Abstract
Apolipoprotein E (apoE) mediates the redistribution of lipids among cells and is expressed at highest levels in brain and liver. Human apoE exists in three major isoforms encoded by distinct alleles (ɛ2, ɛ3, and ɛ4). Compared with APOE ɛ2 and ɛ3, APOE ɛ4 increases the risk of cognitive impairments, lowers the age of onset of Alzheimer’s disease (AD), and decreases the response to AD treatments. Besides age, inheritance of the APOE ɛ4 allele is the most important known risk factor for the development of sporadic AD, the most common form of this illness. Although numerous hypotheses have been advanced, it remains unclear how APOE ɛ4 might affect cognition and increase AD risk. To assess the effects of distinct human apoE isoforms on the brain, we have used the neuron-specific enolase (NSE) promoter to express human apoE3 or apoE4 at similar levels in neurons of transgenic mice lacking endogenous mouse apoE. Compared with NSE-apoE3 mice and wild-type controls, NSE-apoE4 mice showed impairments in learning a water maze task and in vertical exploratory behavior that increased with age and were seen primarily in females. These findings demonstrate that human apoE isoforms have differential effects on brain function in vivo and that the susceptibility to apoE4-induced deficits is critically influenced by age and gender. These results could be pertinent to cognitive impairments observed in human APOE ɛ4 carriers. NSE-apoE mice and similar models may facilitate the preclinical assessment of treatments for apoE-related cognitive deficits.
Apolipoprotein E (apoE) functions as an important carrier protein in the redistribution of lipids among cells (1). The brain is second only to the liver with respect to apoE expression levels, and evidence has been accumulating rapidly that apoE plays a critical role in central nervous system (CNS) integrity, function, and repair after injury (2–5). Human apoE exists in three major isoforms (E2, E3, and E4) encoded by three APOE alleles (ɛ2, ɛ3, and ɛ4). The APOE ɛ4 allele is the main known genetic risk factor for the most common form of Alzheimer’s disease (AD), whereas the more frequent ɛ3 allele and very rare ɛ2 allele provide relative protection from this illness (6). In addition, apoE4 has been related to reduced cognitive function in people without frank dementia (7, 8). Despite these provocative associations, it remains unknown how APOE genotype affects CNS function.
The potential physiological importance of murine apoE has been assessed by comparison of wild-type mice with ApoE knockout mice (9). Although ApoE knockout mice showed no obvious abnormalities in CNS development, neurodegenerative alterations became detectable in their brains at 5–6 months of age (10). These mice also had diminished regenerative capacity after perforant pathway lesions (11) and spatial learning deficits (12–14).
The expression of human apoE isoforms in neurons may be relevant to AD. In wild-type rodent brains, apoE is detected primarily in astrocytes, whereas in human brains it is detected in both astrocytes and neurons (15–17). Striking increases in neuronal immunostaining for apoE have been documented after CNS injuries in humans and rodents (18, 19). We hypothesized that impaired cognitive performance and increased risk for AD in APOE ɛ4 carriers could be caused by effects exerted by apoE4 within neurons. To test this hypothesis, we investigated the behavioral effects of neuronal expression of human apoE3 or apoE4 at similar levels in ApoE knockout mice.
MATERIALS AND METHODS
Transgenic Mice.
Neuron-specific enolase (NSE)-apoE3 and NSE-apoE4 transgenes, constructed as described (20), were microinjected individually into ICR one-cell embryos by standard procedures. Twenty-one transgenic founder mice (9 NSE-apoE3 and 12 NSE-apoE4) were identified by Southern blot analysis. NSE-apoE3 and NSE-apoE4 lines with similar levels of human apoE expression in the brain and cerebrospinal fluid were identified by RNase protection assay and Western blot analysis (21). These lines were crossed with ApoE knockout mice (9) obtained from N. Maeda (University of North Carolina, Chapel Hill). After elimination of wild-type murine ApoE alleles in two generations of breedings among the resulting offspring, transgenic mice were crossed with ApoE knockout mice (C57BL/6J-Apoetm1Unc) from The Jackson Laboratories to generate NSE-apoE3 and NSE-apoE4 mice. These crosses also yielded nontransgenic ApoE knockout littermates. Behavioral testing (see below) revealed no significant differences between female ApoE knockout mice derived from the NSE-apoE4 line (n = 6) and age- and sex-matched ApoE knockout mice derived from the NSE-apoE3 line (n = 8) or obtained from The Jackson Laboratories (C57BL/6J-Apoetm1Unc) (n = 8) (data not shown). Therefore, these three cohorts were combined (as “knockout mice”) in our statistical analyses. Nontransgenic C57BL/6J mice (from The Jackson Laboratories) served as wild-type controls.
