Abstract
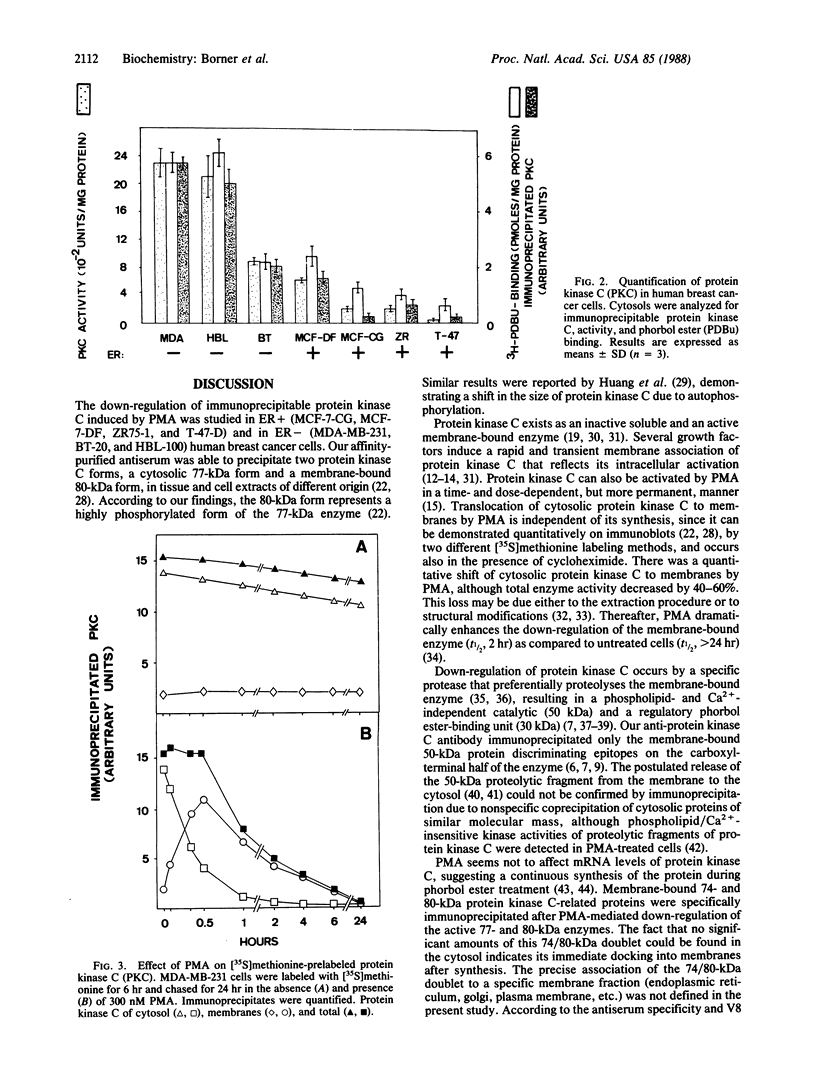
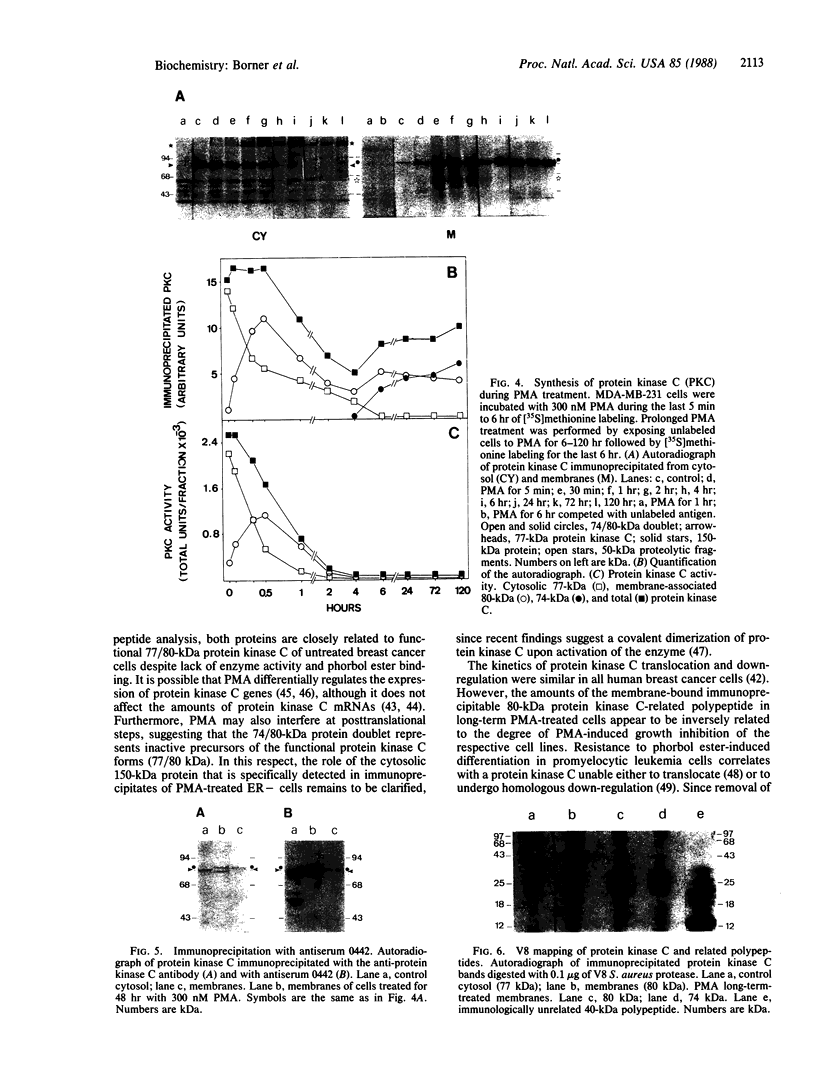
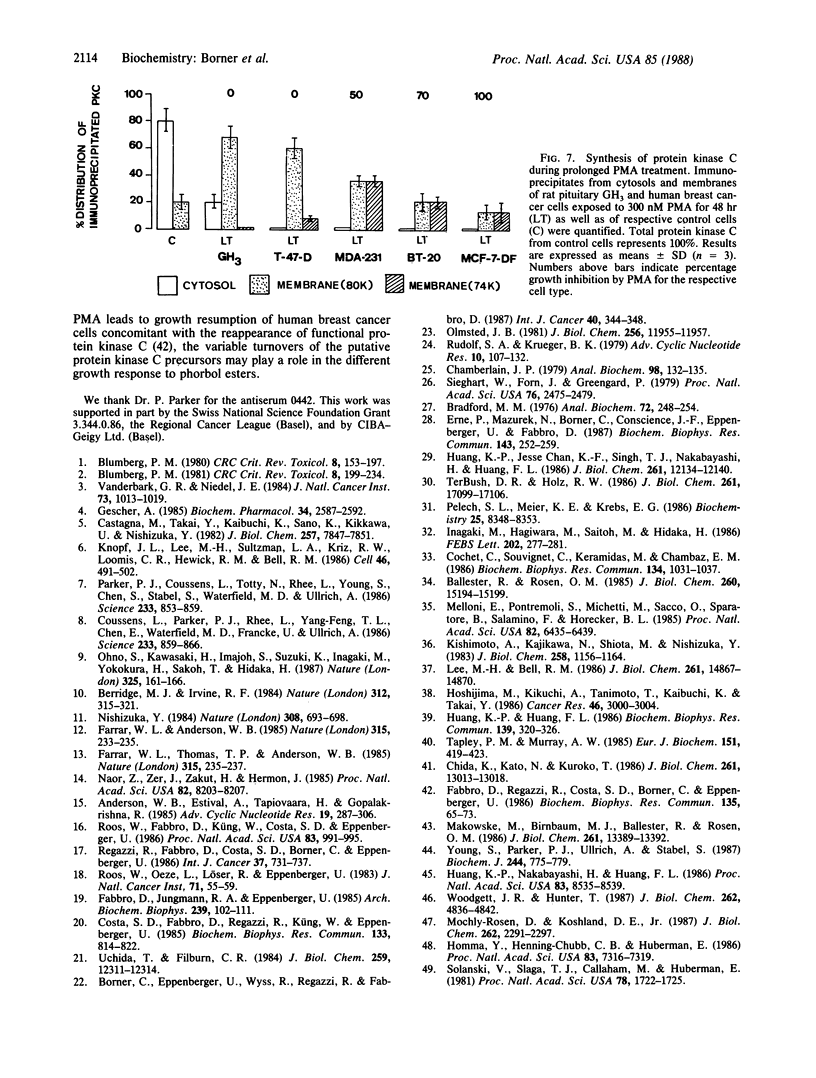
The phorbol 12-myristate 13-acetate (PMA)-dependent down-regulation of immunoprecipitable protein kinase C was studied in human breast cancer cell lines that display different growth inhibitions toward the tumor promoter. PMA induces translocation of [35S]methionine-prelabeled cytosolic protein kinase C to membranes, followed by complete degradation of the enzyme (t1/2, 2 hr). PMA does not affect the protein kinase C synthesis; 20-80% of total protein kinase C of control cells was still immunoprecipitable as membrane-bound 74- and 80-kDa protein kinase C-related polypeptides if cells were allowed to incorporate [35S]methionine during PMA exposure for greater than 6 hr. These two proteins lack protein kinase activity and phorbol ester binding but reveal V8 peptide patterns identical to the active forms of protein kinase C (77/80 kDa) of PMA-untreated cells. The amounts of the immunoprecipitable membrane-bound 80-kDa protein kinase C-related polypeptide synthesized during the prolonged PMA treatment appear to inversely correlate with the extent of PMA-mediated growth inhibition of the respective human breast cancer cell line. These data suggest that after homologous down-regulation, functional protein kinase C (77/80 kDa) is replaced by a population of membrane-associated but enzymatically inactive protein kinase C-related polypeptides (74/80 kDa).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Estival A., Tapiovaara H., Gopalakrishna R. Altered subcellular distribution of protein kinase C (a phorbol ester receptor). Possible role in tumor promotion and the regulation of cell growth: relationship to changes in adenylate cyclase activity. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:287–306. [PubMed] [Google Scholar]
- Blumberg P. M. In vitro studies on the mode of action of the phorbol esters, potent tumor promoters, part 2. Crit Rev Toxicol. 1981 Jun;8(3):199–234. doi: 10.3109/10408448109109658. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M. In vitro studies on the mode of action of the phorbol esters, potent tumor promoters: part 1. Crit Rev Toxicol. 1980 Dec;8(2):153–197. doi: 10.3109/10408448009037493. [DOI] [PubMed] [Google Scholar]
