of both OsHKT2;1- and OsHKT2;2-expressing oocytes (Fig. 5), consistent with tobacco BY2 cell transport analyses. When a 10-fold increase in the external Na+ concentration was imposed in the presence of 1 mm K+, both OsHKT2;1- and OsHKT2;2-expressing oocytes showed substantial positive shifts in the reversal potential, 19.8 ± 1.96 mV and 23.0 ± 1.10 mV, respectively (Fig. 5, C–H). These results are consistent with the results of ion accumulation and tracer influx analyses using transgenic BY2 cells (Figs. 2–4) and previous studies showing a low K+ permeability of OsHKT2;1 compared with OsHKT2;2 (Horie et al., 2001; Garciadeblás et al., 2003).
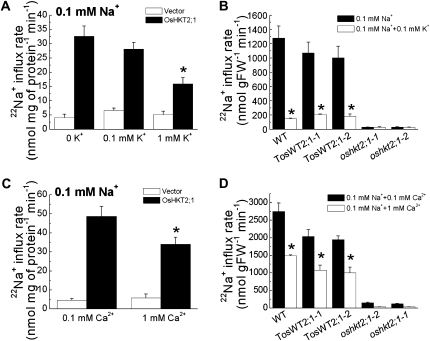
External K+ and Ca2+ Inhibit OsHKT2;1-Mediated Na+ Influx into Plant Cells and Xenopus Oocytes
To determine whether K+ inhibits OsHKT2;1-mediated Na+ influx into plant cells, we performed 22Na+ tracer influx experiments using OsHKT2;1-expressing BY2 cells at 0.1 mm external Na+ with or without external K+. The presence of 0.1 mm external K+ had no significant impact on Na+ influx of OsHKT2;1-expressing BY2 cells. However, the addition of 1 mm extracellular K+ resulted in an approximately 51% to 55% reduction in OsHKT2;1-mediated Na+ influx into BY2 cells (Fig. 6A). We further tested the effect of extracellular K+ addition on OsHKT2;1-dependent Na+ influx into K+-starved intact rice roots. A previous study demonstrated that Na+ influx into K+-starved rice roots at low external Na+ concentrations is primarily dependent on the OsHKT2;1 transporter (Horie et al., 2007). We analyzed 22Na+ influx analyses using the wild-type japonica rice cv Nipponbare, oshkt2;1 mutants that are disrupted in the OsHKT2;1 gene by an insertion of the endogenous retrotransposon Tos17, and their corresponding wild-type “TosWT” plants (Hirochika, 1997, 2001; Miyao et al., 2003; Horie et al., 2007). Experiments were performed at 0.1 mm external Na+ with or without 0.1 mm external K+. Interestingly, the presence 0.1 mm external K+ triggered strong reductions in the OsHKT2;1-dependent Na+ influx into roots of wild-type plants, with approximately 80% to 88% reductions (Fig. 6B). Note, however, that a remarkable difference in the sensitivity of the OsHKT2;1-dependent Na+ influx to external K+ was found between the Na+ influx profiles from BY2 cells and those from intact rice root cells (Fig. 6, A and B).
Figure 6.
External K+ and Ca2+ inhibit OsHKT2;1-mediated Na+ influx into plant cells. A, OsHKT2;1-mediated Na+ influx into BY2 cells at 0.1 mm external Na+ showed significant decreases in the presence of 1 mm K+. Short-term 22Na+ influx analyses are shown using transgenic BY2 cell lines (n = 6; ±sd; time = 15 min; * P < 0.001 compared with OsHKT2;1-mediated Na+ influx rate from a 0.1 mm Na+ buffer without added K+). K+ was added to the influx buffer at 0.1 and 1 mm. B, The presence of 0.1 mm external K+ in influx buffer inhibited OsHKT2;1-mediated Na+ influx into K+-starved rice roots of wild-type (WT) plants. Short-term 22Na+ influx assays at 0.1 mm external Na+ using wild-type, oshkt2;1 mutant, and TosWT rice plants in the presence or absence of 0.1 mm external K+ (n = 3; ±sd; time = 20 min; * P < 0.02, 0.1 mm Na+ versus 0.1 mm Na+ + 0.1 mm K+). FW, Fresh weight. C, The presence of 1 mm Ca2+ in influx buffer inhibited OsHKT2;1-mediated Na+ influx into BY2 cells. Short-term 22Na+ influx analyses are shown using transgenic BY2 cell lines (n = 6; ±sd; time = 15 min; * P < 0.001, 0.1 mm Na+ + 0.1 mm Ca2+ versus 0.1 mm Na+ + 1 mm Ca2+). D, An increase in the external Ca2+ concentration of the influx buffer led to significant decreases in OsHKT2;1-dependent Na+ influx into K+-starved wild-type rice roots. Short-term 22Na+ influx assays were performed at 0.1 mm external Na+ using wild-type, oshkt2;1 mutant, and TosWT rice plants in the presence of either 0.1 or 1 mm external Ca2+ (n = 3; ±sd; time = 20 min; * P < 0.02, 0.1 mm Na+ + 0.1 mm Ca2+ versus 0.1 mm Na+ + 1 mm Ca2+).
Ca2+ is known to partially inhibit Na+ influx into plant roots (Amtmann et al., 1997; Tyerman et al., 1997; Davenport and Tester, 2000; Maathuis and Sanders, 2001). We next tested whether the presence of external Ca2+ has any impact on OsHKT2;1-mediated Na+ influx into plant cells. In the standard uptake buffer solution, 1 mm CaCl2 is included as a component (see “Materials and Methods”). One millimolar Ca2+ in the influx buffer caused a 30% to 32% decrease in OsHKT2;1-mediated Na+ influx into BY2 cells compared with 0.1 mm Ca2+ (Fig. 6C). Ten-day-old wild-type Nipponbare, TosWT, and oshkt2;1 rice plants were also analyzed for the effect of external Ca2+ concentrations on OsHKT2;1-dependent Na+ influx into rice roots. Approximately 46% to 48% reductions in OsHKT2;1-dependent Na+ influx into wild-type rice roots were found in response to an increase in the external Ca2+ concentration from 0.1 to 1 mm (Fig. 6D).