All NSE-apoE mice used in this study were heterozygous for the NSE-apoE transgene on a homozygous ApoE knockout background. The genotype of transgenic and nontransgenic mice was confirmed by Southern blot analysis of genomic tail DNA by using a DNA probe for human APOE (20) and by slot blot analysis using as probe templates the apoE3 or apoE4 minigene isolated from the transgene constructs. NSE-apoE3 and NSE-apoE4 mice were differentiated by PCR. Inclusion of the human APOE intron 3 in the NSE-apoE4 but not in the NSE-apoE3 construct did not affect transgene expression but allowed differentiation of NSE-apoE3 from NSE-apoE4 mice by PCR: the amplicon generated with intron 3—spanning primers (forward primer, nt 3158–3175; reverse primer, nt 3815–3834; GenBank accession number M10065) was 670 bp in NSE-apoE4 mice and 100 bp in NSE-apoE3 mice. The ApoE knockout status of the mice was also confirmed by PCR with mouse ApoE-specific primers (forward primer, nt 2389–2413; reverse primer, nt 2833–2856; GenBank accession number D00466) and a neomycin-specific primer (reverse primer, nt 2016–2038 of pSV2.neo plasmid; GenBank accession number U02434). This primer combination generates a 221-bp amplicon for homozygous ApoE knockout mice and a 467-bp amplicon for wild-type mice (22). For all PCRs, proteinase K-digested tail tissue (2 μl, 1:100) was subjected to the touchdown PCR procedure (23) in a total reaction volume of 22 μl with 0.2 μM of each primer, dNTPs (dATP, dCTP, dGTP, dTTP, 200 μM each), and 0.15 μl of AmpliTaq GoldR DNA polymerase (Perkin–Elmer). The PCR was run on a GeneAmp PCR System 9600 (Perkin–Elmer) thermocycler, and the products were analyzed on 1.5% agarose gels.
Behavioral Testing.
To minimize the effects of social influences on behavior, mice were housed individually under conditions of constant temperature (18°C), light from 6:00 a.m. to 6:00 p.m., and free access to food and water. Mice that were analyzed in all three behavioral paradigms (n = 162) were tested first in the open field, then in the water maze, and last in the passive avoidance test (see below) to minimize the effect of stress on behavioral results. Before each assessment of mice in the open field, rotorod, or passive avoidance tests, the equipment was cleaned with 1 mM acetic acid to remove odors.
Open field activity.
Mice were placed individually into brightly lit automated activity cages equipped with rows of infrared photocells interfaced with a computer (San Diego Instruments, San Diego, CA). After a 1-min adaptation period, open field activity was recorded during three consecutive 10-min periods. Recorded beam breaks were used to calculate active times, path lengths, and the number and duration of rearing events (raising of both forefeet off the ground and extension of the body).
Rotorod test.
Mice were evaluated for motor deficits with the rotorod test (San Diego Instruments). After a 1-min adaptation period on the rod at rest, the rod was accelerated by 5 rpm every 15 sec, and the length of time mice remained on the rod (fall latency) recorded.
Water maze test.
The ability of mice to locate a hidden platform submerged in a pool (61 cm in diameter) filled with opaque water (24°C) was tested in two blocks (separated by a 2-h interval) per day for 4 days. Each block consisted of two consecutive trials. The platform location was changed daily, and the starting point at which the mouse was placed into the water was changed for each trial. Mice that failed to find the platform within 120 sec were put on it for 15 sec. On day 5, the ability of the mice to locate a clearly visible platform was tested to exclude differences in vision, swim speed, and motivation. Because the time required to reach the hidden platform (latency) depends on path length as well as swim speed, all three parameters were recorded with an EthoVision video tracking system (Noldus Instruments, Sterling, VA) set to analyze two images per second. Because swim speeds did not differ significantly among age- and sex-matched mice of the different genotypes analyzed in this study, latencies were used here to illustrate differences in water maze performance.
Passive avoidance.
Passive avoidance learning was measured with a step-through box (San Diego Instruments) consisting of a brightly lit compartment and a dark compartment connected with a sliding door. Mice were placed individually in the bright compartment. When the mouse entered the dark compartment, the sliding door was closed, and the mouse received a slight foot shock (0.3 mA, 1 sec). Twenty-four hours later, the mouse was again placed into the bright compartment, and the latency in re-entering the dark compartment was recorded up to 300 sec (criterion).
Immunohistochemistry.
Mice were anesthetized with chloral hydrate and flush-perfused transcardially with 0.9% saline. Brains were postfixed in phosphate-buffered (pH 7.4) 4% paraformaldehyde at 4°C for 48 h before vibratome sectioning at 40 μm. Endogenous peroxidase activity was quenched by incubation in 0.3% H2O2 in PBS for 20 min. Sections were pretreated for 4 min in 1 μg/ml proteinase K in a buffer containing 250 mM NaCl, 25 mM EDTA, and 50 mM Tris⋅HCl (pH 8) to facilitate penetration of antibodies. They were next incubated for 1 h with 15% normal donkey serum (Jackson ImmunoResearch) in PBS and then for 1 h with polyclonal goat anti-human apoE (Calbiochem), diluted 1:4,000 (immunofluorescent staining) or 1:10,000 (immunoperoxidase staining) in PBS. Sections were then washed twice in PBS and incubated for 1 h with fluorescein isothiocyanate-labeled (Jackson ImmunoResearch) or biotin-labeled (Vector Laboratories) anti-goat to detect antigen-bound anti-human apoE. After three washes in PBS, immunofluorescently labeled sections were mounted in VectaShield (Vector Laboratories) and viewed with a MRC-1024 laser scanning confocal microscope (Bio-Rad) mounted on an Optiphot-2 microscope (Nikon). For immunoperoxidase staining, secondary antibody binding was detected with the ABC-Elite kit (Vector Laboratories).
Statistical Analysis.
Data were analyzed with SuperANOVA (Abacus Concepts, Berkeley, CA). The statistical significance of differences among means was assessed by ANOVA and, as indicated, by Dunnett’s or Tukey–Kramer posthoc tests. Learning curves for the water maze test were compared by repeated-measures ANOVA using contrasts to assess differences between specific groups of mice. The null hypothesis was rejected at the 0.05 level.
RESULTS AND DISCUSSION
Comparable Expression of ApoE3 or ApoE4 in Brains of NSE-apoE Transgenic Mice.
