Abstract
Background
Accumulating evidence from postmortem and magnetic resonance imaging (MRI) studies suggests that abnormalities of medial temporal lobe structures are critically involved in the pathogenesis of schizophrenia. It is still unclear, however, whether certain abnormalities are already present in individuals at ultra high-risk (UHR) for transition into psychosis. Recent studies involving patients at UHR showed contradictory results for hippocampal volume, and only 1 study reported that amygdalar volume was unchanged between healthy patients and those at UHR. Furthermore, no subregions of the hippocampus have been investigated in people at UHR.
Methods
We recruited 29 UHR patients, 23 first-episode patients and 29 age-and sex-matched healthy controls. We measured hippocampal and amygdalar volumes from MRI scans by use of BRAINS2 to manually trace the regions of interest. The hippocampi were divided in 2 regions: head and corpus/tail.
Results
Patients at UHR had significantly smaller volumes of the hippocampus corpus and tail bilaterally, but not of the head, compared with healthy controls. Group differences for the right hippocampus corpus and tail volume remained significant after we controlled for whole brain volume and other covariates. We found that UHR patients who later developed psychosis had smaller right hippocampus corpus and tail volumes than did those who did not develop psychosis. First-episode patients had significantly smaller left amygdalar volumes than did healthy individuals or those at UHR.
Limitations
Our study had a small sample size, and we were unable to control for the effects of medication.
Conclusion
Our findings suggest that parts of the hippocampal–amygdalar complex are involved in the pathogenesis of schizophrenia. Reduction of hippocampus corpus and tail volumes may be indicative of the prodromal phase of schizophrenia and represent risk factors for transition into psychosis. Further investigations are needed to determine whether structural changes of the left amygdala play a role during transition from the prodromal phase to the first manifest episode of schizophrenia.
Introduction
Accumulating evidence from neuropsychological and imaging studies suggests that the hippocampal and entorhinal regions of the brain are critically involved in the pathogenesis of schizophrenia. Recent meta-analyses of studies involving the use of voxel-based morphometry revealed that patients with schizophrenia have structural deficits in the temporal lobe areas, especially in the hippocampus.1–3 The hypothesis that abnormalities of medial temporal lobe structures are key components of the schizophrenic disease process is supported by evidence indicating that even first-episode patients show a loss of grey matter of the hippocampal–amygdalar complex in both hemispheres.4 Similarly, bipolar patients with psychotic symptoms differ from patients without psychotic symptoms and controls in that they show comparable decreased left hippocampal volumes similar to schizophrenia patients, relative to healthy controls.5
Whether such volume reductions — particularly of grey matter — of hippocampal and amygdalar structures represent trait markers of psychosis and may be present before the onset of psychotic symptoms or in first-degree relatives is a matter of debate. McDonald and colleagues6 reported no structural changes of the hippocampal region in unaffected first-degree relatives of schizophrenia patients. In contrast, Seidman and coworkers7 described smaller left hippocampal volumes particularly in nonpsychotic first-degree relatives from families in which more than one person had schizophrenia. Left hippocampus volume was shown to be significantly associated with verbal memory, perhaps reflecting a vulnerability marker of schizophrenia. O’Driscoll and colleagues8 and Ongur and colleagues9 reported similar results.
The situation becomes more puzzling, however, when individuals at ultra high-risk (UHR) of schizophrenia are included. Because schizophrenia does not break out abruptly but rather develops through a perennial prodromal phase, it may be important to intervene early and to identify the neurobiological markers and the pathophysiology of schizophrenia. There are many definitions of the initial prodrome of schizophrenia, but they all describe a period that begins with the first changes in a person and extends up to the development of the first psychotic symptom.10 So-called prodromal symptoms include attenuated positive symptoms (illusions, ideas of reference, magical thinking, superstitiousness), brief limited intermitted psychotic symptoms (hallucination and delusion lasting less then 7 days), so-called cognitive basic symptoms (thought interference, tendency of self reference, changed language expression) and social withdrawal.10–12
Early and late neurodevelopmental disturbances in schizophrenia and their functional consequences have been investigated, and results show that structural brain abnormalities are already apparent in the premorbid stage.13 In a large study comparing people at UHR with first-episode patients and healthy controls, Phillips and colleagues14 reported reduced left and right hippocampal volumes in the UHR group and in first-episode patients compared with controls. Surprisingly, in UHR individuals who later developed a psychotic disorder, the volume of the left hippocampus was larger than that in the nonpsychotic UHR and first-episode patients. In addition, the same group15 found that the volume of the left hippocampus in UHR individuals with a family history of schizophrenia was larger than that in UHR individuals without a familial disposition. In an enlarged sample of individuals at UHR, hippocampal and amygdalar sizes did not differ from controls, irrespective of whether or not the UHR participants later developed a psychotic disorder.16 In the same study, robust results of hippocampal volume reductions were found in patients with chronic schizophrenia and first-episode schizophrenia. These findings therefore suggest that structural changes of the medial temporal lobe may emerge only after the onset of psychotic symptoms and that environmental factors play a decisive role in the development of structural brain anomalies.