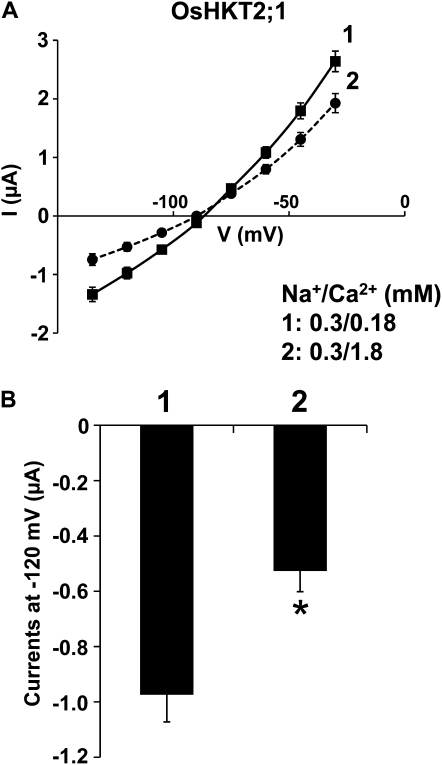
Voltage clamp experiments were performed to determine whether Ca2+ may more directly inhibit OsHKT2;1-mediated Na+ transport. OsHKT2;1-mediated currents were recorded while bathing oocytes in a 0.3 mm Na+ solution containing 0.18 or 1.8 mm Ca2+. Average current-voltage curves revealed a moderate Ca2+ inhibition of OsHKT2;1-mediated Na+ currents in Xenopus oocytes (Fig. 7A). A comparison of current amplitudes at −120 mV from OsHKT2;1-expressing oocytes exhibited an approximately 46% reduction in the OsHKT2;1-mediated currents by a 10-fold increase in the external Ca2+ concentration (P < 0.01; Fig. 7B), which was comparable to results from transgenic BY2 cells and intact rice roots (Fig. 6, C and D). Taken together, these influx analyses in tobacco BY2 cells, intact rice roots, and Xenopus oocytes show that OsHKT2;1-mediated Na+ influx into plant roots is reduced by the presence of K+ and Ca2+ (Figs. 5–7).
Figure 7.
Increasing the external Ca2+ concentration reduces OsHKT2;1-mediated Na+ currents in Xenopus oocytes. A, Average current-voltage curves from OsHKT2;1-expressing oocytes bathed in a 0.3 mm Na+ solution containing either 0.18 mm Ca2+ (solid line, no. 1) or 1.8 mm Ca2+ (dashed line, no. 2). Currents were measured using a step command with 15-mV decrements (see “Materials and Methods”). Error bars represent se (n = 13–15) from two independent experiments. B, Current amplitudes recorded at −120 mV, derived from OsHKT2;1-expressing oocytes in the presence of 0.18 mm Ca2+ (no. 1; n = 15) or 1.8 mm Ca2+ (no. 2; n = 13), which were extracted from the data sets presented in A. Error bars represent se (* P < 0.01, 0.18 mm Ca2+ versus 1.8 mm Ca2+).
DISCUSSION
Several cDNAs encoding plant HKT transporters have been identified from different plant species, and their ion selectivities have been primarily characterized in Xenopus oocytes and yeast (Schachtman and Schroeder, 1994; Rubio et al., 1995, 1999; Gassmann et al., 1996; Fairbairn et al., 2000; Uozumi et al., 2000; Horie et al., 2001; Golldack et al., 2002; Garciadeblás et al., 2003; Su et al., 2003; Haro et al., 2005; Ren et al., 2005; Takahashi et al., 2007; Jabnoune et al., 2009). These past analyses led to the findings that plant HKT transporters can be divided into two different classes based on their Na+/K+ selectivities, a more Na+- over K+-selective transport and Na+-K+ cotransport depending on ionic conditions (Rubio et al., 1995; Horie et al., 2001; Mäser et al., 2002b), with exceptions (Golldack et al., 2002). A later phylogenetic analysis of HKT transporter proteins further supported the classification of HKT transporters into two groups, which correlates with the differential Na+/K+ selectivities of HKT transporters found in heterologous systems (Mäser et al., 2002b; Platten et al., 2006).
The differential cation selectivities of HKT transporters, however, have not yet been analyzed and compared in plant cells. Our findings here reveal that OsHKT2;1 mediates Na+ influx but little Rb+ uptake into plant BY2 cells. Under identical experimental conditions, the closely related OsHKT2;2 transporter mediates Na+-K+ cotransport in plant cells, consistent with findings in heterologous systems (Horie et al., 2001; Mäser et al., 2002b), with analogous correlating findings for the wheat TaHKT2;1 transporter (Rubio et al., 1995; Gassmann et al., 1996). Furthermore, at millimolar Na+ concentrations, OsHKT2;2 (Fig. 3C)and TaHKT2;1 can transport Na+ into plant cells in the absence of external K+, as has been shown in Xenopus oocytes and yeast (Rubio et al., 1995; Gassmann et al., 1996; Horie et al., 2001). These findings show a close correlation to the cation transport selectivities of plant transporters in heterologous systems.
Are Class 2 Plant HKT Transporters Able to Mediate K+ Transport in Plant Cells?
HKT transporters identified to date can be divided into two subgroups, class 1 and class 2 HKT transporters (Mäser et al., 2002b; Platten et al., 2006). The class 1 HKT transporters, such as AtHKT1;1, OsHKT1;5 (SKC1), TmHKT1;4, TmHKT1;5, and TaHKT1;5, were demonstrated or suggested to play crucial roles in salinity resistance mediating Na+ removal from the xylem during salinity stress in different plant species (Uozumi et al., 2000; Mäser et al., 2002a; Ren et al., 2005; Sunarpi et al., 2005; Horie et al., 2006, 2009; Huang et al., 2006; Byrt et al., 2007; Davenport et al., 2007; Møller et al., 2009). In contrast, however, physiological functions and the ion selectivities of the class 2 HKT transporters have rarely been studied in plants. TaHKT2;1-antisense wheat plants showed enhanced growth and reduced Na+/K+ ratios under salinity stress and had lower sodium contents in roots, but no apparent K+ transport-related phenotypes were found in whole roots despite the findings that TaHKT2;1 showed Na+/K+ permeability and, at high Na+ concentrations, Na+-selective influx in heterologous systems (Laurie et al., 2002). The OsHKT2;1 transporter is an unusual class 2 HKT transporter, as it retains a Ser residue in the first p-loop region where a Gly residue is conserved in typical class 2 HKT transporters, which correlates with Na+ selectivity and a lower K+ permeability of OsHKT2;1 (Horie et al., 2001; Mäser et al., 2002b; Garciadeblás et al., 2003; Tholema et al., 2005). Analyses of oshkt2;1 null mutant rice plants further revealed that OsHKT2;1 did not contribute measurably to whole-root K+ influx into K+-starved rice roots, in contrast to a major contribution of OsHKT2:1 to Na+ influx (Horie et al., 2007; Fig. 6, B and D).