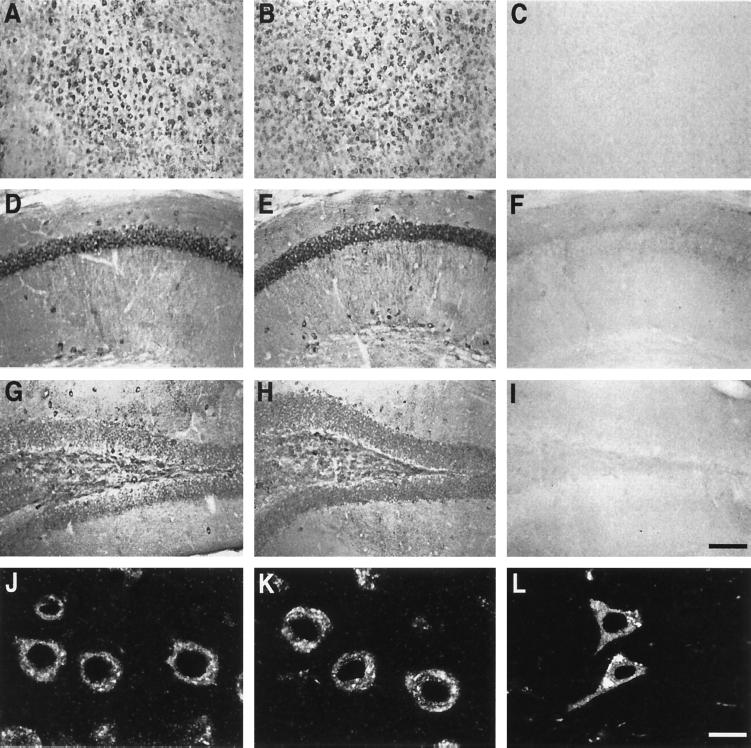
The NSE promoter (24) was used to express minigenes encoding human apoE3 or apoE4 (20) in the brains of transgenic mice lacking murine apoE (21). For behavioral analysis, two lines of NSE-apoE mice were selected that showed similar cerebral levels of apoE3 or apoE4 by RNase protection assay and Western blot analysis (21). To ensure that potential behavioral differences between these lines reflect differences in the biological activity of these apoE isoforms rather than differences in transgene expression patterns, we used antibodies that recognize both apoE3 and apoE4 to map the distribution of human apoE. Both lines showed a similar widespread neuronal expression of human apoE immunoreactivity, most prominently in the neocortex and hippocampus (Fig. 1).
Figure 1.
Immunocytochemical detection of human apoE in neurons of NSE-apoE3 and NSE-apoE4 mice and a human with AD. (A–I) Immunoperoxidase staining for human apoE revealed widespread neuronal labeling, including neocortex (A–C), hippocampal CA1 region (D–F), and dentate gyrus (G–I), in NSE-apoE3 (A, D, and G) and NSE-apoE4 (B, E, and H) mice. No apoE labeling was seen in corresponding brain regions of a knockout control lacking NSE-apoE transgenes (C, F, and I). (J–L) Immunofluorescence staining for human apoE strongly labeled neocortical neurons in NSE-apoE3 (J) and NSE-apoE4 (H) mice, and in a human AD case (APOE ɛ3/ɛ4) (L). (Bars: A–I, 160 μm; J–L, 10 μm.)
Differential Effects of ApoE3 and ApoE4 on Acquisition of a Water Maze Task.
The ability to remember and process spatial information, which is impaired in humans with AD (25), can be tested readily in mice. Various versions of the Morris water maze test (26) have been used to reveal behavioral deficits in mouse models (12–14, 27–29). Our version of this test was based on pilot experiments which showed that it was sensitive to the in vivo CNS effects of apoE3 and apoE4. The target platform was hidden under the surface of opaque water, the location at which mice entered the pool was changed between trials, and the platform location was changed between days. Mice were tested in four trials per day on four consecutive days. Trials were divided into two sessions (blocks) of two consecutive trials with 2 h between blocks. To find the platform, mice must relate their position in the pool to constant extramaze cues and quickly store, retrieve, and utilize information on where the platform is located and where it is not. Day-to-day improvements likely reflect a combination of general task learning and consolidated memory for constant extramaze cues, which should facilitate the development of more efficient search strategies.
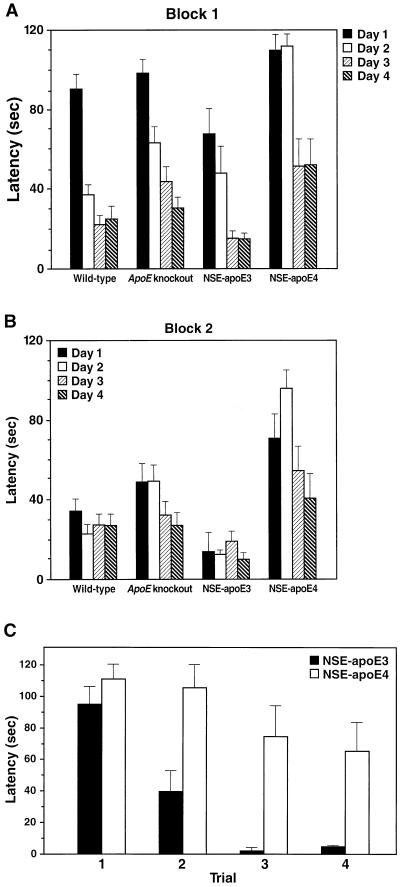
Epidemiological studies in humans suggest that apoE4 interacts with female gender to increase the risk of AD and decrease responsiveness to treatment (6, 30). Therefore, we first tested four groups of 6-month-old female mice: NSE-apoE3 and NSE-apoE4 mice, their nontransgenic ApoE knockout littermates, and wild-type mice (Fig. 2). NSE-apoE4 mice showed striking impairments in learning the water maze task. In contrast, NSE-apoE3 mice performed as well as, if not better than, wild-type mice. On day 2, none of eight NSE-apoE4 mice tested located the platform in the first trial of block 1, and only half of them found it in the first trial of block 2, whereas five of six NSE-apoE3 mice found the platform in the first trial of block 1, and all six found it in the first trial of block 2. Decreases in learning capacity of knockout mice below wild-type levels were subtler than those in NSE-apoE4 mice, and the difference was statistically significant only in block 1 (Fig. 2). The learning impairment of NSE-apoE4 mice was also evident when the water maze performance was compared across the four successive trials of day 1: wild-type, knockout, and NSE-apoE3 mice showed significant improvements (P = 0.001), whereas NSE-apoE4 mice did not. These data suggest that apoE4, but not apoE3, induces significant impairments in spatial learning and memory.