- Cochet C., Souvignet C., Keramidas M., Chambaz E. M. Altered catalytic properties of protein kinase C in phorbol ester treated cells. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1031–1037. doi: 10.1016/0006-291x(86)90355-4. [DOI] [PubMed] [Google Scholar]
- Costa S. D., Fabbro D., Regazzi R., Küng W., Eppenberger U. The cytosolic phorboid receptor correlates with hormone dependency in six mammary carcinoma cell lines. Biochem Biophys Res Commun. 1985 Dec 17;133(2):814–822. doi: 10.1016/0006-291x(85)90977-5. [DOI] [PubMed] [Google Scholar]
- Coussens L., Parker P. J., Rhee L., Yang-Feng T. L., Chen E., Waterfield M. D., Francke U., Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986 Aug 22;233(4766):859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Erne P., Mazurek N., Borner C., Conscience J. F., Eppenberger U., Fabbro D. Translocation of protein kinase C is not required to inhibit the antigen-induced increase of cytosolic calcium in a mast cell line. Biochem Biophys Res Commun. 1987 Feb 27;143(1):252–259. doi: 10.1016/0006-291x(87)90658-9. [DOI] [PubMed] [Google Scholar]
- Fabbro D., Jungmann R. A., Eppenberger U. Subcellular distribution of protein kinase C of GH3 cells: quantitation and characterization by polyacrylamide gel electrophoresis. Arch Biochem Biophys. 1985 May 15;239(1):102–111. doi: 10.1016/0003-9861(85)90816-1. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Anderson W. B. Interleukin-2 stimulates association of protein kinase C with plasma membrane. Nature. 1985 May 16;315(6016):233–235. doi: 10.1038/315233a0. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Thomas T. P., Anderson W. B. Altered cytosol/membrane enzyme redistribution on interleukin-3 activation of protein kinase C. Nature. 1985 May 16;315(6016):235–237. doi: 10.1038/315235a0. [DOI] [PubMed] [Google Scholar]
- Gescher A. Antiproliferative properties of phorbol ester tumour promoters. Biochem Pharmacol. 1985 Aug 1;34(15):2587–2592. doi: 10.1016/0006-2952(85)90552-0. [DOI] [PubMed] [Google Scholar]