In this study, we analyzed the Na+/K+ transport selectivity in plant BY2 cells of OsHKT2;2, a class 2 HKT transporter with the typical four Gly residues of this subfamily (Horie et al., 2001; Mäser et al., 2002b). OsHKT2;2-expressing BY2 cells mediate Na+-stimulated Rb+ (K+) uptake (Fig. 4). Kinetic analyses show an apparent affinity of OsHKT2;2-mediated Rb+ (K+) influx into BY2 cells of approximately 0.035 mm Rb+ and a Vmax of approximately 19 nmol mg−1 protein min−1 (Table I). This is to our knowledge the first study that analyzes the Na+ and K+ (Rb+) transport activities and kinetics of a “four-Gly”-containing class 2 HKT transporter in plant cells. These findings provide evidence that class 2 plant HKT transporters are able to mediate K+ transport in plant cells, as suggested by ion selectivity analyses in eukaryotic heterologous expression systems, including the reproduced K+ permeability of OsHKT2;2 expressed in Xenopus oocytes, presented in this study (Fig. 5, B, D, F, and H).
A study of the barley HvHKT2;1 and wheat TaHKT2;1 transporters expressed in yeast has suggested that the transport activities of these two class 2 HKT transporters in yeast are variable, showing Na+ or K+ unitransport or Na+-K+ cotransport depending on proposed differences in the 5′ translation start sites of HKT proteins at nonconventional (non-ATG) translation start sites (Haro et al., 2005). More importantly, the K+ transport function of HKT transporters characterized in Xenopus oocytes and yeast were further questioned as possible artifacts and concluded not to occur in plant cells (Haro et al., 2005). The basis for these observations may be complex. However, several important limitations indicate that further analyses are needed, including that (1) the proposed non-ATG translational start of HKT proteins was not analyzed or confirmed; (2) the possibility of differential mRNA expression levels of the different 5′ untranslated region constructs in yeast appeared likely and was not tested at the mRNA level (Haro et al., 2005); (3) bidirectional extracellular cation depletion was used as a method to deduce HKT transport selectivities (Haro et al., 2005), rather than the more robust unidirectional tracer flux analyses (Epstein et al., 1963); and (4) the non-ATG translation start model has now been proposed to be unlikely by the same group (Bañuelos et al., 2008).
Notwithstanding the above limitations, the study of Haro et al. (2005) called for analysis of HKT transporter selectivities in plant cells. Our study shows that a typical class 2 HKT transporter, OsHKT2;2, mediates inward K+ (Rb+) transport, showing a clear Na+/K+ cotransport activity in plant BY2 cells (Figs. 3 and 4), correlating well with the results from voltage clamp experiments shown in Figure 5 (B, D, F, and H), which reproduced Na+-K+ transport activity found in prior heterologous system studies (Horie et al., 2001; Mäser et al., 2002b), but not correlating with predictions of Haro et al. (2005). HKT transporters are likely to have several specialized physiological roles in plants. Further studies using gene knockout plants will be required to elucidate the physiological functions of four-Gly-containing class 2 HKT transporters. Our study provides evidence that the eukaryotic heterologous systems, Xenopus oocytes and yeast, provide facile systems for primary analyses of the ion selectivities of HKT transporters and of other transporters/channels as well (Bichet et al., 2003; MacKinnon, 2004).
Selectivity of OsHKT2;1
Biophysical reversal potential analyses using OsHKT2;1 cRNA-injected oocytes incubated in the normal ND96 solution (96 mm Na+) show a small K+ permeability of OsHKT2;1 (Fig. 5, A, C, E, and G), consistent with previous findings of small reversal potential shifts upon shifting the extracellular K+ concentration (Horie et al., 2001). These findings are also consistent with data suggesting a possible small K+ permeability of the Arabidopsis ortholog AtHKT1;1, based on Escherichia coli complementation analyses (Uozumi et al., 2000). Larger K+-induced reversal potential shifts were observed in a recent study of OsHKT2;1 (Jabnoune et al., 2009). However, here we also show that the external K+-induced shifts in reversal potentials depended on the pretreatment of oocytes in low-Na+ (0.5 mm Na+) or high-Na+ (96 mm Na+) buffer (Fig. 5, C, E, and G). These preincubation buffers are expected to shift the intracellular Na+ concentrations of oocytes, in particular when oocytes express highly Na+-permeable channels/transporters like OsHKT2;1, compared with those incubated in a low-Na+ buffer (Kellenberger et al., 1998).
The small K+ permeability of OsHKT2;1 found in oocytes in this study when oocytes were preincubated in 96 mm Na+ (Fig. 5A) is comparable to the findings reported by Horie et al. (2001), but it appears to differ from the lack of clear Rb+ permeability found in OsHKT2;1-expressing BY2 cells (Fig. 4, A–C) and also from the lack of K+ uptake complementation upon OsHKT2;1 expression in yeast (Horie et al., 2001; Garciadeblás et al., 2003) and the main in vivo Na+ uptake activity of OsHKT2;1 in rice roots (Horie et al., 2007). Note, however, that the K+-to-Rb+ selectivity of HKT2 transporters is relatively high (Schachtman and Schroeder, 1994; Rubio et al., 1995; Gassmann et al., 1996; Horie et al., 2001). Therefore, Rb+ accumulation and Rb+ influx analyses using transgenic BY2 cells may overlook the relatively small K+ permeability of OsHKT2;1. Furthermore, our findings that the K+ permeability of OsHKT2;1 depends on pretreatment conditions of oocytes (Fig. 5, A, C, E, and G) and can show no clear K+ permeability in OsHKT2;1-expressing oocytes incubated in a low-Na+ buffer (Fig. 5, C, E, and G) suggest that the limited K+ permeability of OsHKT2;1 depends on ionic conditions. These data are also consistent with early findings that HKT transporters shift their selectivity depending on ion conditions (Rubio et al., 1995, 1999; Gassmann et al., 1996). These findings correlate with data suggesting that HKT transporters are multi-ion occupancy pores (Corratgé et al., 2007). Multi-ion occupancy of transporters/channels is known to cause shifts in the relative permeabilities of these proteins, as demonstrated in classical experiments (Hille, 2001).
