Abstract
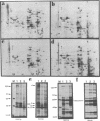
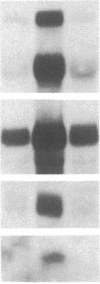
Freshly isolated adult rat hepatocytes exhibit a flat, extended morphology when cultured on dried rat tail collagen in the presence of growth factors; they actively synthesize DNA and express high levels of cytoskeletal mRNAs and proteins (actin, tubulin, cytokeratins, vinculin, alpha-actinin, and desmoplakin), while exhibiting low levels of liver-specific mRNAs (albumin, alpha 1-inhibitor III, and alpha 1-antitrypsin) and limited synthesis and secretion of albumin. Hepatocytes cultured on hydrated gel matrix from the Engelbreth-Holm-Swarm (EHS) mouse tumor form small spherical aggregates and exhibit low DNA, cytoskeletal mRNA, and protein synthesis, while at the same time exhibiting elevated liver-specific mRNAs and albumin production; these cells, therefore, more nearly conform to the program of gene expression seen within the normal animal. Hepatocytes on hydrated rat tail collagen resemble those on dry collagen when cultured at low density, but at high density they form compact trabecular aggregates, synthesize negligible amounts of DNA, and maintain a pattern of gene expression resembling that of hepatocytes seeded on the EHS matrix. If cell morphology is compact, as on EHS or on hydrated rat tail collagen when densely populated, DNA synthesis and expression of cytoskeletal genes are low, while liver-specific mRNAs are abundant. When cells are extended the opposite is the case. Without the growth supplement DNA synthesis is low throughout but gene expression is little affected. These studies point to the importance of cell-cell and cell-matrix interactions in determining the differentiated phenotype of hepatocytes, and they reveal an inverse relationship between cytoskeletal and liver-specific protein expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ze'ev A., Amsterdam A. In vitro regulation of granulosa cell differentiation. Involvement of cytoskeletal protein expression. J Biol Chem. 1987 Apr 15;262(11):5366–5376. [PubMed] [Google Scholar]
- Ben-Ze'ev A., Amsterdam A. Regulation of cytoskeletal proteins involved in cell contact formation during differentiation of granulosa cells on extracellular matrix. Proc Natl Acad Sci U S A. 1986 May;83(9):2894–2898. doi: 10.1073/pnas.83.9.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Cell configuration-related control of vimentin biosynthesis and phosphorylation in cultured mammalian cells. J Cell Biol. 1983 Sep;97(3):858–865. doi: 10.1083/jcb.97.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Arenson D. M., Maher J. J., Roll F. J. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987 Mar;79(3):801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Exton J. H. Assessment of effects of vasopressin, angiotensin II, and glucagon on Ca2+ fluxes and phosphorylase activity in liver. Methods Enzymol. 1985;109:550–558. doi: 10.1016/0076-6879(85)09113-3. [DOI] [PubMed] [Google Scholar]
- Bond J. F., Farmer S. R. Regulation of tubulin and actin mRNA production in rat brain: expression of a new beta-tubulin mRNA with development. Mol Cell Biol. 1983 Aug;3(8):1333–1342. doi: 10.1128/mcb.3.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. F., Robinson G. S., Farmer S. R. Differential expression of two neural cell-specific beta-tubulin mRNAs during rat brain development. Mol Cell Biol. 1984 Jul;4(7):1313–1319. doi: 10.1128/mcb.4.7.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron J. M., Jones A. L., Kirschner M. W. Autoregulation of tubulin synthesis in hepatocytes and fibroblasts. J Cell Biol. 1985 Nov;101(5 Pt 1):1763–1772. doi: 10.1083/jcb.101.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., Darnell J. E., Jr Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983 Sep;3(9):1552–1561. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., Harrelson A. L., Darnell J. E., Jr Dependence of liver-specific transcription on tissue organization. Mol Cell Biol. 1985 Oct;5(10):2623–2632. doi: 10.1128/mcb.5.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981 Aug;25(2):537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- DeLisle A. J., Graves R. A., Marzluff W. F., Johnson L. F. Regulation of histone mRNA production and stability in serum-stimulated mouse 3T6 fibroblasts. Mol Cell Biol. 1983 Nov;3(11):1920–1929. doi: 10.1128/mcb.3.11.