In light of these conflicting results, we were interested in investigating hippocampal and amygdalar volumes in an independent sample of individuals at UHR of schizophrenia and in first-episode patients. Because the hippocampus is a complex and widely distributed structure, we also focused on anatomic subdivisions, which have not yet been investigated in UHR individuals. Specifically, we hypothesized that both UHR and first-episode patients would have smaller hippocampal volumes than would healthy controls and that the hippocampal volumes would be intermediate in size in UHR people. We also explored the differences in amygdalar volume between the diagnostic groups.
Methods
Participants
In total, 81 participants (29 UHR patients, 23 first-episode schizophrenia patients and 29 healthy controls) aged 18–38 years (mean 25.7, standard deviation [SD] 5.2 yr) took part in this study. Table 1 summarizes the participants’ demographic and clinical characteristics. The UHR participants were recruited from the Early Recognition and Intervention Center, Department of Psychiatry and Psychotherapy of the Charité Berlin (Central Campus) and were diagnosed according to the Structured Interview for Prodromal Symptoms.17 We included patients who had at least 1 attenuated positive symptom with a severity level of at least 3 (Box 1). First-episode schizophrenia patients (diagnosed by DSM-IV18) were in-patients or had frequent contact with the out-patient clinic.
Table 1.
Demographic and clinical characteristics of the 81 study participants
| Characteristic | Group; mean (SD)* |
Statistics |
|||
|---|---|---|---|---|---|
| Ultra hih-risk, n = 29 |
First-episode schizophrenia, n = 23 |
Control, n = 29 |
Test | p value | |
| Age, yr | 25.3 (4.3) | 26.4 (6.1) | 25.7 (5.2) | F2,78 = 0.3 | 0.92 |
| Sex, male:female | 19:10 | 16:7 | 17:12 | χ2 = 0.70 | 0.87 |
| Handedness: right, left, mixed or unknown | 24, 2, 3 | 13, 5, 5 | 23, 5, 1 | χ2 = 7.33 | 0.23 |
| Short-duration antipsychotic medication, yes:no | 11:18 | 6:17 | — | χ2 = 3.42 | 0.57 |
| PANSS positive | 11.9 (3.4) | 19.3 (5.1) | t50 = −6.17 | < 0.001 | |
| PANSS negative | 13.6 (5.2) | 18.0 (5.1) | t50 = −3.08 | 0.003 | |
| Whole-brain volume, cm3 | 1259 (146) | 1214 (107) | 1262 (99) | F2,78 = 1.22 | 0.75 |
NS = not significant; PANSS = Positive and Negative Syndrome Scale;25 SD = standard deviation.
Unless otherwise indicated.
Box 1. Inclusion and exclusion criteria.