Inhibitory Effects of External K+ and Ca2+ on OsHKT2;1-Mediated Na+ Influx into Plant Cells
Potassium is an essential macronutrient for plant growth and the most abundant cation in plant cells. In spite of the fact that K+ and Na+ are chemically similar, the majority of plants, including glycophytes, preferentially absorb and accumulate K+ to maintain growth (Flowers and Läuchli, 1983). The K+ selectivities of some plant K+ transporters are disturbed by the presence of millimolar concentrations of Na+ (Santa-María et al., 1997; Fu and Luan, 1998). OsHKT2;1-mediated Na+ transport is also inhibited by the presence of K+ in yeast cells (Garciadeblás et al., 2003). These findings motivated us to analyze OsHKT2;1-mediated Na+ influx into plant BY2 cells and also into intact rice roots in the presence or absence of external K+. External K+ significantly inhibited Na+ influx in OsHKT2;1-expressing BY2 cells and K+-starved rice roots (Fig. 6, A and B). Interestingly, however, the sensitivity of OsHKT2;1-mediated Na+ influx into rice roots to external K+ was remarkably higher than that found in BY2 cells (Fig. 6, A and B). Tracer influx analyses using rice plants indicated that 0.1 mm external K+ was sufficient to trigger large approximately 80% to 88% reductions in Na+ influx via OsHKT2;1 in intact rice roots (Fig. 6B). In contrast, 1 mm K+ was needed to inhibit approximately 51% to 55% of OsHKT2;1-mediated Na+ influx into BY2 cells (Fig. 6A). These results suggest several possibly additive mechanisms controlling the activity of OsHKT2;1 in the presence of K+: (1) a simple competition of the two similar ions Na+ and K+ at the selectivity pore of OsHKT2;1; (2) posttranslational down-regulation of OsHKT2;1 by K+ in rice roots (a likely posttranslational down-regulation of OsHKT2;1 was found in response to elevated Na+ concentrations [Horie et al., 2007]); and (3) in addition, the addition of 0.1 to 1.0 mm K+ would cause depolarization, which could contribute to the reduction in OsHKT2;1-mediated Na+ influx.
Ca2+ is also an essential divalent cation, which acts as a second messenger in diverse signal transduction pathways. In plant stress physiology, Ca2+ is also known to partially inhibit or reduce toxic Na+ influx via voltage-independent channels or nonselective cation channels that have been suggested to mediate Na+ influx, leading to salinity stress (Amtmann et al., 1997; Roberts and Tester, 1997; Tyerman et al., 1997; Buschmann et al., 2000; Davenport and Tester, 2000; Maathuis and Sanders, 2001). Classical plant physiological studies have shown that increasing external concentrations of Ca2+ dramatically reduced Na+ influx into nutrient-starved (K+-starved) barley roots (Rains and Epstein, 1967). The transcript level of the barley HvHKT2;1 gene that is a close ortholog of the OsHKT2;1 gene has been found to be up-regulated in barley roots in response to K+ starvation (Wang et al., 1998). These findings led us to analyze the effect of external Ca2+ on OsHKT2;1-mediated Na+ influx into BY2 cells. Interestingly, an increase in the Ca2+ concentration of the uptake buffer from 0.1 to 1 mm led to significant partial reductions in the OsHKT2;1-mediated Na+ influx into both BY2 cells and intact rice roots (Fig. 6, C and D). Current-voltage relationships from OsHKT2;1-expressing oocytes bathed in a solution containing 0.18 or 1.8 mm Ca2+ showed a moderate inhibitory effect of Ca2+ on the Na+ transport activity of OsHKT2;1 (Fig. 7A). Relatively little influence on the reversal potentials of OsHKT2;1-expressing oocytes in low- and high-Ca2+ solutions (Fig. 7A) and a significant 46% reduction in OsHKT2;1-mediated currents at −120 mV (P < 0.01; Fig. 7B) upon a 10-fold increase in the external Ca2+ concentration suggest that high Ca2+ concentrations can inhibit OsHKT2;1-mediated Na+ currents in oocytes. Taken together, the presented data sets reveal that not only K+ but also high concentrations of Ca2+ reduce Na+ transport by OsHKT2;1.
CONCLUSION
In conclusion, although OsHKT2;1 and OsHKT2;2, two highly homologous HKT transporters, share 93% identical cDNA sequence and 91% identical amino acid sequence (Horie et al., 2001), they exhibit differential Na+/K+ transport selectivities when expressed in tobacco BY2 cells, which correlate remarkably well with ion selectivity studies of these transporters in Xenopus oocytes and yeast. Moreover, ionic conditions affect the K+/Na+ selectivities of HKT transporters, as was found for the wheat TaHKT2;1 transporter (Rubio et al., 1995), supporting the model that HKT transporters are multi-ion pores (Gassmann et al., 1996; Corratgé et al., 2007). Furthermore, interesting inhibition and possible down-regulation of OsHKT2;1-mediated Na+ influx by K+ and Ca2+ in rice roots is shown here. The genome of japonica rice cv Nipponbare includes seven functional OsHKT genes encoding five class 1 and two class 2 HKT transporters (Garciadeblás et al., 2003). Other than OsHKT1;5 (Ren et al., 2005) and OsHKT2;1 (Horie et al., 2007), little in planta genetic mutant information exists on the physiological functions of the remaining HKT transporters in rice plants. Elucidation of the physiological roles of other HKT members in rice will be important for understanding Na+/K+ homeostasis mechanisms mediated by both class 1 and class 2 HKT transporter proteins in plants. Further studies of loss-of-function mutant plants will be required to draw a complete picture of plant HKT transporter functions.
MATERIALS AND METHODS
Transformation of BY2 Cells
Transformation of tobacco (Nicotiana tabacum ‘Bright-Yellow 2’) cells was carried out as described previously (Nakayama et al., 2000). Briefly, 4-d-old cells were coincubated with 100 μL of solution containing Agrobacterium tumefaciens strain C58 carrying the indicated DNA constructs at 25°C in the dark for 2 d. The cells were washed and then plated on modified LS medium (Nagata et al., 1981) containing 100 μg mL−1 kanamycin and 250 μg mL−1 carbenicillin. The plates were placed at 25°C in the dark for 3 to 4 weeks. Kanamycin-resistant calli were transferred onto solid LS medium and placed at 25°C in the dark for 2 weeks. A part of each callus was then transferred into liquid LS medium containing 100 μg mL−1 kanamycin and 250 μg mL−1 carbenicillin for 12 to 14 d. When the growth of each cell line became stable in liquid culture, the cells were maintained as described below.