Figure 2.
Differential effects of human apoE3 and apoE4 on water maze performance in 6-month-old female mice. The time required to locate a hidden platform submerged in a pool of water (latency) was compared among wild-type (n = 16), knockout (n = 16), NSE-apoE3 (n = 6), and NSE-apoE4 (n = 8) mice. Mice were tested on 4 consecutive days in two sessions (blocks) per day with a 2-h interval between blocks. The average latency recorded for each mouse in two successive trials per block was used to calculate group means ± SEM. Mice that failed to locate the platform were assigned a latency value of 120 sec. For block 1 (A), repeated-measures ANOVA revealed highly significant differences in day-to-day learning capacities among genotypes (P = 0.001), specifically between NSE-apoE4 and NSE-apoE3 (P = 0.0001), NSE-apoE4 and wild-type (P = 0.0001), and knockout and wild-type (P = 0.02) mice. Comparison of genotypes on individual days by Tukey–Kramer posthoc test revealed significant differences between NSE-apoE4 and NSE-apoE3 on day 1 (P < 0.05) and between NSE-apoE4 and all other genotypes on day 2 (P < 0.01). For block 2 (B), differences in day-to-day learning capacities among genotypes were even more significant (P = 0.0008 by repeated-measures ANOVA), specifically between NSE-apoE4 and NSE-apoE3 (P = 0.0002), NSE-apoE4 and wild-type (P = 0.0006), and NSE-apoE4 and knockout (P = 0.018) mice. Comparison of genotypes on individual days by Tukey–Kramer posthoc test revealed that NSE-apoE4 differed significantly from NSE-apoE3 (P < 0.01) and wild-type (P < 0.05) mice on day 1 and from all other genotypes on day 2 (P < 0.01). There were also significant differences in learning curves on day 1 (C). In contrast to NSE-apoE3 mice, NSE-apoE4 mice did not improve significantly in their ability to locate the platform over the different trials on day 1, even though the platform was kept in the same location. Differences among groups similar to those described above for latencies were also observed for path lengths (data not shown).
Water maze performance can also be influenced by nonspatial cognitive deficits, such as reduced adaptability to new situations, as well as by emotional and motor peculiarities such as unusual swim patterns and passive floating (29). Reduced adaptive behavior may well have contributed to the poor performance of 6-month-old female NSE-apoE4 mice over different days. However, these mice showed no improvement over the four trials on day 1, even though the platform location remained constant. Therefore, reduced adaptability alone is not likely to account for their impaired performance. Moreover, all four groups of mice swam continuously at similar speeds (Table 1) and in similar patterns, and there were no significant periods of passive floating, possibly because the water temperature was low enough (24°C) to maintain escape motivation. On day 5 of the training cycle, the ability of the mice to locate a clearly visible platform was tested to exclude basic deficits in vision, motivation, motor strength, or coordination. No significant differences in time to locate the platform were identified (Table 1).
Table 1.
Basic visual and motor skills of 6-month-old female mice
| Mice | Latency to reach visible platform,† sec | Swim speed,‡ cm/sec | Rotorod fall latency, sec |
|---|---|---|---|
| Wild-type | 18 ± 3 (n = 16) | 15 ± 4 (n = 16) | 39 ± 3 (n = 4) |
| ApoE knockout | 18 ± 4 (n = 16) | 20 ± 4 (n = 16) | 54 ± 7 (n = 14) |
| NSE–apoE3 | 16 ± 8 (n = 6) | 19 ± 8 (n = 6) | 106 ± 19 (n = 6)* |
| NSE–apoE4 | 16 ± 7 (n = 8) | 18 ± 4 (n = 8) | 68 ± 12 (n = 9) |
Data represent group means ± SEM; n indicates number of mice.
P < 0.001 versus wild-type, P < 0.05 versus knockout (by Tukey–Kramer posthoc test).
Determined on day 5 of the water maze test.
Group means were calculated from average swim speeds of individual mice recorded in 16 trials.
To establish that the differences in water maze performance were not caused by differences in genetic background, we compared the water maze performance of 6-month-old female nontransgenic ApoE knockout controls obtained from the NSE-apoE4 (n = 6) or NSE-apoE3 (n = 8) lines or from The Jackson Laboratories (C57BL/6J-Apoetm1Unc) (n = 8). No significant differences in water maze performance were identified (data not shown). Therefore, potential minor differences in background genes did not account for the significant behavioral differences between NSE-apoE3 and NSE-apoE4 transgenic mice.
