Abstract
While interspecific variation in the temperature response of photosynthesis is well documented, the underlying physiological mechanisms remain unknown. Moreover, mechanisms related to species-dependent differences in photosynthetic temperature acclimation are unclear. We compared photosynthetic temperature acclimation in 11 crop species differing in their cold tolerance, which were grown at 15°C or 30°C. Cold-tolerant species exhibited a large decrease in optimum temperature for the photosynthetic rate at 360 μL L−1 CO2 concentration [Opt (A360)] when growth temperature decreased from 30°C to 15°C, whereas cold-sensitive species were less plastic in Opt (A360). Analysis using the C3 photosynthesis model shows that the limiting step of A360 at the optimum temperature differed between cold-tolerant and cold-sensitive species; ribulose 1,5-bisphosphate carboxylation rate was limiting in cold-tolerant species, while ribulose 1,5-bisphosphate regeneration rate was limiting in cold-sensitive species. Alterations in parameters related to photosynthetic temperature acclimation, including the limiting step of A360, leaf nitrogen, and Rubisco contents, were more plastic to growth temperature in cold-tolerant species than in cold-sensitive species. These plastic alterations contributed to the noted growth temperature-dependent changes in Opt (A360) in cold-tolerant species. Consequently, cold-tolerant species were able to maintain high A360 at 15°C or 30°C, whereas cold-sensitive species were not. We conclude that differences in the plasticity of photosynthetic parameters with respect to growth temperature were responsible for the noted interspecific differences in photosynthetic temperature acclimation between cold-tolerant and cold-sensitive species.
The temperature dependence of leaf photosynthetic rate shows considerable variation between plant species and with growth temperature (Berry and Björkman, 1980; Cunningham and Read, 2002; Hikosaka et al., 2006). Plants native to low-temperature environments and those grown at low temperatures generally exhibit higher photosynthetic rates at low temperatures and lower optimum temperatures, compared with plants native to high-temperature environments and those grown at high temperatures (Mooney and Billings, 1961; Slatyer, 1977; Berry and Björkman, 1980; Sage, 2002; Salvucci and Crafts-Brandner, 2004b). For example, the optimum temperature for photosynthesis differs between temperate evergreen species and tropical evergreen species (Hill et al., 1988; Read, 1990; Cunningham and Read, 2002). Such differences have been observed even among ecotypes of the same species (Björkman et al., 1975; Pearcy, 1977; Slatyer, 1977).
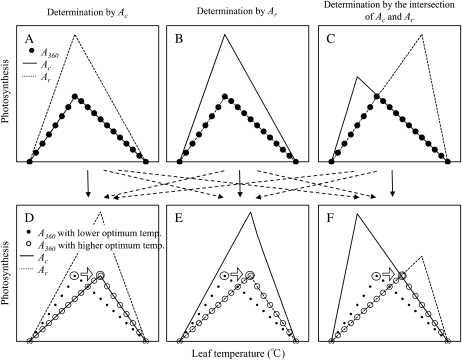
Temperature dependence of the photosynthetic rate has been analyzed using the biochemical model proposed by Farquhar et al. (1980). This model assumes that the photosynthetic rate (A) is limited by either ribulose 1,5-bisphosphate (RuBP) carboxylation (Ac) or RuBP regeneration (Ar). The optimum temperature for photosynthetic rate in C3 plants is thus potentially determined by (1) the temperature dependence of Ac, (2) the temperature dependence of Ar, or (3) both, at the colimitation point of Ac and Ar (Fig. 1; Farquhar and von Caemmerer, 1982; Hikosaka et al., 2006).
Figure 1.
A scheme illustrating the shift in the optimum temperature for photosynthesis depending on growth temperature. Based on the C3 photosynthesis model, the A360 (white and black circles) is limited by Ac (solid line) or Ar (broken line). The optimum temperature for the photosynthetic rate is potentially determined by temperature dependence of Ac (A), temperature dependence of Ar (B), or the intersection of the temperature dependences of Ac and Ar (C). When the optimum temperature for the photosynthetic rate shifts to a higher temperature, there are also three possibilities determining the optimum temperature: temperature dependence of Ac (D), temperature dependence of Ar (E), or the intersection of the temperature dependences of Ac and Ar (F). Especially in the case that the optimum temperature is determined by the intersection of the temperature dependences of Ac and Ar, the optimum temperature can shift by changes in the balance between Ac and Ar even when the optimum temperatures for these two partial reactions do not change.
In many cases, the photosynthetic rate around the optimum temperature is limited by Ac, and thus the temperature dependence of Ac determines the optimum temperature for the photosynthetic rate (Hikosaka et al., 1999, 2006; Yamori et al., 2005, 2006a, 2006b, 2008; Sage and Kubien, 2007; Sage et al., 2008). As the temperature increases above the optimum, Ac is decreased by increases in photorespiration (Berry and Björkman, 1980; Jordan and Ogren, 1984; von Caemmerer, 2000). Furthermore, it has been suggested that the heat-induced deactivation of Rubisco is involved in the decrease in Ac at high temperature (Law and Crafts-Brandner, 1999; Crafts-Brandner and Salvucci, 2000; Salvucci and Crafts-Brandner, 2004a; Yamori et al., 2006b). Numerous previous studies have shown changes in the temperature dependence of Ac with growth temperature (Hikosaka et al., 1999; Bunce, 2000; Yamori et al., 2005). Also, the temperature sensitivity of Rubisco deactivation may differ between plant species (Salvucci and Crafts-Brandner, 2004b) and with growth temperature (Yamori et al., 2006b), which may explain variation in the optimum temperature for photosynthesis (Fig. 1, A and D).
Ar is more responsive to temperature than Ac and often limits photosynthesis at low temperatures (Hikosaka et al., 1999, 2006; Sage and Kubien, 2007; Sage et al., 2008). Recently, several researchers indicated that Ar limits the photosynthetic rate at high temperature (Schrader et al., 2004; Wise et al., 2004; Cen and Sage, 2005; Makino and Sage, 2007). They suggested that the deactivation of Rubisco at high temperatures is not the cause of decreased Ac but a result of limitation by Ar. However, it remains unclear whether limitation by Ar is involved in the variation in the optimum temperature for the photosynthetic rate (Fig. 1, B and E).
A shift in the optimum temperature for photosynthesis can result from changes in the balance between Ar and Ac, even when the optimum temperatures for these two partial reactions do not change (Fig. 1, C and F; Farquhar and von Caemmerer, 1982). The balance between Ar and Ac has been shown to change depending on growth temperature (Hikosaka et al., 1999; Hikosaka, 2005; Onoda et al., 2005a; Yamori et al., 2005) and often brings about a shift in the colimitation temperature of Ar and Ac. Furthermore, recent studies have shown that plasticity in this balance differs among species or ecotypes (Onoda et al., 2005b; Atkin et al., 2006; Ishikawa et al., 2007). Plasticity in this balance could explain interspecific variation in the plasticity of photosynthetic temperature dependence (Farquhar and von Caemmerer, 1982; Hikosaka et al., 2006), although there has been no evidence in the previous studies that the optimum temperature for photosynthesis occurs at the colimitation point of Ar and Ac.