Ultra high-risk patients
Inclusion criteria
-
Attenuated psychotic symptoms
- Presence of at least 1 of the following symptoms: ideas of reference, odd beliefs or magical thinking, unusual perceptual experiences, odd thinking and speech, suspiciousness or paranoid ideation, odd behaviour or appearance (SIPS score of 3, 4 or 5)
- Symptoms occurring at least several times a week
- Duration of mental state change of at least 1 week within the last 3 months
-
No substance abuse
- For cannabis, there had to be a drug-free period of at least 3 months if a symptom was not definitely present before the use of any drug. The respondent could, however, be included, if the symptom was present before cannabis use
- If a symptom was definitely present before the use of cannabis, the respondent with present abuse could be included
- For hallucinogenics and amphetamines, the drug-free period had to be 3 months (i.e., new symptoms had to still present after this period)
Exclusion criteria
-
Acute psychosis
- Presence of at least 1 of the following psychotic symptoms: delusions, formal thought disorders or hallucinations (PANSS score P1–P3, P5–P6 ≥ 4 within the last 3 months)
- Symptoms occurring at least several times a week
- Duration of mental state change of longer than 1 week
First-episode schizophrenia patients
Inclusion criteria
Acute psychosis
Exclusion criteria
Same as for ultra high-risk patients
All patients and healthy controls
Exclusion criteria
Organic mental disorder
History of alcohol dependence
Cannabis abuse in the last 3 months, except if the psychotic symptoms were experienced before the onset of cannabis abuse
Verbal IQ below 85
Gross abnormalities in a brain MRI scan
IQ = intelligence quotient; MRI = magnetic resonance imaging; PANSS = Positive and Negative Syndrome Scale;25 SIPS = Structured Interview for Prodromal Symptoms.17
Before being included in the study, participants underwent a medical examination and blood and urine tests to exclude physical health problems. We screened the healthy controls by use of the Mini International Neuropsychiatric Interview19 for mental diseases in their history or that of their family. We excluded any individual with a history of neurologic or severe somatic illness, head injury or alcohol dependence. We also excluded those with a history of cannabis abuse during the 3 months before scanning, except for those whose psychotic symptoms began before the onset of cannabis abuse. Patients with prodromal symptoms received a diagnosis of schizotypal disorder (ICD-10 F21, DSM-IV-TR 301.22).
We determined handedness using the Edinburgh Handedness Inventory,20 and we estimated premorbid verbal IQ using a German screening instrument (MWT-B) that resembles the Spot-the-Word-Test.21 This study was approved by the local ethics committee (Medical Faculty, Charité Berlin) and was carried out in accordance with the Declaration of Helsinki (Edinburgh 2000 version). All participants gave their written informed consent after the study design and procedures had been fully explained to them. A psychiatrist who was independent from the study confirmed after his own investigation that the patients with schizophrenia were capable of giving informed consent.
Structural MRI scanning
We performed magnetic resonance imaging (MRI) using a Siemens MAGNETOM Symphony 1.5-T scanner. We acquired a 3-dimensional structural MRI scan for each participant by use of a T1-weighted magnetization-prepared rapid-acquisition gradient-echo (MPRAGE) sequence (repetition time 2280 ms, echo time 3.93 ms, TI 1100 ms, flip angle 15°, matrix size 256 × 256 and a field of view of 256 × 256 mm2, which gave 160 transversal slices with a thickness of 1 mm with a voxel size of 1 × 1 × 1 mm3).
A neuroradiologist reviewed all MRI brain scans; no gross abnormalities (e.g., tumour, space-occupying cystic lesion greater 10 mm, signs of bleeding, contusion, infarction, major grey or white matter lesions) were reported.
Data analysis
We analyzed the data using BRAINS2 (Brain Research: Analysis of Images, Networks and Systems; Iowa Mental Health Clinical Research Center, www.psychiatry.uiowa.edu). BRAINS2 allows manual tracing of regions of interest (ROIs) and calculates the volume of the ROIs. Manual tracing was made using double-amplified images. In cases where the anatomic borders of the target structures were blurred, we used 4-fold–enlarged images. Calculations of whole brain volume were made with SPM2 (www.fil.ion.ucl.ac.uk/spm/).22
Hippocampus
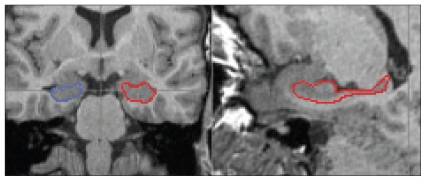
By use of the software MRIcro (www.sph.sc.edu/comd/rorden/mricro.html), we tipped the MRI scans along the longitudinal axis of the hippocampus in horizontal line to get a better rating of the hippocampal borders.23 To draw the anatomic borders of the hippocampus, we used the scripts of Pantel and colleagues24 and Pruessner and colleagues.25 We generated the hippocampal volume using the coronal slices. In the coronal images, the identification of the hippocampal borders, especially the differentiation of the hippocampus and amygdala, is very difficult. To facilitate the delineation in coronal images, we marked the hippocampal borders in the sagittal slices. These marked borders could also be seen in the coronal images. The drawings in the sagittal slices were obtained from lateral to medial MRI slices.23 The hippocampus was divided into 2 regions: head and corpus/tail (Fig. 1). The border between the 2 subregions was defined by the sulcus hippocampus, which was clearly visible on the sagittal images.