Dependence of Detrimental ApoE4 Effects on Age and Gender.
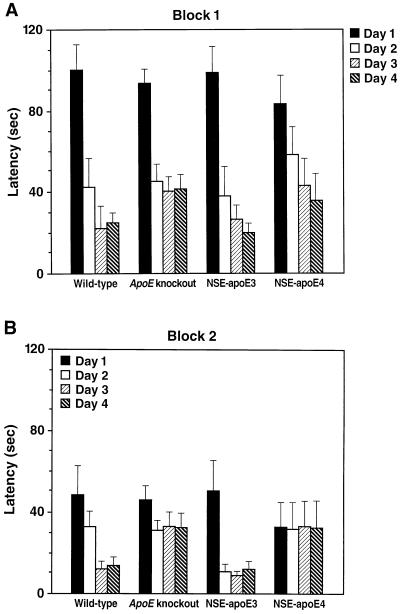
Because age is an important risk factor for the development of AD, we next assessed the effect of age on apoE4-induced impairments in spatial learning and memory. Comparison of 3-month-old female wild-type, knockout, NSE-apoE3, and NSE-apoE4 mice in the water maze revealed no significant differences among groups in block 1 and only subtle impairments in NSE-apoE4 and knockout mice in block 2 (Fig. 3). These results indicate that the detrimental functional effects of apoE4 described above are strongly dependent on age.
Figure 3.
Water maze performance of young mice is independent of cerebral apoE expression. Three-month-old female wild-type (n = 6), knockout (n = 19), NSE-apoE3 (n = 5), and NSE-apoE4 (n = 7) mice were compared in the water maze as described in Fig. 2. In block 1 (A), mice of all genotypes learned the task without significant differences identified among groups by repeated-measures ANOVA. In block 2 (B), significant day-to-day improvements were seen only in wild-type (p = 0.008) and NSE-apoE3 (p = 0.001) mice, but not in knockout or NSE-apoE4 mice. However, the differences in learning curves among groups in block 2 were not significant by repeated-measures ANOVA. The average swim speeds among groups of mice did not differ significantly, and the four groups showed similar performance in locating the visible platform on day 5 (data not shown).
Next, we tested the performance of 6-month-old male wild-type, knockout, NSE-apoE3, and NSE-apoE4 mice in the water maze. In contrast to the results in 6-month-old female mice, there were no significant differences among these groups of males (data not shown). Other cohorts of male ApoE knockout mice have shown small (12) or severe (13) learning deficits compared with wild-type controls. Differences in mouse strains, environmental factors such as diets, and water maze tests may have contributed to these divergent findings (31).
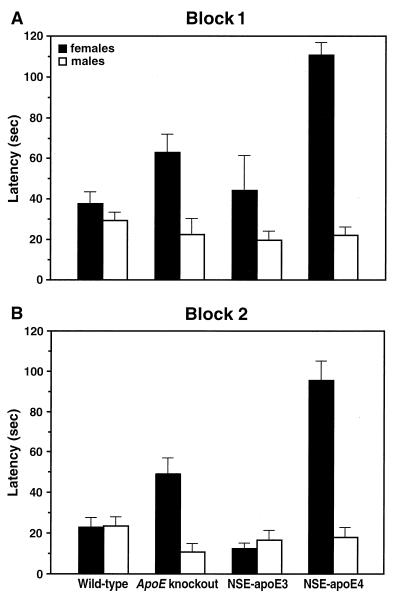
Day-to-day learning capacity in the water maze test differed significantly between 6-month-old males and females in the NSE-apoE4 (P = 0.0001, for Blocks 1 and 2) and knockout groups (P = 0.013 for block 1, P = 0.0006 for block 2), but not in the NSE-apoE3 and wild-type groups. The gender effect is illustrated for the second day of testing (Fig. 4). These results indicate that female gender significantly increases the susceptibility to apoE-dependent impairments in spatial learning. This finding may seem at odds with the beneficial effects estrogens may have on cognitive impairments in AD (32). However, although estrogen enhances verbal memory skills, it does not improve performance in visual-spatial memory tasks (33). In fact, lower estrogen levels may enhance spatial cognitive performance in humans (34).
Figure 4.
Gender dependence of apoE effects on water maze performance in 6-month-old mice. Wild-type (21 males, 16 females), knockout (14 males, 16 females), NSE-apoE3 (14 males, 6 females), and NSE-apoE4 (13 males, 8 females) mice were compared in the water maze as described in Fig. 2. Data represent results obtained in block 1 (A) and block 2 (B) on the second day of testing. For each of these blocks, two-factor ANOVA revealed highly significant effects of genotype and gender (P = 0.0001) as well as a highly significant interaction between the effects of genotype and gender (P = 0.0001). Significant differences between males and females were identified only in knockout and NSE-apoE4 mice (P = 0.0001) but not in wild-type or NSE-apoE3 mice. Average swim speeds and ability to locate a visible platform on day 5 did not differ significantly between age-matched male and female mice (data not shown).
No ApoE Effects on Passive Avoidance Learning.