Temperature tolerance differs between species and, with growth temperature, even within species from the same functional group (Long and Woodward, 1989). Bunce (2000) indicated that the temperature dependences of Ar and Ac to growth temperature were different between species from cool and warm climates and that the balance between Ar and Ac was independent of growth temperature for a given plant species. However, it was not clarified what limited the photosynthetic rate or what parameters were important in temperature acclimation of photosynthesis. Recently, we reported that the extent of temperature homeostasis of leaf respiration and photosynthesis, which is assessed as a ratio of rates measured at their respective growth temperatures, differed depending on the extent of the cold tolerance of the species (Yamori et al., 2009b). Therefore, comparisons of several species with different cold tolerances would provide a new insight into interspecific variation of photosynthetic temperature acclimation and their underlying mechanisms. In this study, we selected 11 herbaceous crop species that differ in their cold tolerance (Yamori et al., 2009b) and grew them at two contrasting temperatures, conducting gas-exchange analyses based on the C3 photosynthesis model (Farquhar et al., 1980). Based on these results, we addressed the following key questions. (1) Does the plasticity in photosynthetic temperature acclimation differ between cold-sensitive and cold-tolerant species? (2) Does the limiting step of photosynthesis at several leaf temperatures differ between plant species and with growth temperature? (3) What determines the optimum temperature for the photosynthetic rate among Ac, Ar, and the intersection of the temperature dependences of Ac and Ar?
RESULTS
Temperature Dependence of Photosynthesis
Temperature dependences of dark respiration (Rd) and photosynthesis (A) at high light and 360 μL L−1 CO2 (A360) differed between plant species and with growth temperature (Supplemental Fig. S1). When plants were grown at 15°C, the mean optimum temperature for A360 [Opt (A360)] was significantly lower in cold-tolerant species (22.3°C ± 2.2°C) than in cold-sensitive species (28.3°C ± 2.4°C; Table I). Conversely, when plants were grown at 30°C, the mean Opt (A360) was similar between cold-tolerant species (28.4°C ± 1.9°C) and cold-sensitive species (31.2°C ± 2.2°C). All species showed an increase in Opt (A360) with increasing growth temperature, except for Vicia faba (Supplemental Table S1). Cold-tolerant species were more responsive to the growth temperatures than cold-sensitive species (Table I).
Table I.
Opt (A360), Opt (Ac), Opt (Ar), Jmax/Vcmax, and Ci
The data shown are mean values for each species (see Supplemental Table S1 for each species). A repeated-measures ANOVA was used to test for statistical differences. P values from repeated-measures ANOVA are shown for differences between growth temperatures and between plant types and for interactions. NS, Not significant.
| Species or Variable | Opt (A360) | Opt (Ac) | Opt (Ar) | Jmax/Vcmax | Ci (25°C) |
|---|---|---|---|---|---|
| °C | °C | °C | μL L−1 | ||
| Cold-sensitive species | |||||
| 15°C plant | 28.3 ± 2.4 | 27.6 ± 3.3 | 28.7 ± 1.8 | 1.48 ± 0.30 | 277.2 ± 26.9 |
| 30°C plant | 31.2 ± 2.2 | 32.2 ± 3.1 | 32.7 ± 1.1 | 1.40 ± 0.21 | 294.8 ± 21.8 |
| Cold-tolerant species | |||||
| 15°C plant | 22.3 ± 2.2 | 22.6 ± 2.3 | 26.8 ± 1.9 | 1.95 ± 0.16 | 281.5 ± 29.2 |
| 30°C plant | 28.4 ± 1.9 | 28.1 ± 1.5 | 30.9 ± 1.3 | 1.57 ± 0.16 | 303.1 ± 18.5 |
| P from repeated-measures ANOVA | |||||
| Cold tolerance | <0.001 | <0.001 | <0.01 | <0.001 | NS |
| Growth temperature | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Interaction |
<0.01 |
<0.01 |
NS |
<0.01 |
NS |
The temperature dependences of Ac and Ar also differed between plant species and with growth temperature (Supplemental Figs. S1 and S2). When plants were grown at 15°C, the mean Opt (Ac) was lower in cold-tolerant species (22.6°C ± 2.3°C) than in cold-sensitive species (27.6°C ± 3.3°C; Table I). When plants were grown at 30°C, the mean Opt (Ac) was also lower in cold-tolerant species (28.1°C ± 1.5°C) than in cold-sensitive species (32.2°C ± 3.1°C). When plants were grown at 15°C, the mean Opt (Ar) was lower in cold-tolerant species (26.8°C ± 1.9°C) than in cold-sensitive species (28.7°C ± 1.8°C; Table I). When plants were grown at 30°C, the mean Opt (Ar) was also slightly lower in cold-tolerant species (30.9°C ± 1.3°C) than in cold-sensitive species (32.7°C ± 1.1°C).
We analyzed the ratio of the chloroplast electron transport rate to the maximum RuBP carboxylation rate (Jmax/Vcmax) at 25°C, which is a measure of the balance between RuBP regeneration and carboxylation (Table I; Supplemental Table S1). Although the Jmax/Vcmax ratio varied depending on measurement temperature, the general trends were not affected by the measurement temperature; the correlation coefficients (r) were 0.77, 0.82, and 0.97 for the relationships between 15°C and 30°C, between 15°C and 25°C, and between 25°C and 30°C, respectively. In cold-sensitive species, the mean Jmax/Vcmax ratio was not significantly affected by growth temperature, whereas in cold-tolerant species, the mean Jmax/Vcmax ratio increased at low growth temperature. The intercellular CO2 concentration (Ci) at 25°C was less in 15°C plants than in 30°C plants but was independent of cold tolerance (Table I; for temperature dependence of Ci, see Supplemental Fig. S1).
Limiting Step of the Temperature Dependence of A360
The limiting step of A360 was analyzed based on the CO2 dependence of the photosynthetic rate. Figure 2 shows three typical examples of the limiting step of A360 obtained in this study: Secale cereale grown at 15°C and Cucumis sativus and Nicotiana tabacum grown at 30°C (for each species, see Supplemental Fig. S2). Figure 2, A to C, show the ratio of A360 to A200. Dotted lines denote the ratio of the Ac at 360 to the Ac at 200 μL L−1 CO2 concentration (Ac360/Ac200 ratio), and continuous lines denote the ratio of the Ar at 360 to the Ar at 200 μL L−1 CO2 concentration (Ar360/Ar200; see “Materials and Methods”). Similarly, the ratios of A1500 to A360 are shown in Figure 2, D to F. We further analyzed whether of Ac or Ar limited A360 using obtained parameter values, based on the C3 photosynthesis model (Fig. 2, G–I; see “Materials and Methods”). We regarded that Ar (or Ac) limited photosynthesis if the difference between Ar (or Ac) and A360 was smaller than 5%.
Figure 2.
Three examples of limiting steps of the A360. We classified plants into three groups with respect to the limiting step of A360: type 1, A360 is limited by Ac at all temperatures in S. cereale grown at 15°C (A, D, and G); type 2, A360 is limited by both Ac and Ar in C. sativus grown at 30°C (B, E, and H); type 3, A360 is limited by Ar at all temperatures in N. tabacum grown at 30°C (C, F, and I). Black circles with bars denote the mean measured photosynthetic rates with sd. The ratios of A360 to A200 (A–C) and A1500 to A360 (D–F) are shown. Lines denote the ratio of the photosynthetic rate assuming the limitation by Ac (dotted lines; see Eq. 2 in Supplemental Appendix S1) or the limitation by Ar (continuous lines; see Eq. 3 in Supplemental Appendix S1). The limiting step of A360 was also analyzed by the model of Farquhar et al. (1980) with the obtained parameters (G–I).