Fig. 1.
Coronal (left) and sagittal (right) 1-mm magnetic resonance imaging slices showing the hippocampi. The right hippocampus is circled in blue and the left hippocampus in red.
Amygdala
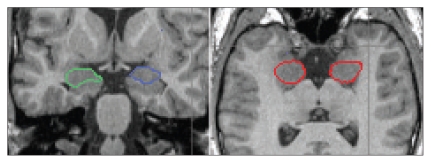
To draw the anatomic borders of the amygdala, we used the scripts of Pantel and colleagues24 and Pruessner and colleagues.25 In contrast to their methods, we marked the amygdalar borders in the axial and sagittal images first. These marked borders could also be seen in the coronal slices, and they helped us to draw the boundaries in the coronal slices that were done from posterior to anterior MRI slices. We determined the amygdalar volume using the coronal slices23 (Fig. 2).
Fig. 2.
Coronal (left) and axial (right) 1-mm magnetic resonance imaging slices showing the amygdalae. The right amygdala is circled in green (and red in the axial slice) and the left amygdala is circled in blue (and red in the axial slice).
Interrater and intrarater reliability
To test the intrarater reliability, we measured the ROI volume twice for 5 brains. The period between the first and second measurement was at least 3 weeks. The interrater reliability was determined by measuring the volumes of the ROIs of the 5 brains by an independent second investigator. Neither investigator knew the patients’ names or which group the images were from.
Statistical analysis
We analyzed the volumes of the ROIs separately for the left and right side. To test for differences between the groups for volume, we used analysis of variance (ANOVA) with independent-samples t test as post-hoc tests. Because we found no differences in whole brain volume, age, sex, medication and handedness, we used the uncorrected values in the primary analyses. To control for these variables in the secondary analyses, we performed analyses of covariance (ANCOVA) with group as the independent variable and total brain volume, sex or medication as the covariates.
We calculated the intraclass coefficients for intrarater and interrater reliability. To test for associations between ROI volume and psychopathology, we used Spearman nonparametric correlations. We assessed the group differences for age and whole brain volume using ANOVA and the group differences in sex, handedness and medication using the χ2 test. We compared the clinical parameters (Positive and Negative Syndrome Scale [PANSS26] between groups using independent-samples t tests. For comparisons between groups with small sample sizes, we used nonparametric statistics (Mann–Whitney U test). Owing to the exploratory character of the hippocampal subdivision analyses, we did not correct for multiple comparisons. Values are expressed as mean and standard deviation (SD).
Results
Of the patients with prodromal symptoms, 14 had light-to-middle depressive syndrome and 1 had an obsessive–compulsive disorder. Less than half of the UHR patients (11/29) had taken either risperidone or olanzapine in low dosages and only for a few days (mean 1.9, SD 1.2 d); the others were free of medication. Six of the 23 patients with first-episode schizophrenia had taken typical or atypical neuroleptics at clinical dosages but only for a few days (mean 2.6, SD 1.9 d). The others had never taken a neuroleptic.
Within 9 months after MRI scanning, 2 of the 29 UHR patients made the transition to schizophrenic psychosis, and we lost contact with 6 patients, probably because they transitioned into full-blown psychosis (assume based on the patients’ clinical information). Thus, we were able to include 8 converters (5 male, 3 female; mean age 24.4, SD 5.1 yr) and 21 patients (14 male, 7 female; mean age 25.9, SD 4.0 yr) who remained clinically stable over the observation period of 9 months (conversion rate 28%).
Intrarater and interrater reliability for determining the size and extension of the ROIs, whole brain volumes and grey matter volumes were within acceptable ranges (intraclass correlation coefficients ranged from 0.64 [p < 0.05] to 0.89 [p < 0.001]). For the amygdala, intrarater reliability was 0.92 (p = 0.001) and interrater reliability was 0.85 (p = 0.003). For the hippocampus, intrarater reliability was 0.97 (p = 0.001) for the whole hippocampus, 0.86 (p = 0.001) for the corpus/tail and 0.96 (p = 0.001) for the head. The interrater reliability was 0.85 (p = 0.001) for the whole hippocampus, 0.74 (p = 0.02) for the corpus/tail and 0.78 (p = 0.01) for the head.