- Homma Y., Henning-Chubb C. B., Huberman E. Translocation of protein kinase C in human leukemia cells susceptible or resistant to differentiation induced by phorbol 12-myristate 13-acetate. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7316–7319. doi: 10.1073/pnas.83.19.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshijima M., Kikuchi A., Tanimoto T., Kaibuchi K., Takai Y. Formation of a phorbol ester-binding fragment from protein kinase C by proteolytic digestion. Cancer Res. 1986 Jun;46(6):3000–3004. [PubMed] [Google Scholar]
- Huang K. P., Chan K. F., Singh T. J., Nakabayashi H., Huang F. L. Autophosphorylation of rat brain Ca2+-activated and phospholipid-dependent protein kinase. J Biol Chem. 1986 Sep 15;261(26):12134–12140. [PubMed] [Google Scholar]
- Huang K. P., Nakabayashi H., Huang F. L. Isozymic forms of rat brain Ca2+-activated and phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8535–8539. doi: 10.1073/pnas.83.22.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M., Hagiwara M., Saitoh M., Hidaka H. Protein kinase C negatively modulated by phorbol ester. FEBS Lett. 1986 Jul 7;202(2):277–281. doi: 10.1016/0014-5793(86)80701-3. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Shiota M., Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983 Jan 25;258(2):1156–1164. [PubMed] [Google Scholar]
- Knopf J. L., Lee M. H., Sultzman L. A., Kriz R. W., Loomis C. R., Hewick R. M., Bell R. M. Cloning and expression of multiple protein kinase C cDNAs. Cell. 1986 Aug 15;46(4):491–502. doi: 10.1016/0092-8674(86)90874-3. [DOI] [PubMed] [Google Scholar]
- Makowske M., Birnbaum M. J., Ballester R., Rosen O. M. A cDNA encoding protein kinase C identifies two species of mRNA in brain and GH3 cells. J Biol Chem. 1986 Oct 15;261(29):13389–13392. [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Sparatore B., Salamino F., Horecker B. L. Binding of protein kinase C to neutrophil membranes in the presence of Ca2+ and its activation by a Ca2+-requiring proteinase. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6435–6439. doi: 10.1073/pnas.82.19.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D., Koshland D. E., Jr Domain structure and phosphorylation of protein kinase C. J Biol Chem. 1987 Feb 15;262(5):2291–2297. [PubMed] [Google Scholar]