In S. cereale grown at 15°C, the A360/A200 ratio was very close to the dotted line (Ac360/Ac200) at all leaf temperatures (Fig. 2A), whereas the A1500/A360 ratio was greater than the continuous line (Ar1500/Ac360) at all leaf temperatures (Fig. 2D). This indicates that in S. cereale grown at 15°C, A360 was solely limited by Ac at all leaf temperatures (Fig. 2G). In C. sativus at 30°C, A360 was colimited by Ac and Ar at low temperature below 25°C, whereas above 25°C, it was limited by Ar (Fig. 2H). In N. tabacum grown at 30°C, A360 was solely limited by Ar at all leaf temperatures (Fig. 2I). Since the A1500/A360 ratio was within 1.0 ± 0.1 at 10°C to 15°C, A360 was limited by inorganic phosphate (Pi) regeneration in N. tabacum grown at 30°C. We classified plants into three groups with respect to the limiting step of A360: type 1, A360 is limited by Ac at any temperature; type 2, A360 is colimited by both Ac and Ar or the limiting step was different depending on measurement temperature; and type 3, A360 is limited by Ar at any temperature (Fig. 2).
Figure 3 summarizes the differences in the limiting steps of A360 depending on plant species and growth temperature. In cold-tolerant species, the limiting step of A360 varied with growth temperature. Most of the cold-tolerant species grown at 15°C belonged to type 1, whereas all the cold-tolerant species grown at 30°C belonged to type 2. On the other hand, in most of the cold-sensitive species, the limiting step of A360 was independent of growth temperature. In most of the cold-sensitive species, A360 was limited by Ar across a broad temperature range, irrespective of growth temperatures (type 2 or 3). It is obvious that in plants with a high Jmax/Vcmax ratio (i.e. cold-tolerant species grown at low temperature), the limitation of A360 by Ar was alleviated. In N. tabacum and Solanum lycopersicum grown at 30°C, A360 was limited by Pi regeneration capacity at temperatures below 15°C.
Figure 3.
Summary of differences in the limiting step of the temperature dependence of A360 by Ac or Ar by plant species and growth temperature. This figure was summarized from Figure 2 and Supplemental Figure S2. The Jmax/Vcmax ratio at 25°C were adopted from Supplemental Table S1. We classified plants into three groups with respect to the limiting step of A360: type 1, A360 is limited by Ac at any temperature; type 2, A360 is limited by both Ac and Ar; type 3, A360 is limited by Ar at any temperature. White stars indicate the optimum temperature for A360. White crosses indicate that A360 was limited by Pi regeneration capacity.
Both in cold-sensitive species grown at 15°C and cold-tolerant species grown at 30°C, A360 at the growth temperature was colimited by both Ac and Ar. Conversely, A360 at the growth temperature was limited by Ar in two cold-sensitive species (N. tabacum and Oryza sativa) grown at 30°C and by Ac in most of the cold-tolerant species grown at 15°C.
Determination of the Opt (A360)
Opt (A360) strongly correlated both with Opt (Ac) and with Opt (Ar) (Fig. 4, A–C). To judge whether Ar or Ac limits photosynthetic rate at Opt (A360), we calculated Ar and Ac at Opt (A360). A360 at Opt (A360) was correlated both with Ac360 and with Ar360 at Opt (A360) in both cold-sensitive and cold-tolerant species (Fig. 4, D and E). In cold-sensitive species, A360 was lower than Ac360 but similar to Ar360 at Opt (A360) (Fig. 4, E and F). On the other hand, in cold-tolerant species, A360 was lower than Ar360 but similar to Ac360 at Opt (A360) (Fig. 4, D and F). These results indicate that photosynthetic rate at optimum temperature was limited by Ar in cold-sensitive species but by Ac in cold-tolerant species; thus, the determinant of optimum temperature of photosynthesis differed between cold-tolerant and cold-sensitive species.
Figure 4.
Relationships between Opt (A360) and Opt (Ac) (A), between Opt (A360) and Opt (Ar) (B), between Opt (Ac) and Opt (Ar) (C), between A360 at the optimum temperature and Ac360 at Opt (A360) (D), between A360 at the optimum temperature and Ar360 at Opt (A360) (E), and between Ar360 at Opt (A360) and Ac360 at Opt (A360) (F). Cold-sensitive species grown at 30°C (white circles), cold-sensitive species grown at 15°C (white squares), cold-tolerant species grown at 30°C (black circles), and cold-tolerant species grown at 15°C (black squares) are shown. The correlation lines are shown as solid lines for cold-sensitive species and as dashed lines for cold-tolerant species. Values are means; n = 3 to 5.
Syndrome of the Temperature Acclimation of Photosynthesis
In this study, growth temperature caused changes in various physiological characteristics, but the extent of plasticity differed depending on plant species and physiological characteristics. Here, we applied a principal component analysis to the parameters obtained in this study and our previous study (Yamori et al., 2009b). We used differences in Opt (A360) depending on growth temperature [ΔOpt (A360)], the ratio of Jmax/Vcmax at 25°C for 15°C plants to that for 30°C plants (Jmax/Vcmax ratio at 25°C), the ratio of leaf mass per area (LMA) for 15°C plants to that for 30°C plants (LMA), the ratio of nitrogen content per unit area (Narea), the ratio of Rubisco content (Rubisco content), the ratio of A360 at 15°C for 15°C plants to that at 30°C for 30°C plants (A360 at the growth temperature), the ratio of the maximum catalytic turnover rate of Rubisco (kcat) activity (Rubisco kcat at the growth temperature), the ratio of Rd (Rd at the growth temperature), and the ratio of NAD-malic enzyme (ME) activity (NAD-ME at the growth temperature). The first (axis 1) and second (axis 2) axes explained 62.0% and 16.2% of the total variation, respectively (Table II). Eight out of nine parameters were significantly correlated with axis 1, with the relationship between Rubisco kcat at the growth temperature and axis 1 being marginally significant (P < 0.1). Axis 2 did not correlate with most parameters except for Rubisco kcat at the growth temperature. Thus, axis 2 reflects mainly Rubisco kcat at the growth temperature, and the trend of interspecific variation in Rubisco kcat at the growth temperature was somewhat different from that in other parameters.
Table II.
Correlation coefficients and probabilities for plant attributes linked to the first principal components for photosynthetic parameters concerned with temperature acclimation
Variables taken into account are as follows: the differences in Opt (A360) depending on growth temperature [ΔOpt (A360)], the ratio of Jmax/Vcmax at 25°C for 15°C plants to that at 25°C for 30°C plants (Jmax/Vcmax ratio at 25°C), the ratio of leaf mass per area for 15°C plants to that for 30°C plants (LMA), the ratio of nitrogen content per unit area for 15°C plants to that for 30°C plants (Narea), the ratio of Rubisco content for 15°C plants to that for 30°C plants (Rubisco content), the ratio of A360 at 15°C for 15°C plants to that at 30°C for 30°C plants (A360 at the growth temperature), the ratio of Rubisco kcat activity at 15°C for 15°C plants to that at 30°C for 30°C plants (Rubisco kcat activity at the growth temperature), the ratio of Rd at 15°C for 15°C plants to that at 30°C for 30°C plants (Rd at the growth temperature), and the ratio of NAD-ME activity at 15°C for 15°C plants to that at 30°C for 30°C plants (NAD-ME activity at the growth temperature). Differences between the mean data for each species were regressed against the scores of Axis 1 and Axis 2. + P < 0.10, * P < 0.05, ** P < 0.01.