There were no differences between the UHR group, first-episode group and control group for age, sex distribution or handedness. Whole-brain volume did not differ significantly between the 3 groups (Table 1).
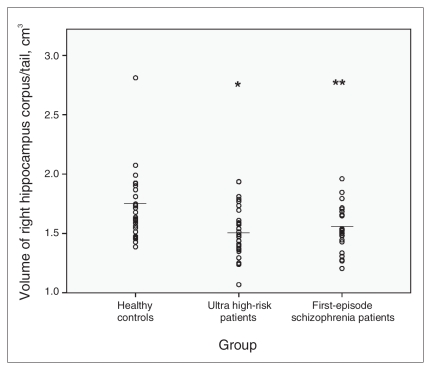
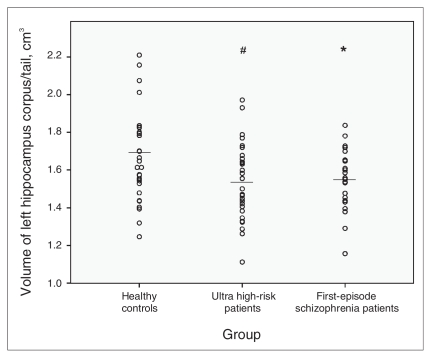
We observed significant differences among the 3 groups in the size of the right hippocampal corpus and tail (F2,78 = 5.40, p = 0.006), and the differences approached significance for the volumes of the left hippocampal corpus and tail (F2,78 = 2.84, p = 0.064). Exploratory analysis revealed differences in the volume of the left amygdala among the groups (F2,78 = 3.17, p = 0.047). We found no differences in the size of the right amygdala and in the volume of the right and left hippocampus. Post-hoc t tests revealed that the UHR patients had significantly smaller volumes of the right (t56 = −2.90, p = 0.005) (Fig. 3) and left hippocampal corpus and tail (t56 = −2.09, p = 0.047) (Fig. 4) than did the controls.
Fig. 3.
Mean right hippocampal corpus/tail volumes, with p values from post-hoc t tests. *Significant at p < 0.05 compared with healthy controls. **Significant at p < 0.01 compared with healthy controls.
Fig. 4.
Mean left hippocampal corpus/tail volumes, with p values from post-hoc t tests. #Significant at p < 0.10 compared with healthy controls. *Significant at p < 0.05 compared with healthy controls.
Ultra high-risk converters had significantly smaller volumes of the right hippocampal corpus and tail than did nonconverting UHR patients (mean 1.42 [SD 0.21] v. 1.58 [SD 0.18] cm3; Z = −2.23, p = 0.037) After we corrected the values for sex, the difference was still significant (Z = −2.47, p = 0.031), whereas the difference for the left side was not significant (mean 1.43 [SD 0.21] v. 1.53 [SD 0.22] cm3; Z = −1.37, p = 0.13). There was no difference in amygdalar volume (mean 1.82 [SD 0.34] v. 1.91 [SD 0.29] cm3; p = 0.83). Converters were slightly more ill compared with nonconverters (PANSS positive: 12.4 [SD 3.4] v. 10.4 [SD 3.0]; Z = −1.74, p < 0.10; PANSS negative: 15.1 [SD 5.0] v. 9.3 [SD 2.8]; Z = −4.01, p < 0.01).
First-episode patients differed from controls not only in their right (t50 = −2.31, p = 0.025) and left hippocampal corpus and tail volumes (t50 = −1.83, p = 0.061) but also in the left amygdalar volume (t50 = −2.27, p = 0.028), which was considerably smaller in first-episode patients than in controls (Fig. 5). The UHR and first-episode patients differed significantly only in left amygdalar volume, with first-episode patients having smaller volumes (t50 = 2.27, p = 0.028). We found no differences in right or left hippocampal volume between UHR and first-episode patients.
Fig. 5.
Mean left amygdala volumes, with p values from post-hoc t tests. *Significant at p < 0.05 compared with healthy controls.