Culture Conditions and Maintenance of Tobacco BY2 Cells
Tobacco BY2 cells were maintained in modified LS liquid medium (Nagata et al., 1981) at 25°C in the dark in an incubator shaker (Innova 4430; New Brunswick Scientific) at 130 rpm. The liquid culture medium contained (mm): 19 KNO3, 20 NH4NO3, 2.7 KH2PO4, 0.005 KI, 0.10 H3BO3, 0.10 MnSO4-5H2O, 0.03 ZnSO4-7H2O, 0.0010 Na2MoO4-2H2O, 0.00010 CuSO4-5H2O, 0.00010 CoCl2-6H2O, 3.0 CaCl2-2H2O, 1.5 MgSO4-7H2O, 0.10 Fe(III)-EDTA, 88 Suc, 0.56 myoinositol, 0.003 thiamine-HCl, and 0.00090 2,4-dichlorophenoxyacetic acid, pH 5.8, with KOH. One to 2 mL of suspension cell culture solution was transferred into 50 mL of LS liquid medium once per week. To maintain transformed BY2 cells, 100 μg mL−1 kanamycin was added in liquid medium.
Plant Materials and Growth
Japonica rice (Oryza sativa ‘Nipponbare’) was used in this study. Rice plants were prepared as described previously (Horie et al., 2007). The line numbers of Tos17-insertion rice mutants used in this study are NF1009 for oshkt2;1-1 and TosWT2;1-1 and ND3042 for oshkt2;1-2 and TosWT2;1-2, respectively (Horie et al., 2007).
Real-Time PCR Assays
To analyze the expression levels of OsHKT2;1 and OsHKT2;2 in tobacco BY2 cell lines and vector control lines, quantitative real-time PCR analyses were performed. Total RNA samples were isolated from 7-d-old suspension cells using the RNeasy Plant Mini Kit (Qiagen) followed by DNase digestion and RNA purification, and then the first-strand cDNA was reverse transcribed with a first-strand cDNA synthesis kit (GE Healthcare) at 37°C for 1 h. Specific primers were designed for OsHKT2;1 and OsHKT2;2 transcripts: for OsHKT2;1, forward (5′-GCATATTCACCCATTCTGGATTCAGT-3′) and reverse (5′-CGATGGTGATGAGGCTGGAAAGT-3′); and for OsHKT2;2, forward (5′-GCATGTTCACCCATTCTGGATCCAAC-3′) and reverse (5′-GGTGCTGAGGCCGGAAACG-3′). Amplification of the NtEF1α (forward, 5′-GCTGTGAGGGACATGCGTCAAA-3′; and reverse, 5′-GTAGTAGATATCGCGAGTACCACCA-3′) mRNA was used as an internal quantitative control. Real-time PCR was performed using a LightCycler Carousel-Based System (Roche) and LightCycler FastStart DNA MasterPLUS SYBR Green I Master Mix (Roche). PCR amplification was carried out with an initial step at 95°C for 10 min followed by 45 cycles of 10 s at 95°C and 1 min at 60°C. An amplification of target genes was monitored every cycle by SYBR Green fluorescence. Three independent replicates were performed for each line.
Ion Content Determinations
Four-day-old OsHKT2;1, OsHKT2;2, and vector control BY2 cells were pretreated with 19 mm NH4+ and 0 mm K+ for 8 h. NH4+ was added to inhibit residual K+ uptake from LS growth medium. After washing five times with uptake buffer {2 mm MES-1,3-bis[tris(hydroxymethyl-9-methylamino)]propane (BTP), pH 5.8, 1 mm CaCl2, and 0.17 m mannitol}, cells were treated with uptake buffer containing 0.1 mm Na+ and 0 mm K+ and 0.1 mm Rb+ and 0.01 mm Na+ for 0, 30, and 60 min. Then, the cells were washed with washing/uptake buffer again three times. Total protein contents were determined by the Bradford method and used to normalize ion concentration data from ICP-OES analyses (Bradford, 1976; Mendoza-Cózatl et al., 2002; Mendoza-Cózatl and Moreno-Sanchez, 2005). The rest of the cells were dried at 80°C in an oven for 2 d. Dry samples were digested in nitric acid (trace metal grade [Fisher]; Gong et al., 2003) and analyzed by ICP-OES (Perkin-Elmer Optima 3000XL; Applied Biosystems) at the Scripps Institution of Oceanography, University of California, San Diego (Gong et al., 2004).
Tracer Influx Analyses
Four-day-old OsHKT2;1, OsHKT2;2, and vector control BY2 cells were treated and washed as described in “Ion Content Determinations.” The basic influx buffer was composed of 2 mm MES-BTP, pH 5.8, 1 mm CaCl2, and 0.17 m mannitol supplemented with either nonradioactive NaCl and 22NaCl (Perkin-Elmer) or nonradioactive RbCl and 86RbCl (Perkin-Elmer) for Na+ and Rb+ influx assays, respectively (Gierth et al., 2005; Horie et al., 2007). Cells were incubated for 15 min except for time-dependent influx assays. After treatments, the cells were washed with a nonradioactive washing buffer and collected with a 0.45-μm HVHP membrane filter (Millipore). The washed cells were transferred to a scintillation vial, and radioactivity was measured with a scintillation counter (LS6500; Beckman). In K+ inhibition assays, OsHKT2;1-mediated 22Na+ influx rates were analyzed and compared after the OsHKT2;1-expressing BY2 cells and vector controls were exposed to either an influx buffer with no added K+ or with added K+ buffer, 0.1 mm external K+ or 1 mm external K+. The Ca2+ concentration was lowered to 0.1 mm from the standard (1 mm Ca2+) basic influx buffer as indicated. OsHKT2;1-mediated 22Na+ influx rates were analyzed and compared after OsHKT2;1-expressing BY2 cells and vector controls were exposed to either a buffer with decreased Ca2+ concentration (0.1 mm Ca2+) or a 1 mm Ca2+ buffer.
22Na+ tracer influx experiments using rice plants were performed as described previously (Horie et al., 2007) with minor modifications. Briefly, 10-d-old rice plants grown in 1 mm CaSO4 solution (K+ starvation) were used for influx analyses. The basic influx buffer was composed of 2 mm MES-BTP, pH 5.5, 1 mm CaCl2 supplemented with cold NaCl, and 22NaCl (Amersham Bioscience). In K+ inhibition analyses, either 0.1 mm or 1 mm KCl was added to the basic influx buffer. In Ca2+ inhibition analyses, a modified influx buffer that contains 0.1 mm CaCl2 but maintains the same other components as the basic influx buffer was prepared and used. Plants were incubated for 20 min in each influx experiment, and radioactivity from excised roots was measured as described above.