| Parameter | Axis 1 | Axis 2 | Jmax/Vcmax | LMA | Narea | A360 | Rubisco | kcat | Rd | NAD-ME | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔOpt (A360) | −0.73 | * | 0.57 | + | 0.79 | * | 0.57 | + | 0.53 | + | 0.53 | + | 0.73 | * | 0.00 | 0.64 | * | 0.30 | ||
| Jmax/Vcmax ratio at 25°C | −0.82 | ** | 0.19 | 0.85 | ** | 0.53 | + | 0.46 | 0.68 | * | 0.39 | 0.47 | 0.59 | + | ||||||
| LMA | −0.88 | ** | −0.08 | 0.63 | * | 0.50 | 0.79 | * | 0.57 | + | 0.40 | 0.86 | ** | |||||||
| Narea | −0.69 | * | 0.38 | 0.42 | 0.75 | * | 0.08 | 0.42 | 0.48 | |||||||||||
| A360 at growth temperature | −0.82 | ** | −0.17 | 0.70 | * | 0.58 | + | 0.93 | ** | 0.66 | * | |||||||||
| Rubisco content | −0.91 | ** | 0.23 | 0.29 | 0.67 | * | 0.70 | * | ||||||||||||
| kcat at growth temperature | −0.57 | + | −0.80 | * | 0.39 | 0.85 | ** | |||||||||||||
| Rd at growth temperature | −0.77 | * | 0.04 | 0.51 | ||||||||||||||||
| NAD-ME at growth temperature | −0.84 | ** | −0.48 | |||||||||||||||||
| Contribution (%) |
62.0 |
16.2 |
||||||||||||||||||
Correlations between the parameters were significant and positive in many cases (Table II). In particular, ΔOpt (A360) was strongly correlated with Jmax/Vcmax ratio at 25°C (Fig. 5). Both ΔOpt (A360) and Jmax/Vcmax ratio at 25°C were strongly correlated with Rubisco content. Species with a greater ΔOpt (A360) tended to maintain A360 and Rd at the growth temperature. Thus, species with a greater ΔOpt (A360) tended to have greater change in Jmax/Vcmax ratio, LMA, Narea, Rubisco content, Rubisco kcat, and NAD-ME and consequently more stable A360 and Rd at the growth temperature (greater ability of temperature homeostasis).
Figure 5.
Relationships between ΔOpt (A360) and Jmax/Vcmax ratio at 25°C (A), between ΔOpt (A360) and A360 at the growth temperature (B), between ΔOpt (A360) and Rd at the growth temperature (C), between Rubisco contents and ΔOpt (A360) (D), and between Rubisco contents and Jmax/Vcmax ratio at 25°C (E). ΔOpt (A360) is the difference in the optimum temperature for the Opt (A360) depending on growth temperature, A360 (or Rd) at growth temperature is the ratio of A360 (or Rd) measured at 15°C in 15°C plants to that measured at 30°C in 30°C plants, Jmax/Vcmax ratio at 25°C is the ratio of Jmax/Vcmax for 15°C plants to that for 30°C plants at 25°C, and Rubisco content is the ratio of Rubisco content for 15°C plants to that for 30°C plants. Cold-sensitive species are C. sativus (white circles), N. tabacum (white triangles), O. sativa (white squares), and S. lycopersicum (white diamonds); cold-tolerant species are S. cereale (black circles), Solanum tuberosum (gray circles), S. oleracea (gray squares), T. aestivum (spring; black squares), T. aestivum (winter; black diamonds), Triticosecale (black triangles), and V. faba (gray triangles). The correlation lines are shown. Values are means; n = 3 to 5.
DISCUSSION
Interspecific Variation in Temperature Acclimation of Photosynthesis
We found significant interspecific variation in photosynthetic temperature acclimation depending on the extent of cold tolerance (Table I; Supplemental Table S1). Inherent ability for photosynthetic acclimation to the high temperature was similar between cold-sensitive and cold-tolerant species, as they all had similar Opt (A360). By contrast, when plants were grown at low temperature, cold-tolerant species exhibited a greater ability to shift Opt (A360) to lower temperatures than cold-sensitive species. Only S. lycopersicum, a cold-sensitive species, showed a large extent of flexibility in its temperature acclimation of photosynthesis. S. lycopersicum is relatively tolerant of low temperature for a cold-sensitive species (Yamori et al., 2009b), but variation in cold tolerance has been reported for a given species (Brüggemann and Linger, 1994; Saruyama and Tanida, 1995; Yu et al., 2002).
Growth temperature-dependent changes in optimum photosynthetic temperature were greater in plants native to desert habitats than in plants native to coastal habitats (Björkman et al., 1975; Pearcy, 1977; Mooney et al., 1978) and were greater in temperate evergreen species than in tropical evergreen species (Hill et al., 1988; Read, 1990; Cunningham and Read, 2002). They suggested that these differences in the phenotypic plasticity were attributed to the extent of the daily and seasonal temperature variations. However, our study clearly shows that the inherent ability of photosynthetic temperature acclimation differed depending on the cold tolerance. Cold-tolerant species were not only able to survive stressful low temperature conditions (i.e. chilling and freezing; Thomashow, 1998, 1999) but were also able to photosynthetically acclimate to low growth temperatures, allowing the plants to perform efficient photosynthesis and maintain their growth.
In this study, we selected two growth temperatures, 15°C/10°C and 30°C/25°C. It may be that cold-sensitive species have a greater capacity for photosynthetic acclimation to growth temperatures higher than 30°C/25°C compared with cold-tolerant species. Further studies are necessary to reveal differences in the inherent capacity for photosynthetic temperature acclimation to high temperatures between cold-sensitive and cold-tolerant species.
What Determines the Temperature Dependence of Photosynthesis?
The limiting step of A360 differed depending on plant species and growth temperature (Figs. 2 and 3; Supplemental Fig. S1) and could be classified into three groups: type 1, A360 is limited by Ac at all temperatures; type 2, A360 is colimited by Ac and Ar or the limiting step changes depending on measurement temperature; and type 3, A360 is limited by Ar at all temperatures (Fig. 3). It has been reported that the Ci affects the temperature dependence of A360 (Yamori et al., 2006a). Although Ci was slightly lower in 15°C plants than in 30°C plants, the differences in Ci between plant species and between growth temperatures were generally small (Table I; Supplemental Fig. S1). Differences in the limiting step of A360 depending on plant species and growth temperature were attributable to differences in the Jmax/Vcmax ratio, which may be a result of different nitrogen partitioning within the photosynthetic apparatus. Cold-tolerant species grown at 15°C had much higher Jmax/Vcmax ratio than cold-sensitive species grown at either 30°C or 15°C (Table I; Fig. 3; Supplemental Table S1), suggesting that they invested more nitrogen in RuBP regeneration processes (electron transport, ATP synthase, and Calvin cycle except for Rubisco) than in Rubisco. As a result, in cold-tolerant species grown at 15°C, A360 tended to be limited by Ac at all temperatures, with Opt (A360) determined by its temperature dependence. Additionally, in cold-tolerant species grown at high temperatures, Opt (A360) was also determined by the temperature dependence of Ac. However, in cold-sensitive species, A360 tended to be limited by Ar at all temperatures, with Opt (A360) determined by its temperature dependence. In cold-tolerant species, Opt (Ac) greatly altered depending on growth temperature, whereas in cold-sensitive species, Opt (Ar) altered only slightly. Therefore, the differences in the limiting step of A360 at the optimum temperature and the plasticity in parameters with respect to growth temperature caused interspecific differences in Opt (A360).