We found no significant correlation coefficients for whole brain volume and any of the ROI parameters (p > 0.05). Our analyses of variance using whole brain volume as a covariate revealed that group differences for right hippocampal corpus and tail volume remained significant (F2,78 = 5.06, p = 0.009). There was only a marginal influence of whole brain volume on left hippocampal corpus and tail size (F2,78 = 2.48, p = 0.091) as well as on the left amygdala (F2,78 = 3.06, p = 0.053) (Table 2). Although medication (2-level factor: medication taken or not) in the 2 patient groups significantly influenced group comparisons of the left hippocampal corpus and tail volume (F2,50 = 1.54, p = 0.22); no such effect was observed for right hippocampal corpus and tail volume (F2,50 = 4.00, p = 0.022) or left amygdalar size (F2,50 = 3.35, p = 0.040).
Table 2.
Values of hippocampal and amygdalar volumes for ultra high-risk, first-episode and healthy participants
| Brain region | Group; volume, cm3, mean (SD) |
ANCOVA effect |
|||||
|---|---|---|---|---|---|---|---|
| Ultra high risk | First-episode schizophrenia | Healthy controls | Whole brain volume |
Group |
|||
| F278 | P | F278 | p | ||||
| Amygdala | |||||||
| Left | 1.86 (0.31) | 1.69 (0.24) | 1.83 (0.20) | 9.78 | 0.001 | 3.06 | 0.057 |
| Right | 1.87 (0.28) | 1.74 (0.25) | 1.82 (0.20) | 6.55 | 0.014 | 1.46 | 0.23 |
| Hippocampus | |||||||
| Corpus tail right | 1.51 (0.22) | 1.53 (0.19) | 1.70 (0.29) | 2.51 | 0.13 | 5.06 | 0.009 |
| Head right | 2.26 (0.31) | 2.10 (0.31) | 2.18 (0.37) | 7.81 | 0.001 | 1.56 | 0.21 |
| Total right | 3.77 (0.43) | 3.64 (0.37) | 3.88 (0.53) | 8.01 | 0.001 | 0.84 | 0.54 |
| Corpus tail left | 1.54 (0.20) | 1.55 (0.16) | 1.66 (0.25) | 4.42 | 0.047 | 2.48 | 0.09 |
| Head left | 2.09 (0.25) | 1.95 (0.32) | 1.96 (0.36) | 9.88 | 0.001 | 2.14 | 0.14 |
| Total left | 3.62 (0.39) | 3.50 (0.36) | 3.62 (0.51) | 8.38 | 0.001 | 0.18 | 0.84 |
ANCOVA = analysis of covariance; SD = standard deviation.
The main results did not change when we performed ANCOVA using sex or age as covariates. When we performed a multivariate ANCOVA (general linear model) analysis with “right/left hippocampus corpus/tail” as repeated measurements within the participant factor, “group” and “sex” as between-subject factors and “total brain volume” as a covariate, the effect for “group” was still significant (F2,76 = 4.22, p = 0.018). When we performed the same analysis for “left/right amygdala,” there was no longer a significant effect of group (F2,76 = 1.96, p = 0.14). Finally, we found no significant differences in size between the left and right amygdala (t79 = 0.73, p = 0.47) and hippocampal corpus/tail (t79 = 0.11, p = 0.92). There were no significant correlations between general, positive or negative symptomatology, as measured with PANSS, and any of the volume parameters for the ROIs.
Discussion
In the present study, we sought to determine the volumes of hippocampal subdivisions and the amygdala on both sides of the brain in individuals at UHR of schizophrenia and in first-episode patients and matched controls. Consistent with some, but not all, previous studies, we found bilateral reduced hippocampal volume in the corpus and tail (pronounced on the right side) in both UHR and first-episode patients compared with controls. Ultra-high risk converters who developed psychosis within 9 months after MRI scanning had smaller hippocampal corpus and tail volumes than did UHR patients who remained clinically stable.