OsHKT2 Gene Expression and Electrophysiology in Xenopus Oocytes
OsHKT2;1 and OsHKT2;2 cRNAs were transcribed from linearized plasmid constructs pXβG-ev1∷OsHKT2;1 and OsHKT2;2 (Horie et al., 2001) using the mMESSAGE mMACHINE in vitro transcription kit (Ambion), and 12.5 ng of each OsHKT2 cRNA was injected into Xenopus laevis oocytes. Oocytes were kept for 1 ± 2 d at 18°C in either a normal ND96 solution including 96 mm NaCl or a modified ND96 solution composed of 92 mm Tris-HCl, 0.5 mm NaCl, 2 mm KCl, 1 mm CaCl2, 2 mm MgCl2, and 10 mm HEPES-Tris, pH 7.4. K+/Na+ selectivity experiments shown in Figure 5 were independently reproduced at the University of California, San Diego, by S.X. and D.E.B. and at Okayama University by T.H. and M.K. Two-electrode voltage clamp experiments were performed using a TEV-200 amplifier (Dagan Corporation) or a dual-electrode voltage clamp amplifier (Nihon Kohden). Detailed descriptions of electrophysiological experiments are as reported by Rubio et al. (1995) and Horie et al. (2001). Oocytes were perfused with a solution containing 6 mm MgCl2, 1.8 mm CaCl2, 10 mm MES-BTP, pH 5.5, 180 mm d-mannitol, and the indicated concentrations of Na- and K-Glu. The ionic strength of the solutions for different Na+ and K+ concentrations was kept constant by adding Tris-Glu. PCLAMP10 (Axon Instrument) or Lab-Trax-4/16 (World Precision Instruments) was used for electrophysiological measurements. A voltage ramp was generated from 0 to −150 mV at a rate of 0.15 mV ms−1. For Ca2+ inhibition analysis, OsHKT2;1 cRNA-injected oocytes were incubated in a high-Na+ solution for more than 24 h. Upon recording of OsHKT2;1-mediated currents, the perfusion solution described above was modified by replacing 1.62 mm CaCl2 with additional MgCl2, leading to the final concentrations of CaCl2 and MgCl2 of 0.18 and 7.62 mm, respectively. Voltage steps were applied from 0 to −150 mV in −15-mV decrements. Microelectrodes were filled with 3 m KCl. A 3 m KCl agar bridge was used as a bath electrode. All experiments were performed at room temperature (23°C).
Acknowledgments
We thank Dr. David Mendoza-Cozatl (University of California, San Diego) for helpful advice and discussion on tracer influx analyses using liquid-cultured plant cells. We also thank Drs. Takuya Furuichi and Takayuki Sasaki (Research Institute for Bioresources, Okayama University) for helpful discussion on voltage clamp experiments. The original heterozygous Tos17-insertion rice mutants (Horie et al., 2007) were provided by Drs. H. Hirochika and A. Miyao (National Institute of Agrobiological Sciences, Japan).
This work was supported by the U.S. Department of Energy (grant no. DOE–DE–FG02–03ER15449 to J.I.S.), the National Institutes of Health (grant no. ES010337 to J.I.S.), and a China Scholarship Council scholarship, China, at the University of California, San Diego, to X.Y.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Julian I. Schroeder (julian@biomail.ucsd.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
Open Access articles can be viewed online without a subscription.
References
- Aharon GS, Apse MP, Duan SL, Hua XJ, Blumwald E (2003) Characterization of a family of vacuolar Na+/H+ antiporters in Arabidopsis thaliana. Plant Soil 253 245–256 [Google Scholar]
- Amtmann A, Laurie S, Leigh R, Sanders D (1997) Multiple inward channels provide flexibility in Na+/K+ discrimination at the plasma membrane of barley suspension culture cells. J Exp Bot 48 481–497 [DOI] [PubMed] [Google Scholar]
- Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285 1256–1258 [DOI] [PubMed] [Google Scholar]
- Apse MP, Blumwald E (2007) Na+ transport in plants. FEBS Lett 581 2247–2254 [DOI] [PubMed] [Google Scholar]
- Bañuelos MA, Garciadeblas B, Cubero B, Rodriguez-Navarro A (2002) Inventory and functional characterization of the HAK potassium transporters of rice. Plant Physiol 130 784–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bañuelos MA, Haro R, Fraile-Escanciano A, Rodriguez-Navarro A (2008) Effects of polylinker uATGs on the function of grass HKT1 transporters expressed in yeast cells. Plant Cell Physiol 49 1128–1132 [DOI] [PubMed] [Google Scholar]
- Berthomieu P, Conejero G, Nublat A, Brackenbury WJ, Lambert C, Savio C, Uozumi N, Oiki S, Yamada K, Cellier F, et al (2003) Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J 22 2004–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichet D, Haass FA, Jan LY (2003) Merging functional studies with structures of inward-rectifier K+ channels. Nat Rev Neurosci 4 957–967 [DOI] [PubMed] [Google Scholar]
- Blumwald E, Poole RJ (1985) Na+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris. Plant Physiol 78 163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E, Poole RJ (1987) Salt tolerance in suspension-cultures of sugar-beet: induction of Na+/H+ antiport activity at the tonoplast by growth in salt. Plant Physiol 83 884–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Buschmann PH, Vaidyanathan R, Gassmann W, Schroeder JI (2000) Enhancement of Na+ uptake currents, time-dependent inward-rectifying K+ channel currents, and K+ channel transcripts by K+ starvation in wheat root cells. Plant Physiol 122 1387–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrt CS, Platten JD, Spielmeyer W, James RA, Lagudah ES, Dennis ES, Tester M, Munns R (2007) HKT1;5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiol 143 1918–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Zhou MX, Newman IA, Mendham NJ, Zhang GP, Shabala S (2007) Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct Plant Biol 34 150–162 [DOI] [PubMed] [Google Scholar]
- Clarkson DT, Hanson JB (1980) The mineral-nutrition of higher plants. Annu Rev Plant Physiol Plant Mol Biol 31 239–298 [Google Scholar]
- Corratgé C, Zimmermann S, Lambilliotte RRL, Plassard C, Marmeisse R, Thibaud JB, Lacombe B, Sentenac H (2007) Molecular and functional characterization of a Na+-K+ transporter from the Trk family in the ectomycorrhizal fungus Hebeloma cylindrosporumk. J Biol Chem 282 26057–26066 [DOI] [PubMed] [Google Scholar]
- Davenport RJ, Munoz-Mayor A, Jha D, Essah PA, Rus A, Tester M (2007) The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ 30 497–507 [DOI] [PubMed] [Google Scholar]