- Naor Z., Zer J., Zakut H., Hermon J. Characterization of pituitary calcium-activated, phospholipid-dependent protein kinase: redistribution by gonadotropin-releasing hormone. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8203–8207. doi: 10.1073/pnas.82.23.8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ohno S., Kawasaki H., Imajoh S., Suzuki K., Inagaki M., Yokokura H., Sakoh T., Hidaka H. Tissue-specific expression of three distinct types of rabbit protein kinase C. Nature. 1987 Jan 8;325(7000):161–166. doi: 10.1038/325161a0. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Meier K. E., Krebs E. G. Rapid microassay for protein kinase C translocation in Swiss 3T3 cells. Biochemistry. 1986 Dec 30;25(26):8348–8353. doi: 10.1021/bi00374a002. [DOI] [PubMed] [Google Scholar]
- Regazzi R., Fabbro D., Costa S. D., Borner C., Eppenberger U. Effects of tumor promoters on growth and on cellular redistribution of phospholipid/Ca2+-dependent protein kinase in human breast cancer cells. Int J Cancer. 1986 May 15;37(5):731–737. doi: 10.1002/ijc.2910370514. [DOI] [PubMed] [Google Scholar]
- Roos W., Fabbro D., Küng W., Costa S. D., Eppenberger U. Correlation between hormone dependency and the regulation of epidermal growth factor receptor by tumor promoters in human mammary carcinoma cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):991–995. doi: 10.1073/pnas.83.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos W., Oeze L., Löser R., Eppenberger U. Antiestrogenic action of 3-hydroxytamoxifen in the human breast cancer cell line MCF-7. J Natl Cancer Inst. 1983 Jul;71(1):55–59. [PubMed] [Google Scholar]
- Sieghart W., Forn J., Greengard P. Ca2+ and cyclic AMP regulate phosphorylation of same two membrane-associated proteins specific to nerve tissue. Proc Natl Acad Sci U S A. 1979 May;76(5):2475–2479. doi: 10.1073/pnas.76.5.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Filburn C. R. Affinity chromatography of protein kinase C-phorbol ester receptor on polyacrylamide-immobilized phosphatidylserine. J Biol Chem. 1984 Oct 25;259(20):12311–12314. [PubMed] [Google Scholar]
- Vandenbark G. R., Niedel J. E. Phorbol diesters and cellular differentiation. J Natl Cancer Inst. 1984 Nov;73(5):1013–1019. [PubMed] [Google Scholar]
- Young S., Parker P. J., Ullrich A., Stabel S. Down-regulation of protein kinase C is due to an increased rate of degradation. Biochem J. 1987 Jun 15;244(3):775–779. doi: 10.1042/bj2440775. [DOI] [PMC free article] [PubMed] [Google Scholar]