In contrast to our original assumption, we did not find any differences in hippocampal volume between the 2 diagnostic groups. However, the volume of the left amygdala was reduced in first-episode patients compared with UHR patients and controls, whereas amygdalar volume did not differ between UHR patients and healthy controls. These findings were largely independent of whole brain volume, handedness, age, sex, psychopathology and medication use. Notably, whole brain volumes did not differ between UHR, first-episode patients and healthy controls, suggesting normal total brain volume in the early stages of schizophrenia. Interestingly, we found no significant group differences for the hippocampus head. This finding is in line with 2 previous studies,27,28 which also failed to determine changes in the hip-pocampal head volume in patients with schizophrenia, yet revealed differences in the shape of the hippocampus in schizophrenia patients relative to controls. Although macroscopic and microscopic alterations of hippocampal structures may not be associated, we speculate that the negative finding with regard to the hippocampal head in UHR and first-episode patients suggests that the histological CA1 region is unaffected in schizophrenia.29
Neurodevelopmental models of schizophrenia suggest that cell migration, particularly in the medial temporal lobes and limbic system, is impaired during fetal brain growth in individuals who later develop schizophrenia.30,31 Although a loss of cortical and subcortical neuronal mass, especially in the limbic system, has been well established in patients with chronic schizophrenia,1,32 morphological changes in the hippocampus and amygdala — key areas involved in the schizophrenic disease process — have not unequivocally been demonstrated in early stages of schizophrenia.33,34 In the last couple of years, several studies have been published both in support of or refuting the assumption of volume reductions in these areas in the prodromal stages as compared with first-episode patients with schizophrenia.14,16 For example, Pantelis and colleagues35,36 hypothesized that morphological brain changes are critical in the onset of psychosis. Accordingly, it is assumed that early neurodevelopmental insults interact with postpubertal brain maturation (in addition to which nonspecific stress acts as precipitating factor) to produce structural and functional alterations in subsequent stages of the disorder, especially located within the temporal and frontal lobes.37
In contrast, a longitudinal study did not find progressive brain changes in these areas in multiple-episode patients with schizophrenia.38 Even more recently, another longitudinal study revealed that the greater severity of morphological changes in adolescent-onset schizophrenia evolved into a more typical adult-onset pattern over time, suggesting a delay and alteration of the brain maturation processes rather than a simple progressive loss of brain tissue.39 Thus, exactly what happens during the transition period from prodromal to manifest psychosis is unclear. Our findings can tentatively be interpreted in line with those of Pantelis and colleagues35,36 as providing evidence for morphological alterations in parts of the medial temporal lobe that are critically involved in the pathogenesis of schizophrenia and already quantifiable before the onset of full-blown psychosis.
Moreover, our findings indicate a possible progression of the disease process from the prodromal stages to the first clinical manifestation of schizophrenic disorder, because a reduction of the amygdalar volume was found only in first-episode patients, whereas reduction of hippocampal volume was found in both groups. Whether the putative progressive cell loss in the left amygdala is caused by antipsychotic treatment or disease-related processes is currently unknown. However, the amygdala is known to be intimately tied to the processing of fearful stimuli,29,39 and it has been hypothesized that cortisol and glutamate release because of nonspecific stressors may exert neurotoxic effects on the amygdala during the first psychotic episode.40
Limitations
Our study has several limitations. The sample size was fairly small, which might have reduced the statistical power. Accordingly, the number of participants in our study precluded an analysis of schizophrenia subtypes. Moreover, we could not completely exclude an influence of medication. However, most patients in our study received antipsychotic medication for less than 3 days; as such, the possible effect of antipsychotics on hippocampal and amygdalar volumes can be deemed to be negligible.
Also, our study did not include another clinical control group. Therefore, it remains unclear as to what extent our findings are specific to psychosis. For example, according to a recent meta-analysis, hippocampal volume is also reduced in patients with major depression, although this was less pronounced than in patients with psychosis.41 Finally, it cannot be ruled out that our study included a largely schizotypal population that was not so much at UHR of schizophrenia but was manifesting a more stable syndrome indicative of a genetic liability to schizophrenia. Studies involving MRI scanning suggest that patients with schizotypal personality disorder show abnormalities in some, but not all, of the brain regions implicated in schizophrenia. Several studies found reduced temporal cortex volume, particularly in the superior temporal gyrus42,43 but also in the middle and inferior temporal gyrus.44 Reduction of medial temporal volume, including the amygdala and/or hippocampal complex, has not been consistently observed in schizotypal personality disorder.45 Only one study reported reduced hippocampal volume in women with schizotypal personality disorder.46 Follow-up studies of patients with prodromal symptoms showed that those who later made the transition to schizophrenia had several psychiatric diagnosis such as major depression, dysthymic disorder, hypochondriasis, somatization disorder, panic disorder, generalized anxiety disorder, obsessive–compulsive disorder and all kinds of personality disorders.12,47 That is why we did not use these psychiatric disorders as exclusion criteria for the diagnosis of a prodromal state.