- Davenport RJ, Tester M (2000) A weakly voltage-dependent, nonselective cation channel mediates toxic sodium influx in wheat. Plant Physiol 122 823–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E, Elzam OE, Rains DW (1963) Resolution of dual mechanisms of potassium absorption by barley roots. Proc Natl Acad Sci USA 49 684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn DJ, Liu WH, Schachtman DP, Gomez-Gallego S, Day SR, Teasdale RD (2000) Characterisation of two distinct HKT1-like potassium transporters from Eucalyptus camaldulensis. Plant Mol Biol 43 515–525 [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Läuchli A (1983) Sodium versus potassium: substitution and compartmentation. Inorganic Plant Nutrition 15b 651–681 [Google Scholar]
- Fu HH, Luan S (1998) AtKUP1: a dual-affinity K+ transporter from Arabidopsis. Plant Cell 10 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciadeblás B, Senn ME, Banuelos MA, Rodriguez-Navarro A (2003) Sodium transport and HKT transporters: the rice model. Plant J 34 788–801 [DOI] [PubMed] [Google Scholar]
- Gassmann W, Rubio F, Schroeder JI (1996) Alkali cation selectivity of the wheat root high-affinity potassium transporter HKT1. Plant J 10 869–882 [DOI] [PubMed] [Google Scholar]
- Gaxiola RA, Rao R, Sherman A, Grisafi P, Alper SL, Fink GR (1999) The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc Natl Acad Sci USA 96 1480–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierth M, Mäser P (2007) Potassium transporters in plants: involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett 581 2348–2356 [DOI] [PubMed] [Google Scholar]
- Gierth M, Mäser P, Schroeder JI (2005) The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol 137 1105–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golldack D, Su H, Quigley F, Kamasani UR, Munoz-Garay C, Balderas E, Popova OV, Bennett J, Bohnert HJ, Pantoja O (2002) Characterization of a HKT-type transporter in rice as a general alkali cation transporter. Plant J 31 529–542 [DOI] [PubMed] [Google Scholar]
- Gong JM, Lee DA, Schroeder JI (2003) Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc Natl Acad Sci USA 100 10118–10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JM, Waner DA, Horie T, Li SL, Horie R, Abid KB, Schroeder JI (2004) Microarray-based rapid cloning of an ion accumulation deletion mutant in Arabidopsis thaliana. Proc Natl Acad Sci USA 101 15404–15409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway H, Munns R (1980) Mechanisms of salt tolerance in non-halophytes. Annu Rev Plant Physiol Plant Mol Biol 31 149–190 [Google Scholar]
- Haro R, Bañuelos MA, Senn MAE, Barrero-Gil J, Rodríguez-Navarro A (2005) HKT1 mediates sodium uniport in roots: pitfalls in the expression of HKT1 in yeast. Plant Physiol 139 1495–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B (2001) Ion Channels of Excitable Membrane. Sinauer Associates, Sunderland, MA
- Hirochika H (1997) Retrotransposons of rice: their regulation and use for genome analysis. Plant Mol Biol 35 231–240 [PubMed] [Google Scholar]
- Hirochika H (2001) Contribution of the Tos17 retrotransposon to rice functional genomics. Curr Opin Plant Biol 4 118–122 [DOI] [PubMed] [Google Scholar]
- Horie T, Costa A, Kim TH, Han MJ, Horie R, Leung HY, Miyao A, Hirochika H, An G, Schroeder JI (2007) Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J 26 3003–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Hauser F, Schroeder JI (2009) HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci 14 660–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Horie R, Chan WY, Leung HY, Schroeder JI (2006) Calcium regulation of sodium hypersensitivities of sos3 and athkt1 mutants. Plant Cell Physiol 47 622–633 [DOI] [PubMed] [Google Scholar]
- Horie T, Schroeder JI (2004) Sodium transporters in plants: diverse genes and physiological functions. Plant Physiol 136 2457–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Yoshida K, Nakayama H, Yamada K, Oiki S, Shinmyo A (2001) Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa. Plant J 27 129–138 [DOI] [PubMed] [Google Scholar]
- Huang SB, Spielmeyer W, Lagudah ES, James RA, Platten JD, Dennis ES, Munns R (2006) A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat. Plant Physiol 142 1718–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabnoune M, Espeout S, Mieulet D, Fizames C, Verdeil JL, Conejero G, Rodriguez-Navarro A, Sentenac H, Guiderdoni E, Abdelly C, et al (2009) Diversity in expression patterns and functional properties in the rice HKT transporter family. Plant Physiol 150 1955–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Gautschi I, Rossier BC, Schild L (1998) Mutations causing Liddle syndrome reduce sodium-dependent downregulation of the epithelial sodium channel in the Xenopus oocyte expression system. J Clin Invest 101 2741–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kwak JM, Uozumi N, Schroeder JI (1998) AtKUP1: an Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell 10 639–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Lucas WJ (1988) Potassium transport in roots. Adv Bot Res 15 93–178 [Google Scholar]
- Laurie S, Feeney KA, Maathuis FJM, Heard PJ, Brown SJ, Leigh RA (2002) A role for HKT1 in sodium uptake by wheat roots. Plant J 32 139–149 [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Amtmann A (1999) K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot (Lond) 84 123–133 [Google Scholar]
- Maathuis FJM, Sanders D (2001) Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol 127 1617–1625 [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R (2004) Potassium channels and the atomic basis of selective ion conduction. Biosci Rep 24 75–100 [DOI] [PubMed] [Google Scholar]
- Mäser P, Eckelman B, Vaidyanathan R, Horie T, Fairbairn DJ, Kubo M, Yamagami M, Yamaguchi K, Nishimura M, Uozumi N, et al (2002. a) Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKTI1. FEBS Lett 531 157–161 [DOI] [PubMed] [Google Scholar]
- Mäser P, Hosoo Y, Goshima S, Horie T, Eckelman B, Yamada K, Yoshida K, Bakker EP, Shinmyo A, Oiki S, et al (2002. b) Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants. Proc Natl Acad Sci USA 99 6428–6433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Cózatl D, Devars S, Loza-Tavera H, Moreno-Sanchez R (2002) Cadmium accumulation in the chloroplast of Euglena gracilis. Physiol Plant 115 276–283 [DOI] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Moreno-Sanchez R (2005) Cd2+ transport and storage in the chloroplast of Euglena gracilis. Biochim Biophys Acta 1706 88–97 [DOI] [PubMed] [Google Scholar]