We next assessed whether apoE also affects learning in a test that is largely independent of spatial abilities. Unconditioned mice placed into the bright side of a “step-through box” quickly enter the dark compartment. In contrast, mice that have been negatively conditioned (by receiving a mild foot shock after entering the dark compartment for the first time) hesitate to re-enter the dark compartment when tested 24 h later. This latency in re-entering the dark compartment is used as a measure of passive avoidance learning. Testing of 6-month-old female mice (6–16 mice/genotype) showed no significant differences in re-entrance latencies among wild-type (212 ± 32 sec), knockout (215 ± 28 sec), NSE-apoE3 (201 ± 39 sec), and NSE-apoE4 (261 ± 20 sec) mice. Similarly, age-matched male mice showed no significant differences in re-entrance latencies; however, fewer males than females re-entered the dark compartment (data not shown), as previously reported for nontransgenic rodents (35). The finding that apoE4 significantly impaired learning in the water maze but not in the step-through box suggests that apoE4 affects spatial learning more strongly than passive avoidance learning. However, we cannot exclude the possibility that differences in the overall complexity and sensitivity of the two behavioral paradigms contributed to the divergent findings.
Isoform-Specific ApoE Effects on Vertical Exploratory Activity.
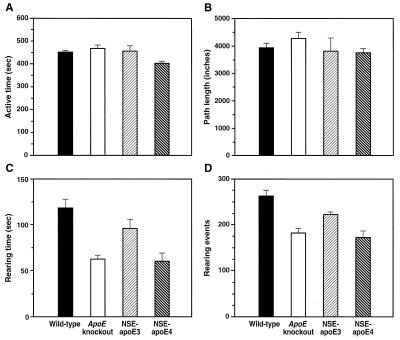
To exclude differences in overall activity that could influence performance on learning tests, we also measured active times and path lengths of the four groups of mice in the open field test (Fig. 5). Only subtle differences were detected in active times, and no differences in path lengths. Thus, differences in overall activity levels do not explain the differences in water maze performance. There was a striking discrepancy between horizontal and vertical exploratory activity. Although path lengths did not differ among groups, NSE-apoE4 and knockout mice, but not NSE-apoE3 mice, had significantly fewer rearing events and shorter rearing times than wild-type controls.
Figure 5.
Effects of cerebral apoE expression on exploratory behavior. Active times (A), path lengths (B), as well as time (C) and frequency (D) of rearing were compared among 6-month-old female wild-type (n = 14), knockout (n = 16), NSE-apoE3 (n = 6), and NSE-apoE4 (n = 8) mice during open field activity. Bar graphs represent results (means ± SEM) obtained during the second of three 10-min observation periods. NSE-apoE4 mice showed mildly reduced active times (A), which differed significantly only from those observed in wild-type mice (P < 0.05 by Dunnett’s but insignificant by Tukey–Kramer posthoc test) and knockout mice (P < 0.05 by Tukey–Kramer posthoc test). ANOVA revealed no significant differences in pathlengths (B) among the four genotypes. Compared with wild-type controls, knockout mice and NSE-apoE4 mice showed significant reductions in frequency (C) and time (D) of rearing (P < 0.01 by Tukey-Kramer posthoc test), whereas NSE-apoE3 mice did not (Tukey–Kramer or Dunnett’s posthoc tests).
Reductions in rearing could reflect impairment of vertical exploratory activity. However, a loss of balance control would also reduce the duration and/or frequency of rearing because the two-legged stance used for rearing is less stable than the four-legged stance used for horizontal movement. Control of body position involves the vestibular part of the inner ear, proprioreceptors in joints and muscles, as well as vision. Six-month-old NSE-apoE4 mice and knockout controls performed normally in tests of vestibular function, including the reaching response (36), air righting reflex (37), and swim patterns (38) (data not shown). In addition, NSE-apoE4 and knockout mice showed no impairments in the rotorod test compared with wild-type controls (Table 1). Knockout controls from the NSE-apoE3 (n = 8) and NSE-apoE4 (n = 6) lines did not differ significantly in rotorod performance (data not shown), indicating that these two lines are well matched with respect to background genes. The superior performance of NSE-apoE3 mice in the rotorod test (Table 1) was not paralleled by increased exploratory behavior compared with wild-type controls (Fig. 5 C and D) or by improved swim speed or capacity to locate the visible platform in the water maze (Table 1).
Because these tests excluded significant impairments in balance, strength, or coordination in NSE-apoE4 and knockout mice, the differences in rearing behavior suggest that vertical exploratory behavior is impaired in ApoE knockout mice and that apoE4 cannot compensate for this deficit. These data raise the intriguing possibility that APOE genotype may also influence exploratory activity in humans, which could affect learning performance.
We found no significant differences in rearing times, rearing events, active times, or path lengths in the open field among 3-month-old female wild-type, knockout, NSE-apoE3, and NSE-apoE4 mice (data not shown). Therefore, the apoE-dependent impairments in rearing behavior are also age-dependent. Compared with age-matched wild-type controls, 6-month-old male NSE-apoE4 and knockout mice displayed subtler reductions in rearing behavior than females (data not shown), further indicating that females are more susceptible than males to apoE-related behavioral impairments.
ApoE-Dependent Neuropathological Alterations.
ApoE knockout mice develop age-dependent neurodegenerative alterations (10), show diminished regenerative capacity after perforant pathway lesions (11), and are more susceptible to injury from focal cerebral ischemia-reperfusion (39). Although these studies have yielded important insights into potential functions of murine apoE, they provide no information on the differential effects of human apoE isoforms on CNS pathobiology. Similarly, although transgenic mice expressing human apoE isoforms in the brain have been generated previously (40, 41), these studies did not determine whether the expression of these molecules had any effects on CNS integrity or function.