It has been suggested that the shift in Opt (A360) with growth temperature is determined by a shift in the intersection between the temperature dependences of Ac and Ar (Fig. 1, C and F; Farquhar and von Caemmerer, 1982). Although the intersection of Ac and Ar was similar to Opt (A360) in some plant species (Figs. 3 and 4), there was little evidence that the intersection of Ac and Ar determined Opt (A360) at both growth temperatures or that the limiting step of A360 at the optimum drastically altered depending on growth temperature (e.g. from Ac to Ar and vice versa). Thus, we conclude that alterations in nitrogen partitioning (i.e. Jmax/Vcmax ratio) did not contribute to the shift of the Opt (A360) with growth temperature in this study.
Both Ac and Ar have been suggested to limit photosynthetic rate at high temperature; one study indicated that heat-induced Rubisco deactivation limits photosynthesis at high temperature (Salvucci and Crafts-Brandner, 2004a), while another indicated that electron transport limits photosynthesis (Sage and Kubien, 2007). If the limiting step of photosynthesis at a certain temperature were identical among species, every species would have a similar CO2 dependence of photosynthetic rate. In this study, however, CO2 dependence differed among species (Fig. 2; Supplemental Fig. S2), suggesting that the limiting step of photosynthesis differs between species, even at high temperatures. Interestingly, our observation that the limiting step differs between cold-sensitive and cold-tolerant species may partly explain the different conclusions between the above-mentioned studies. Previous studies showed that decreases in the photosynthetic rate at high temperature were determined by Ac in cold-tolerant species, such as Spinacia oleracea (Weis, 1981; Yamori et al., 2005, 2006a, 2006b, 2008), Triticum aestivum (Kobza and Edwards, 1987; Law and Crafts-Brandner, 1999), and Deschampsia antarctica (Salvucci and Crafts-Brandner, 2004b), and by Ar in cold-sensitive species, such as Gossypium barbadense (Schrader et al., 2004; Wise et al., 2004), Ipomoea batatas (Cen and Sage, 2005), and O. sativa (Makino and Sage, 2007). Salvucci and Crafts-Brandner (2004b) showed that the optimum temperature for Rubisco activase activity was 10°C lower in plants from cold regions (D. antarctica, Lysipomia pumila, and S. oleracea) than in plants from warm regions (Larrea tridentate, N. tabacum, Simmondsia chinensis, and Gossypium hirsutum). These facts may suggest a generality that the limiting step of photosynthesis at high temperature differs between cold-sensitive and cold-tolerant species.
In this study, we used Rubisco kinetic parameters reported by Bernacchi et al. (2001); however, the possibility that Rubisco kinetic parameters are species specific should be borne in mind. Then, we performed sensitivity analyses of the limiting step of A360 (Supplemental Fig. S3) and analyzed the effects of Rubisco kinetic parameters on the limiting step of A360 using three different Rubisco kinetic parameters: (1) the Rubisco kinetic parameters obtained from Bernacchi et al. (2001); (2) the Rubisco kinetic parameters multiplied by 1.15; and (3) the Rubisco kinetic parameters multiplied by 0.85. The limiting step of A360 showed similar results, irrespective of the Rubisco kinetic parameters used (Supplemental Fig. S3). Moreover, we also analyzed the effects of mesophyll conductance (gm) on the limiting step of A360 under two assumptions: (1) infinite gm and (2) finite gm. When we analyzed the limiting step of A360 with assumption of infinite gm, we used Rubisco kinetic parameters from Bernacchi et al. (2001). However, when we took account of gm, we used Rubisco kinetic parameters and gm from Bernacchi et al. (2002). The gm at 25°C was estimated with an assumed relationship between gm and the photosynthetic rate (A) at 25°C (gm = 0.012 × A; Evans and von Caemmerer, 1996) and applied it to the temperature dependence (Bernacchi et al., 2002). Although under assumptions with finite gm A360 tended to be limited by Ar at lower temperatures, the limiting step of A360 was similar, irrespective of whether an infinite or finite gm was posited. Therefore, we conclude that our analyses of the limiting step of A360 are robust.
At low temperature, Pi regeneration capacity often limits photosynthetic rate (Sharkey, 1985; Sage and Sharkey, 1987; Labate and Leegood, 1988; Cen and Sage, 2005; Sage and Kubien, 2007). When the cold-sensitive species N. tabacum and S. lycopersicum were grown at 30°C, Pi regeneration capacity limited A360 at temperatures below 15°C (Figs. 2 and 3; Supplemental Fig. S2), suggesting that cold-sensitive species tended to be limited by Pi regeneration capacity at low temperature compared with cold-tolerant species but that the limitation by Pi regeneration capacity at low temperature was not necessarily common even in cold-sensitive species grown at 30°C.
The Balance between RuBP Carboxylation and Regeneration at the Growth Temperature
The limiting step of A360 at the growth temperature was different depending on plant species and growth temperature (Figs. 2 and 3; Supplemental Fig. S2). Hikosaka (1997) predicted that the optimal photosynthetic rate at the growth temperature could be achieved under conditions where colimitation by Ac and Ar occurs, since this will allow for the maximally efficient use of nitrogen in the production of photosynthetic proteins. In some of the cold-tolerant species grown at 30°C and the cold-sensitive species grown at 15°C, A360 at the growth temperature was colimited by Ac and Ar. However, colimitation of Ac and Ar at the growth temperature was not realized in others. In some of the cold-tolerant species grown at 15°C, A360 at the growth temperature was solely limited by Ac, while in some of the cold-sensitive species grown at 30°C, A360 was solely limited by Ar. This suggests the existence of constraints on nitrogen partitioning among the photosynthetic apparatus and implies that nitrogen investment may not always be optimal in the natural habitat.
Growth temperature affects nitrogen partitioning in the photosynthetic apparatus (Hikosaka, 2005; Onoda et al., 2005a; Yamori et al., 2005), although these changes are not observed in every species (Onoda et al., 2005b; Atkin et al., 2006; Ishikawa et al., 2007). We found that the Jmax/Vcmax ratio, which is a measure of the balance between Ar and Ac, increased with decreasing growth temperature, and the extent was greater in cold-tolerant species. Rubisco content was also found to significantly increase with decreasing growth temperature (Yamori et al., 2009b). This suggests that with decreasing growth temperature, while Rubisco content increased, proteins related to RuBP regeneration increased to a greater extent. Thus, the process of RuBP regeneration is more responsive to growth temperature than that of RuBP carboxylation. Photoinhibition at low temperature occurs in many species, and the extent of cold-induced photoinhibition differs depending on the cold tolerance (Koroleva et al., 1994; Park et al., 1995; Bertin et al., 1997). A higher Jmax/Vcmax ratio in cold-tolerant species grown at low temperature would reduce excess excitation energy by providing a greater sink for photosynthetic electron transport, thereby avoiding photoinhibition in its natural habitat, where temperature and light intensity vary greatly both daily and seasonally (Hikosaka et al., 2006).
Our results were contrary to those of Bunce (2000), who concluded that the balance between Ar and Ac was roughly constant irrespective of growth temperature and plant species. In cool climate species, the balance seems to change with growth temperature (figure 5 in Bunce, 2000). Therefore, it is fair to say that the response of the balance between Ar and Ac to growth temperature would be species specific.