- Miyao A, Tanaka K, Murata K, Sawaki H, Takeda S, Abe K, Shinozuka Y, Onosato K, Hirochika H (2003) Target site specificity of the Tos17 retrotransposon shows a preference for insertion within genes and against insertion in retrotransposon-rich regions of the genome. Plant Cell 15 1771–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller IS, Gilliham M, Jha D, Mayo GM, Roy SJ, Coates JC, Haseloff J, Tester M (2009) Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. Plant Cell 21 2163–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T, Okada K, Takebe I, Matsui C (1981) Delivery of tobacco mosaic-virus RNA into plant protoplasts mediated by reverse-phase evaporation vesicles (liposomes). Mol Gen Genet 184 161–165 [Google Scholar]
- Nakayama H, Yoshida K, Ono H, Murooka Y, Shinmyo A (2000) Ectoine, the compatible solute of Halomonas elongata, confers hyperosmotic tolerance in cultured tobacco cells. Plant Physiol 122 1239–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu XM, Bressan RA, Hasegawa PM, Pardo JM (1995) Ion homeostasis in NaCl stress environments. Plant Physiol 109 735–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platten JD, Cotsaftis O, Berthomieu P, Bohnert H, Davenport RJ, Fairbairn DJ, Horie T, Leigh RA, Lin HX, Luan S, et al (2006) Nomenclature for HKT transporters, key determinants of plant salinity tolerance. Trends Plant Sci 11 372–374 [DOI] [PubMed] [Google Scholar]
- Quintero FJ, Blatt MR (1997) A new family of KC transporters from Arabidopsis that are conserved across phyla. FEBS Lett 415 206–211 [DOI] [PubMed] [Google Scholar]
- Rains DW, Epstein E (1967) Sodium absorption by barley roots: its mediation by mechanism 2 of alkali cation transport. Plant Physiol 42 319–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren ZH, Gao JP, Li LG, Cai XL, Huang W, Chao DY, Zhu MZ, Wang ZY, Luan S, Lin HX (2005) A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet 37 1141–1146 [DOI] [PubMed] [Google Scholar]
- Rigas S, Debrosses G, Haralampidis K, Vicente-Agullo F, Feldmann KA, Grabov A, Dolan L, Hatzopoulos P (2001) TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell 13 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SK, Tester M (1997) A patch clamp study of Na+ transport in maize roots. J Exp Bot 48 431–440 [DOI] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI (1995) Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 270 1660–1663 [DOI] [PubMed] [Google Scholar]
- Rubio F, Schwarz M, Gassmann W, Schroeder JI (1999) Genetic selection of mutations in the high affinity K+ transporter HKT1 that define functions of a loop site for reduced Na+ permeability and increased Na+ tolerance. J Biol Chem 274 6839–6847 [DOI] [PubMed] [Google Scholar]
- Rus A, Baxter I, Muthukumar B, Gustin J, Lahner B, Yakubova E, Salt DE (2006) Natural variants of AtHKT1 enhance Na+ accumulation in two wild populations of Arabidopsis. PLoS Genet 2 1964–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-María GE, Rubio F, Dubcovsky J, Rodríguez-Navarro A (1997) The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell 9 2281–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI (1994) Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 370 655–658 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Ward JM, Gassmann W (1994) Perspectives on the physiology and structure of inward-rectifying K+ channels in higher plants: biophysical implications for K+ uptake. Annu Rev Biophys Biomol Struct 23 441–471 [DOI] [PubMed] [Google Scholar]
- Shabala S (2000) Ionic and osmotic components of salt stress specifically modulate net ion fluxes from bean leaf mesophyll. Plant Cell Environ 23 825–837 [Google Scholar]
- Shi HZ, Ishitani M, Kim CS, Zhu JK (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97 6896–6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HZ, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14 465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Balderas E, Vera-Estrella R, Golldack D, Quigley F, Zhao CS, Pantoja O, Bohnert JH (2003) Expression of the cation transporter McHKT1 in a halophyte. Plant Mol Biol 52 967–980 [DOI] [PubMed] [Google Scholar]
- Sunarpi, Horie T, Motoda J, Kubo M, Yang H, Yoda K, Horie R, Chan WY, Leung HY, Hattori K, et al (2005) Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J 44 928–938 [DOI] [PubMed] [Google Scholar]
- Sze H, Li XH, Palmgren MG (1999) Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell 11 677–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R, Liu S, Takano T (2007) Cloning and functional comparison of a high-affinity K+ transporter gene PhaHKT1 of salt-tolerant and salt-sensitive reed plants. J Exp Bot 58 4387–4395 [DOI] [PubMed] [Google Scholar]
- Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot (Lond) 91 503–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholema N, Vor der Bruggen M, Maser P, Nakamura T, Schroeder JI, Kobayashi H, Uozumi N, Bakker EP (2005) All four putative selectivity filter glycine residues in KtrB are essential for high affinity and selective K+ uptake by the KtrAB system from Vibrio alginolyticus. J Biol Chem 280 41146–41154 [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Skerrett M, Garrill A, Findlay GP, Leigh RA (1997) Pathways for the permeation of Na+ and Cl− into protoplasts derived from the cortex of wheat roots. J Exp Bot 48 459–480 [DOI] [PubMed] [Google Scholar]
- Uozumi N, Kim EJ, Rubio F, Yamaguchi T, Muto S, Tsuboi A, Bakker EP, Nakamura T, Schroeder JI (2000) The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiol 122 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TB, Gassmann W, Rubio F, Schroeder JI, Glass ADM (1998) Rapid up-regulation of HKT1, a high-affinity potassium transporter gene, in roots of barley and wheat following withdrawal of potassium. Plant Physiol 118 651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Hirschi KD, Sze H (2003) Plants pass the salt. Trends Plant Sci 8 200–201 [DOI] [PubMed] [Google Scholar]
- Yokoi S, Quintero FJ, Cubero B, Ruiz MT, Bressan RA, Hasegawa PM, Pardo JM (2002) Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J 30 529–539 [DOI] [PubMed] [Google Scholar]
- Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6 66–71 [DOI] [PubMed] [Google Scholar]