In a recent histopathological comparison of wild-type, knockout, NSE-apoE3, and NSE-apoE4 mice (21), we confirmed that ApoE knockout mice develop an age-dependent loss of synaptophysin-immunoreactive presynaptic terminals and microtubule-associated protein-2-positive neuronal dendrites in the neocortex and hippocampus, two brain structures important for cognitive function. Expression of apoE3 on the murine ApoE knockout background prevented this age-related neuronal pathology, but apoE4 did not. However, NSE-apoE4 mice did not show significantly more neurodegeneration than age-matched knockout controls, and there was no difference between male and female NSE-apoE4 mice with respect to neuronal structure. Therefore, these structural alterations cannot fully account for the apoE4-induced functional CNS impairments revealed by the current study. Additional studies are needed to determine the molecular mechanisms that underlie apoE4-induced cognitive deficits.
Conclusions.
The age- and gender-dependent behavioral effects of human apoE isoforms identified in our investigation may be pertinent to cognitive impairments in humans expressing apoE4. It is unclear whether cognitive impairments in human APOE ɛ4 carriers reflect merely the inability of apoE4 to function as well as apoE3 in the CNS or whether apoE4 has detrimental consequences that go beyond a deficiency in function. Our study demonstrates that apoE3 can more than adequately compensate for the lack of murine apoE in the brain of ApoE knockout mice. In contrast, apoE4 expression failed to prevent the age-dependent impairments of vertical exploratory behavior in ApoE knockout mice. Notably, NSE-apoE4 mice were significantly more impaired in their acquisition of the water maze task than knockout controls, indicating that the expression of apoE4 is actually worse than having no apoE at all, consistent with a pathogenic gain-of-function. Experiments are in progress to determine whether apoE3 or apoE4 effects predominate in mice expressing both isoforms on the murine ApoE knockout background.
Although great caution must be applied in extrapolating from findings in mice to complex human diseases, the results from these analyses may provide guidance in the decision whether to pharmacologically inhibit detrimental apoE4 effects or to simulate apoE3 effects in APOE ɛ4 carriers. The increased susceptibility of females to apoE4-induced behavioral impairments identified here underlines that interactions between APOE genotype and gender must be considered carefully in the design and assessment of AD treatments.
Acknowledgments
We thank S. Ordway and G. Howard for editorial assistance, R. Haines for manuscript preparation, and A. Doupe and L. Tecott for critical reading of the manuscript. This research was funded in part by a Cambridge Neuroscience/Gladstone collaborative research agreement. Matthias Orth was partially supported by the Deutsche Forschungsgemeinschaft.
ABBREVIATIONS
- apoE
apolipoprotein E
- CNS
central nervous system
- AD
Alzheimer’s disease
- NSE
neuron-specific enolase
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Mahley R W. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 2.Strittmatter W J, Roses A D. Annu Rev Neurosci. 1996;19:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- 3.Weisgraber K H, Mahley R W. FASEB J. 1996;10:1485–1494. doi: 10.1096/fasebj.10.13.8940294. [DOI] [PubMed] [Google Scholar]
- 4.Tang M X, Maestre G, Tsai W Y, Liu X H, Feng L, Chung W Y, Chun M, Schofield P, Stern Y, Tycko B, Mayeux R. Ann NY Acad Sci. 1996;802:6–15. doi: 10.1111/j.1749-6632.1996.tb32593.x. [DOI] [PubMed] [Google Scholar]
- 5.Arendt T, Schindler C, Brückner M K, Eschrich K, Bigl V, Zedlick D, Marcova L. J Neurosci. 1997;17:516–529. doi: 10.1523/JNEUROSCI.17-02-00516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrer L A, Cupples L A, Haines J L, Hyman B, Kukull W A, Mayeux R, Myers R H, Pericak-Vance M A, Risch N, van Duijn C M. J Am Med Assoc. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 7.Reed T, Carmelli D, Swan G E, Breitner J C S, Welsh K A, Jarvik G P, Deeb S, Auwerx J. Arch Neurol. 1994;51:1189–1192. doi: 10.1001/archneur.1994.00540240033012. [DOI] [PubMed] [Google Scholar]
- 8.Yaffe K, Cauley J, Sands L, Browner W. Arch Neurol. 1997;54:1110–1114. doi: 10.1001/archneur.1997.00550210044011. [DOI] [PubMed] [Google Scholar]
- 9.Piedrahita J A, Zhang S H, Hagaman J R, Oliver P M, Maeda N. Proc Natl Acad Sci USA. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masliah E, Mallory M, Ge N, Alford M, Veinbergs I, Roses A D. Exp Neurol. 1995;136:107–122. doi: 10.1006/exnr.1995.1088. [DOI] [PubMed] [Google Scholar]