Some species have been shown to alter their Jmax/Vcmax ratio in response to growth light intensity (Evans, 1996; Poorter and Evans, 1998; Evans and Poorter, 2001), whereas others did not (Makino et al., 1997; Hikosaka, 2005; Yamori et al., 2009a). It is possible that there is interspecific variation in the response of Jmax/Vcmax to the growth light intensity. In this study, we grew plants in a laboratory environment. Therefore, it will be important to analyze plants grown under natural conditions and analyze seasonal effects in order to clarify photosynthetic responses to growth temperature under natural conditions.
Syndrome of Temperature Acclimation of Photosynthesis
It is known that plants exhibit a set of characteristic responses to growth irradiance. For example, when plants are grown under shade conditions, plants develop longer internodes and petioles, produce broader, thinner leaves, and reduce the chlorophyll a/b ratio, photosynthetic capacity, and respiratory capacity (Smith, 1982; Smith and Whitelam, 1997; Kim et al., 2005). In this study, we found that cold-tolerant species that could greatly change Opt (A360) depending on growth temperature exhibited considerable plasticity in other photosynthetic parameters; when the growth temperature decreased from 30°C to 15°C, these species decreased Opt (A360) and increased Jmax/Vcmax ratio, LMA, Narea, and Rubisco content, leading to a greater ability for temperature homeostasis of Rd and A360 (Fig. 5; Yamori et al., 2009b). Therefore, plants exhibiting considerable plasticity in a certain parameter also showed great plasticity in other parameters. This set of responses may be regarded as a “syndrome” of temperature acclimation. Alteration of all the parameters, which are independently regulated, may play an important role in a plant's temperature acclimation. Natural selection might favor plasticity in these traits for success in changing environments.
CONCLUSION
We have demonstrated interspecific variation in photosynthetic temperature acclimation among plants with different cold tolerances. Cold-tolerant species drastically altered their Opt (A360) depending on growth temperature, whereas cold-sensitive species were less plastic. There were differences in the limiting step of A360 at optimum temperatures and in the plasticity of the photosynthetic limiting step to growth temperature between cold-tolerant and cold-sensitive species. Cold-tolerant plants showed more flexibility in many photosynthetic parameters concerned with temperature acclimation (syndrome of temperature acclimation of photosynthesis). Thus, differences in the extent of plasticity for all of the photosynthetic parameters with respect to growth temperature caused interspecific differences in photosynthetic temperature acclimation between cold-tolerant and cold-sensitive species.
To facilitate increases in crop yield and to increase tolerance of severe growth environments, genetic manipulation of photosynthesis has become a key target for improvement (Dunwell, 2000; Richards, 2000; Raines, 2006). What determines the maximum photosynthetic rate? Our data indicate that there is no single answer to this question. Even in a single plant species, the limiting step of photosynthesis differed depending on growth and measurement temperatures. Therefore, this study strongly suggests that the impact on the control of carbon fixation by manipulation of one enzyme would differ depending on plant species and growth conditions. More attention should be paid to studying differences in the photosynthesis-limiting step depending on species and growth conditions, as this might provide opportunities for achieving faster improvements in crop production.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Studies were conducted on 11 herbaceous crop species as described previously (Yamori et al., 2009b). For cold-sensitive species, Cucumis sativus (cucumber), Nicotiana tabacum (tobacco), Oryza sativa (rice), and Solanum lycopersicum (tomato) were examined, while for cold-tolerant species, Secale cereale (winter rye), Spinacia oleracea (spinach), Solanum tuberosum (potato), Triticum aestivum (spring wheat), Triticum aestivum (winter wheat), Triticosecale (triticale), and Vicia faba (broad bean) were selected. Day and night lengths were 8 and 16 h, respectively. Photosynthetically active photon flux density during the daytime was 250 μmol m−2 s−1. The day/night air temperatures were either 30°C/25°C or 15°C/10°C, and the relative humidity was 65%. The plants grown at 30°C/25°C and 15°C/10°C are called 30°C plants and 15°C plants, respectively.
Gas-Exchange Measurements
Temperature dependence of the Rd and A at a light intensity of 1,500 μmol m−2 s−1 was measured at 5°C intervals from 10°C to 35°C and at 38°C using the most recently fully expanded leaves, as described previously (Yamori et al., 2005). The vapor pressure deficit was less than 1.0 kPa at 10°C to 30°C and less than 3.0 kPa at 35°C and 38°C. CO2 dependence of A was examined based on measurements at CO2 concentrations in the ambient air (Ca) of 50, 100, 150, 200, 360, and 1,500 μL L−1 (LI-6400; LI-COR). At each CO2 concentration, A and Ci were calculated. The optimum temperature for the photosynthetic parameters was derived from cubic curves that were fitted to the data in Excel (Microsoft).
The limiting steps of the photosynthetic rate were analyzed based on their A-Ci curves using the C3 photosynthesis model (see Supplemental Appendix S1) according to Atkin et al. (2006), Ishikawa et al. (2007), and Makino and Sage (2007). Curve fitting of the A-Ci curve was performed with Kaleidagraph (Synergy Software). The Vcmax and Ac were estimated from the photosynthetic rate at low CO2 concentrations (<200 μL L−1), whereas the Jmax and the Ar were estimated from the photosynthetic rate at 1,500 μL L−1 (Farquhar et al., 1980; von Caemmerer, 2000). We used the temperature dependence of Rubisco kinetic parameters that were obtained at leaf temperatures of 10°C to 40°C in N. tabacum (Bernacchi et al., 2001). We theoretically derived ratios of Ac at 360 μL L−1 CO2 concentration (Ac360) to Ac200 using Equation 2 in Supplemental Appendix S1. If the measured A360/A200 ratio is equal to the Ac360/Ac200 ratio, it indicates that A360 is limited by Ac (A360 = Ac360). If the A360/A200 ratio is lower than the Ac360/Ac200 ratio, it indicates that A360 is limited by Ar (or Pi regeneration limitation). Also, we derived the ratio of Ar at 1,500 μL L−1 CO2 concentration (Ar1500) to Ar360 using Equation 3 in Supplemental Appendix S1. If the A1500/A360 ratio is equal to the Ar1500/Ar360 ratio, it indicates that A360 is limited by Ar (A360 = Ar360), whereas if the A1500/A360 ratio is higher than the Ar1500/Ar360 ratio, it indicates that A360 is limited by Ac. If photosynthesis is limited by Pi regeneration (triose phosphate utilization), the A1500/A360 ratio becomes 1 (Sage, 1990).
Biochemical Analyses
Immediately after gas-exchange measurements, leaf discs were frozen and stored at −80°C until biochemical analysis. The content of Rubisco was determined by the method of Yamori et al. (2005). The frozen leaf sample was ground in liquid nitrogen and homogenized in an extraction buffer containing 100 mm sodium phosphate buffer (pH 7.0), 1.0% (w/v) polyvinylpyrrolidone, 0.1% (v/v) Triton X-100, 1 mm phenylmethylsulfonyl fluoride, and 1.0% β-mercaptoethanol.