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972 Sep;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enat R., Jefferson D. M., Ruiz-Opazo N., Gatmaitan Z., Leinwand L. A., Reid L. M. Hepatocyte proliferation in vitro: its dependence on the use of serum-free hormonally defined medium and substrata of extracellular matrix. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1411–1415. doi: 10.1073/pnas.81.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S. R., Wan K. M., Ben-Ze'ev A., Penman S. Regulation of actin mRNA levels and translation responds to changes in cell configuration. Mol Cell Biol. 1983 Feb;3(2):182–189. doi: 10.1128/mcb.3.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Mayer D., Schmid E., Denk H., Borenfreund E. Differences of expression of cytoskeletal proteins in cultured rat hepatocytes and hepatoma cells. Exp Cell Res. 1981 Aug;134(2):345–365. doi: 10.1016/0014-4827(81)90435-3. [DOI] [PubMed] [Google Scholar]
- Fujita M., Spray D. C., Choi H., Saez J. C., Watanabe T., Rosenberg L. C., Hertzberg E. L., Reid L. M. Glycosaminoglycans and proteoglycans induce gap junction expression and restore transcription of tissue-specific mRNAs in primary liver cultures. Hepatology. 1987 Jan-Feb;7(1 Suppl):1S–9S. doi: 10.1002/hep.1840070702. [DOI] [PubMed] [Google Scholar]
- Guguen-Guillouzo C., Clément B., Baffet G., Beaumont C., Morel-Chany E., Glaise D., Guillouzo A. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp Cell Res. 1983 Jan;143(1):47–54. doi: 10.1016/0014-4827(83)90107-6. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Jefferson D. M., Clayton D. F., Darnell J. E., Jr, Reid L. M. Posttranscriptional modulation of gene expression in cultured rat hepatocytes. Mol Cell Biol. 1984 Sep;4(9):1929–1934. doi: 10.1128/mcb.4.9.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landry J., Bernier D., Ouellet C., Goyette R., Marceau N. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985 Sep;101(3):914–923. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt J., Ng S. Y., Aebi U., Varma M., Latter G., Burbeck S., Kedes L., Gunning P. Expression of transfected mutant beta-actin genes: alterations of cell morphology and evidence for autoregulation in actin pools. Mol Cell Biol. 1987 Jul;7(7):2457–2466. doi: 10.1128/mcb.7.7.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Penman S., Fulton A., Capco D., Ben Ze'ev A., Wittelsberger S., Tse C. F. Cytoplasmic and nuclear architecture in cells and tissue: form, functions, and mode of assembly. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):1013–1028. doi: 10.1101/sqb.1982.046.01.094. [DOI] [PubMed] [Google Scholar]
- Rojkind M., Gatmaitan Z., Mackensen S., Giambrone M. A., Ponce P., Reid L. M. Connective tissue biomatrix: its isolation and utilization for long-term cultures of normal rat hepatocytes. J Cell Biol. 1980 Oct;87(1):255–263. doi: 10.1083/jcb.87.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin K., Hök M., Obrink B., Timpl R. Substrate adhesion of rat hepatocytes: mechanism of attachment to collagen substrates. Cell. 1981 May;24(2):463–470. doi: 10.1016/0092-8674(81)90337-8. [DOI] [PubMed] [Google Scholar]
- Singer P. A., Trevor K., Oshima R. G. Molecular cloning and characterization of the Endo B cytokeratin expressed in preimplantation mouse embryos. J Biol Chem. 1986 Jan 15;261(2):538–547. [PubMed] [Google Scholar]
- Spiegelman B. M., Farmer S. R. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982 May;29(1):53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Ginty C. A. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983 Dec;35(3 Pt 2):657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Fujita M., Saez J. C., Choi H., Watanabe T., Hertzberg E., Rosenberg L. C., Reid L. M. Proteoglycans and glycosaminoglycans induce gap junction synthesis and function in primary liver cultures. J Cell Biol. 1987 Jul;105(1):541–551. doi: 10.1083/jcb.105.1.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain A. J., McGowan J. A., Bucher N. L. Stimulation of DNA synthesis in primary cultures of adult rat hepatocytes by rat platelet-associated substance(s). In Vitro. 1982 Feb;18(2):108–116. doi: 10.1007/BF02796402. [DOI] [PubMed] [Google Scholar]
- Ungar F., Geiger B., Ben-Ze'ev A. Cell contact- and shape-dependent regulation of vinculin synthesis in cultured fibroblasts. 1986 Feb 27-Mar 5Nature. 319(6056):787–791. doi: 10.1038/319787a0. [DOI] [PubMed] [Google Scholar]