- 11.Masliah E, Mallory M, Alford M, Ge N, Mucke L. In: Research Advances in Alzheimer’s Disease and Related Disorders. Iqbal K, Mortimer J, Winblad B, Wisniewski H, editors. New York: Wiley; 1995. pp. 405–414. [Google Scholar]
- 12.Gordon I, Grauer E, Genis I, Sehayek E, Michaelson D M. Neurosci Lett. 1995;199:1–4. doi: 10.1016/0304-3940(95)12006-p. [DOI] [PubMed] [Google Scholar]
- 13.Oitzl M S, Mulder M, Lucassen P J, Havekes L M, Grootendorst J, De Kloet E R. Brain Res. 1997;752:189–196. doi: 10.1016/s0006-8993(96)01448-5. [DOI] [PubMed] [Google Scholar]
- 14.Masliah E, Samuel W, Veinbergs I, Mallory M, Mante M, Saitoh T. Brain Res. 1997;751:307–314. doi: 10.1016/s0006-8993(96)01420-5. [DOI] [PubMed] [Google Scholar]
- 15.Han S-H, Einstein G, Weisgraber K H, Strittmatter W J, Saunders A M, Pericak-Vance M, Roses A D, Schmechel D E. J Neuropathol Exp Neurol. 1994;53:535–544. doi: 10.1097/00005072-199409000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Bao F, Arai H, Matsushita S, Higuchi S, Sasaki H. NeuroReport. 1996;7:1733–1739. doi: 10.1097/00001756-199607290-00008. [DOI] [PubMed] [Google Scholar]
- 17.Metzger R E, LaDu M J, Pan J B, Getz G S, Frail D E, Falduto M T. J Neuropathol Exp Neurol. 1996;55:372–380. doi: 10.1097/00005072-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Kida E, Pluta R, Lossinsky A S, Golabek A A, Choi-Miura N-H, Wisniewski H M, Mossakowski M J. Brain Res. 1995;674:341–346. doi: 10.1016/0006-8993(94)01467-v. [DOI] [PubMed] [Google Scholar]
- 19.Horsburgh K, Nicoll J A R. Neuropathol Appl Neurobiol. 1996;22:342–349. doi: 10.1111/j.1365-2990.1996.tb01113.x. [DOI] [PubMed] [Google Scholar]
- 20.Bellosta S, Nathan B P, Orth M, Dong L-M, Mahley R W, Pitas R E. J Biol Chem. 1995;270:27063–27071. doi: 10.1074/jbc.270.45.27063. [DOI] [PubMed] [Google Scholar]
- 21.Buttini, M., Orth, M., Bellosta, S., Pitas, R. E., Wyss-Coray, T., Mahley, R. W. & Mucke, L. (1998) Neurobiol. Aging 19(Suppl. 4S), S56. [DOI] [PMC free article] [PubMed]
- 22.Anderson R, Higgins G A. Psychopharmacology. 1997;132:135–144. doi: 10.1007/s002130050329. [DOI] [PubMed] [Google Scholar]
- 23.Hecker K H, Roux K H. BioTechniques. 1996;20:478–485. doi: 10.2144/19962003478. [DOI] [PubMed] [Google Scholar]
- 24.Forss-Petter S, Danielson P E, Catsicas S, Battenberg E, Price J, Nerenberg M, Sutcliffe J G. Neuron. 1990;5:187–197. doi: 10.1016/0896-6273(90)90308-3. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence, A. D. & Sahakian, B. J. (1995) Alzheimer’s Dis. Assoc. Disorders 9 (Suppl. 2), 43–49. [PubMed]
- 26.Morris R J. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 27.Moran P M, Higgins L S, Cordell B, Moser P C. Proc Natl Acad Sci USA. 1995;92:5341–5345. doi: 10.1073/pnas.92.12.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klapdor K, Van der Staay F J. Physiol Behav. 1996;60:1247–1254. doi: 10.1016/s0031-9384(96)00224-7. [DOI] [PubMed] [Google Scholar]
- 29.Wolfer D P, Müller U, Stagliar M, Lipp H-P. Brain Res. 1997;771:1–13. doi: 10.1016/s0006-8993(97)00673-2. [DOI] [PubMed] [Google Scholar]
- 30.Farlow M R. Neurology. 1997;48:S30–S34. doi: 10.1212/wnl.48.5_suppl_6.30s. [DOI] [PubMed] [Google Scholar]
- 31.Owen E H, Logue S F, Rasmussen D L, Wehner J M. Neuroscience. 1997;80:1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- 32.Birge S J. Neurology. 1997;48:S36–S41. doi: 10.1212/wnl.48.5_suppl_7.36s. [DOI] [PubMed] [Google Scholar]
- 33.Kampen D L, Sherwin B B. Obstet Gynecol. 1994;83:979–983. doi: 10.1097/00006250-199406000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Janowsky J S, Oviatt S K, Orwoll E S. Behav Neurosci. 1994;108:325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- 35.Beatty W W, Gregoire K C, Parmiter L L. Bull Phychon Soc. 1973;2:99–100. [Google Scholar]
- 36.Steel K P, Bock G R. Behav Neurosci. 1983;97:381–391. doi: 10.1037//0735-7044.97.3.381. [DOI] [PubMed] [Google Scholar]
- 37.Ossenkopp K P, Prkacin A, Hargreaves E L. Pharmacol Biochem Behav. 1990;36:875–881. doi: 10.1016/0091-3057(90)90093-w. [DOI] [PubMed] [Google Scholar]
- 38.Horn K M, DeWitt J R, Nielson H C. Physiol Psychol. 1981;9:371–378. [Google Scholar]
- 39.Laskowitz D T, Sheng H, Bart R D, Joyner K A, Roses A D, Warner D S. J Cerebr Blood Flow Metab. 1997;17:753–758. doi: 10.1097/00004647-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Bowman B H, Yang F, Buchanan J M, Adrian G S, Martinez A O, Jansen L, Zhao M, Atherton S L, Hixson J E. Neurosci Lett. 1996;219:57–59. doi: 10.1016/s0304-3940(96)13166-9. [DOI] [PubMed] [Google Scholar]
- 41.Xu P-T, Schmechel D, Rothrock-Christian T, Burkhart D S, Qiu H L, Popko B, Sullivan P, Maeda N, Saunders A M, Roses A D, Gilbert J R. Neurobiol Dis. 1996;3:229–245. doi: 10.1006/nbdi.1996.0023. [DOI] [PubMed] [Google Scholar]