For determinations of enzyme activities of Rubisco and NAD-ME, the frozen leaf sample was rapidly homogenized in a chilled mortar and pestle with extraction buffer containing 100 mm HEPES-KOH (pH 7.8), 10 mm MgCl2, 5 mm dithiothreitol, and 1 mm EDTA. The homogenate was centrifuged at 16,000g for 30 s at 4°C, and the maximal Rubisco activity of the supernatant was determined by monitoring NADH oxidation at 340 nm, according to the method of Yamori et al. (2006b). After Rubisco was activated for 20 min at 4°C in an activation medium that contained 375 mm HEPES-KOH (pH 7.8), 50 mm MgCl2, and 50 mm NaHCO3, the total activity was assayed in an assay medium containing 100 mm Bicine-KOH (pH 8.2), 20 mm MgCl2, 20 mm NaHCO3, 5 mm ATP, 5 mm creatine phosphate, 60 μm NADH, 0.6 mm RuBP, 10 units mL−1 creatine kinase, 10 units mL−1 3-phosphoglyceric phosphokinase, and 25 units mL−1 glyceraldehyde-3-phosphate dehydrogenase. The kcat was calculated from the Rubisco activity and content. The maximal NAD-ME activity was assayed according to Millar et al. (1998) in a reaction medium consisting of 50 mm MOPS-KOH (pH 6.5), 2 mm NAD+, 0.025% (v/v) Triton X-100, 2 mm MnCl2, 4 mm dithiothreitol, and 10 mm malate.
The leaf dry mass and nitrogen contents were determined on leaf discs taken after the gas-exchange measurements. The leaf discs were dried at 70°C for at least 7 d, and then leaf nitrogen contents were measured with an NC analyzer (CHNOS Elemental Analyzer, Vario EL III; Elementar).
Statistical Analyses
To evaluate whether the inherent ability of temperature acclimation of photosynthesis is different between cold-sensitive and cold-tolerant species, we used a repeated-measures ANOVA by STATVIEW (version 4.58; SAS Institute). Individual data from each plant species were used.
Principal component analysis was conducted with a program provided at http://aoki2.si.gunma-u.ac.jp/lecture/stats-by-excel/vba/html/pca.html.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Temperature dependence of Rd, A360, and Ci.
Supplemental Figure S2. Analyses of limiting steps of A360.
Supplemental Figure S3. Effects of Rubisco kinetics and mesophyll conductance on the limiting step of A360.
Supplemental Table S1. Opt (A360), Opt (Ac), Opt (Ar), and Jmax/Vcmax at 25°C and Ci at 25°C.
Supplemental Appendix S1. C3 photosynthesis model.
Supplementary Material
Acknowledgments
We are grateful to Dr. J. Evans (Australian National University) and Dr. L. Irving (Tohoku University) for valuable comments on the manuscript. We are also grateful to Takuya Kubo (Hokkaido University) for statistical analyses. We thank Dr. T. Yoshihira (Rakuno Gakuen University) for his generous gift of seeds of T. aestivum (spring and winter), Triticosecale, and S. cereale.
This work was supported by a grant from the Japan Society for the Promotion of Science for young research fellows to W.Y.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Wataru Yamori (wataru.yamori@biochem.tohoku.ac.jp).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Atkin OK, Scheurwater I, Pons TL (2006) High thermal acclimation potential of both photosynthesis and respiration in two lowland Plantago species in contrast to an alpine congeneric. Glob Change Biol 12 500–515 [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP (2002) Temperature response of mesophyll conductance: implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol 130 1992–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR Jr, Long SP (2001) Improved temperature responses functions for models of Rubisco-limited photosynthesis. Plant Cell Environ 24 253–259 [Google Scholar]
- Berry JA, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31 491–543 [Google Scholar]
- Bertin P, Bouharmont J, Kinet JM (1997) Somaclonal variation and improvement of chilling tolerance in rice: changes in chilling-induced chlorophyll fluorescence. Crop Sci 37 1727–1735 [Google Scholar]
- Björkman O, Mooney HA, Ehleringer J (1975) Photosynthetic responses of plants from habitats with contrasting thermal environments: comparison of photosynthetic characteristics of intact plants. Year B Carnegie Inst Wash 74 743–748 [Google Scholar]
- Brüggemann W, Linger P (1994) Long-term chilling of young tomato plants under low light. IV. Differential responses of chlorophyll fluorescence quenching coefficients in Lycopersicon species of different chilling sensitivity. Plant Cell Physiol 35 585–591 [Google Scholar]
- Bunce JA (2000) Acclimation of photosynthesis to temperature in eight cool and warm climate herbaceous C3 species: temperature dependence of parameters of a biochemical photosynthesis model. Photosynth Res 63 59–67 [DOI] [PubMed] [Google Scholar]
- Cen YP, Sage RF (2005) The regulation of Rubisco activity in response to variation in temperature and atmospheric CO2 partial pressure in sweet potato. Plant Physiol 139 979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME (2000) Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc Natl Acad Sci USA 97 13430–13435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham SC, Read J (2002) Comparison of temperate and tropical rainforest tree species: photosynthetic responses to growth temperature. Oecologia 133 112–119 [DOI] [PubMed] [Google Scholar]
- Dunwell JM (2000) Transgenic approaches to crop improvement. J Exp Bot 51 487–496 [DOI] [PubMed] [Google Scholar]
- Evans JR (1996) Developmental constraints on photosynthesis: effects of light and nutrition. In NR Baker, ed, Photosynthesis and the Environment. Kluwer, Dordrecht, The Netherlands, pp 281–304
- Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24 755–767 [Google Scholar]
- Evans JR, von Caemmerer S (1996) Carbon dioxide diffusion inside leaves. Plant Physiol 110 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S (1982) Modelling of photosynthetic responses to environmental conditions. In OL Lange, PS Nobel, CB Osmond, H Ziegler, eds, Physiological Plant Ecology. II. Encyclopedia of Plant Physiology, New Series, Vol 12B. Springer-Verlag, Berlin, pp 548–577
- Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149 78–90 [DOI] [PubMed] [Google Scholar]
- Hikosaka K (1997) Modelling optimal temperature acclimation of the photosynthetic apparatus in C3 plants with respect to nitrogen use. Ann Bot (Lond) 80 721–730 [Google Scholar]
- Hikosaka K (2005) Nitrogen partitioning in the photosynthetic apparatus of Plantago asiatica leaves grown at different temperature and light conditions: similarities and differences between temperature and light acclimation. Plant Cell Physiol 46 1283–1290 [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y (2006) Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J Exp Bot 57 291–302 [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Murakami A, Hirose T (1999) Balancing carboxylation and regeneration of ribulose bisphosphate in leaf photosynthesis: temperature acclimation in an evergreen tree, Quercus myrsinaefolia. Plant Cell Environ 22 841–849 [Google Scholar]
- Hill RS, Read J, Busby JR (1988) The temperature-dependence of photosynthesis of some Australian temperate rainforest trees and its biogeographical significance. J Biogeography 15 431–449 [Google Scholar]
- Ishikawa K, Onoda Y, Hikosaka K (2007) Intraspecific variation in temperature dependence of gas exchange characteristics among Plantago asiatica ecotypes from different temperature regimes. New Phytol 176 356–364 [DOI] [PubMed] [Google Scholar]
- Jordan DB, Ogren WL (1984) The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase: dependence on ribulose bisphosphate concentration, pH and temperature. Planta 161 308–313 [DOI] [PubMed] [Google Scholar]
- Kim GT, Yano S, Kozuka T, Tsukaya H (2005) Photomorphogenesis of leaves: shade-avoidance syndrome and diverentiation of sun/shade leaves. Photochem Photobiol Sci 4 770–774 [DOI] [PubMed] [Google Scholar]
- Kobza J, Edwards GE (1987) Influences of leaf temperature on photosynthetic carbon metabolism in wheat. Plant Physiol 83 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroleva OY, Brüggemann W, Krause GH (1994) Photoinhibition, xanthophyll cycle and in vivo chlorophyll fluorescence quenching of chilling-tolerant Oxyria digyna and chilling-sensitive Zea mays. Physiol Plant 92 577–584 [Google Scholar]
- Labate CA, Leegood RC (1988) Limitation of photosynthesis by changes in temperature. Planta 173 519–527 [DOI] [PubMed] [Google Scholar]
- Law RD, Crafts-Brandner SJ (1999) Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol 120 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SF, Woodward FI, editors (1989) Plants and Temperature: Symposia of the Society for Experimental Biology, Vol 42. Company of Biologists, Cambridge, UK
- Makino A, Sage RF (2007) Temperature response of photosynthesis in transgenic rice transformed with ‘sense’ or ‘antisense’ rbcS. Plant Cell Physiol 48 1472–1483 [DOI] [PubMed] [Google Scholar]
- Makino A, Sato T, Nakano H, Mae T (1997) Leaf photosynthesis, plant growth and nitrogen allocation in rice under different irradiances. Planta 203 390–398 [Google Scholar]
- Millar AH, Atkin OK, Menz RI, Henry B, Farquhar GD, Day DA (1998) Analysis of respiratory chain regulation in roots of soybean seedlings. Plant Physiol 117 1083–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney HA, Billings WD (1961) Comparative physiological ecology of arctic and alpine populations of Oxyria digyna. Ecol Monogr 31 1–29 [Google Scholar]
- Mooney HA, Björkman O, Collatz GJ (1978) Photosynthetic acclimation to temperature in the desert shrub, Larrea divaricata. I. Carbon dioxide exchange characteristics of intact leaves. Plant Physiol 61 406–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda Y, Hikosaka K, Hirose T (2005. a) Seasonal change in the balance between capacities of RuBP carboxylation and RuBP regeneration affects CO2 response of photosynthesis in Polygonum cuspidatum. J Exp Bot 56 755–763 [DOI] [PubMed] [Google Scholar]
- Onoda Y, Hikosaka K, Hirose T (2005. b) The balance between RuBP carboxylation and RuBP regeneration: a mechanism underlying the interspecific variation in acclimation of photosynthesis to seasonal change in temperature. Funct Plant Biol 32 903–910 [DOI] [PubMed] [Google Scholar]
- Park HS, Park IH, Moon BY, Lee CB, Lee CH (1995) Differences among three rice cultivars during chilling stress and subsequent recovery period. In P Mathis, ed, Photosynthesis: From Light to Biosphere, Vol IV. Kluwer, Dordrecht, The Netherlands, pp 813–816
- Pearcy RW (1977) Acclimation of photosynthetic and respiratory carbon dioxide exchange to growth temperature in Atriplex lentiformis (Torr.). Plant Physiol 59 795–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H, Evans JR (1998) Photosynthetic nitrogen-use efficiency of species that differ inherently in specific area. Oecologia 116 26–37 [DOI] [PubMed] [Google Scholar]
- Raines CA (2006) Transgenic approaches to manipulate the environmental responses of the C3 carbon fixation cycle. Plant Cell Environ 29 331–339 [DOI] [PubMed] [Google Scholar]
- Read J (1990) Some effects of acclimation temperature on net photosynthesis in some tropical and extra-tropical Australasian Nothofagus species. J Ecol 78 100–112 [Google Scholar]
- Richards RA (2000) Selectable traits to increase crop photosynthesis and yield of grain crops. J Exp Bot 51 447–458 [DOI] [PubMed] [Google Scholar]
- Sage RF (1990) A model describing the regulation of ribulose-1,5-bisphosphate carboxylase, electron transport, and triose phosphate use in response to light intensity and CO2 in C3 plants. Plant Physiol 94 1728–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF (2002) Variation in the kcat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature. J Exp Bot 53 609–620 [DOI] [PubMed] [Google Scholar]
- Sage RF, Kubien DS (2007) The temperature response of C3 and C4 photosynthesis. Plant Cell Environ 30 1086–1106 [DOI] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD (1987) The effect of temperature on the occurrence of O2 and CO2 insensitive photosynthesis in field grown plants. Plant Physiol 84 658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Way DA, Kubien DS (2008) Rubisco, Rubisco activase, and global climate change. J Exp Bot 59 1581–1595 [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ (2004. a) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120 179–186 [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ (2004. b) Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol 134 1460–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saruyama H, Tanida M (1995) Effect of chilling on activated oxygen-scavenging enzymes in low temperature-sensitive and -tolerant cultivars of rice (Oryza sativa L.). Plant Sci 109 105–113 [Google Scholar]
- Schrader SM, Wise RR, Wacholtz WF, Ort DR, Sharkey TD (2004) Thylakoid membrane responses to moderately high leaf temperature in Pima cotton. Plant Cell Environ 27 725–735 [Google Scholar]
- Sharkey TD (1985) Photosynthesis in intact leaves of C3 plants: physics, physiology, and rate limitations. Bot Rev 51 53–105 [Google Scholar]
- Slatyer RO (1977) Altitudinal variation in the photosynthetic characteristics of snow gum, Eucalyptus pauciflora Sieb. ex Spreng. IV. Temperature response of four populations grown at different temperatures. Aust J Plant Physiol 4 583–594 [Google Scholar]
- Smith H (1982) Light quality, photoreception, and plant strategy. Annu Rev Plant Physiol 33 481–518 [Google Scholar]
- Smith H, Whitelam GC (1997) The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant Cell Environ 20 840–844 [Google Scholar]
- Thomashow MF (1998) Role of cold-responsive genes in plant freezing tolerance. Plant Physiol 118 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50 571–599 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S (2000) Biochemical Models of Leaf Photosynthesis. CSIRO Publishing, Collingwood, Australia
- Weis E (1981) Reversible heat-inactivation of the Calvin cycle: a possible mechanism of the temperature regulation of photosynthesis. Planta 151 33–39 [DOI] [PubMed] [Google Scholar]
- Wise RR, Olson AJ, Schrader SM, Sharkey TD (2004) Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant Cell Environ 27 717–724 [Google Scholar]
- Yamori W, Evans JR, von Caemmerer S (2009. a) Effects of growth and measurement light intensities on temperature dependence of CO2 assimilation rate in tobacco leaves. Plant Cell Environ (in press) [DOI] [PubMed]
- Yamori W, Noguchi K, Hanba TY, Terashima I (2006. a) Effects of internal conductance on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant Cell Physiol 47 1069–1080 [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Hikosaka K, Terashima I (2009. b) Cold-tolerant crop species have greater temperature homeostasis of leaf respiration and photosynthesis than cold-sensitive species. Plant Cell Physiol 50 203–215 [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Kashino Y, Terashima I (2008) The role of the electron transport in determining the temperature dependence of photosynthetic rate in spinach leaves grown at contrasting temperatures. Plant Cell Physiol 49 583–591 [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Terashima I (2005) Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant Cell Environ 28 536–547 [Google Scholar]
- Yamori W, Suzuki K, Noguchi K, Nakai M, Terashima I (2006. b) Effects of Rubisco kinetics and Rubisco activation state on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant Cell Environ 29 1659–1670 [DOI] [PubMed] [Google Scholar]
- Yu JQ, Zhou YH, Huang LF, Allen D (2002) Chill-induced inhibition of photosynthesis: genotypic variation within Cucumis sativus. Plant Cell Physiol 43 1182–1188 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.