We did not use diagnostic instruments to analyze the personality structure in detail because no personality disorder (except schizotypal personality disorder) has been reported to have a significant positive correlation with subsequent diagnosis of schizophrenia.12 On the other hand, our study was performed in accordance with the methodological requirements for magnetic resonance–based in vivo hippocampal volumetrics;48 that is, a magnetic field strength of 1.5 T, slice thickness of 1 mm (compared with other studies that used slices of 1.5 mm; for example, Woods and colleagues34 and Velakoulis and colleagues16), discernible anatomic boundaries of the hippocampus and amygdala and use of the BRAINS2 program to provide a simultaneous presentation of the axial, coronal and sagittal views.
Conclusion
Our study shows that morphological changes in key areas of the schizophrenic disease process can already be detected in patients at UHR of schizophrenia. These individuals had similar volume reductions of parts of the hippocampus as did first-episode patients. Unlike first-episode patients, however, people at UHR of psychosis did not have abnormalities in the amygdala. Longitudinal studies with individuals at UHR and after manifestation of full-blown psychosis may reveal whether the amygdala is subject to progressive changes in the early stages of schizophrenia and whether such neuronal losses can be prevented by the early use of antipsychotic and nonpharmacological treatments.
Acknowledgement
This study was supported in part by an unrestricted grant from the Charities Aid Foundation and was, in part, associated with the framework of the European Prediction of Psychosis Study (EPOS, QLRT-2000-01081, European Commission 5th Program).
Footnotes
Competing interests: Andreas Heinz has received research funding from the German Research Foundation and the Bernstein Centre for Computational Neuroscience Berlin (German Federal Ministry of Education and Research), Eli Lilly & Company, Janssen-Cilag and Bristol-Myers Squibb. Dr. Heinz has received speaker honoraria from Janssen-Cilag, Johnson & Johnson, Eli Lilly, Pfizer, Bristol-Myers Squibb, AstraZeneca and Servier. Georg Juckel has received consultancy fees from Janssen-Cilag, AstraZeneca and Eli Lilly. He has received speaker fees and/or educational grants from Janssen-Cilag, AstraZeneca, Eli Lilly, Wyeth and Pfizer.
None declared for Henning Witthaus, Ute Mendes, Martin Brune, Seza Özgürdal, George Bohner, Yehonala Gudlowski, Peter Kalus and Randolf Klingebiel.
Contributors: Drs. Witthaus, Mendes and Juckel contributed to the conception and design of the study and the acquisition, analysis and interpretation of data. Dr. Heinz contributed to the conception and design of the study and the acquisition of data. Dr. Klaus contributed to the conception and design of the article and the analysis and interpretation of data. Drs. Özgürdal, Klingebiel and Bohner contributed to the acquisition of the data. Dr. Juckel wrote the article, which he revised along with the other authors. All authors approved the final version submitted for publication. All authors had full access to all of the data in the study and all analyses
References
- 1.Honea R, Crow TJ, Passingham D, et al. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–45. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 2.Steen RG, Mull C, McClure R, et al. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–8. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 3.Vita A, De Peri L, Silenzi C, et al. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res. 2006;82:75–88. doi: 10.1016/j.schres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Job DE, Whalley HC, McConnell S, et al. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage. 2002;17:880–9. [PubMed] [Google Scholar]
- 5.Strasser HC, Lilyestrom J, Ashby ER, et al. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry. 2005;57:633–9. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 6.McDonald C, Marshall N, Sham PC, et al. Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry. 2006;163:478–87. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- 7.Seidman LJ, Faraone SV, Goldstein JM, et al. Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Arch Gen Psychiatry. 2002;59:839–49. doi: 10.1001/archpsyc.59.9.839. [DOI] [PubMed] [Google Scholar]
- 8.O’Driscoll GA, Florencio PS, Gagnon D, et al. Amygdala-hippocampal volume and verbal memory in first-degree relatives of schizophrenic patients. Psychiatry Res. 2001;107:75–85. doi: 10.1016/s0925-4927(01)00095-6. [DOI] [PubMed] [Google Scholar]
- 9.Ongur D, Cullen TJ, Wolf DH, et al. The neural basis of relational memory deficits in schizophrenia. Arch Gen Psychiatry. 2006;63:356–65. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- 10.Yung AR, McGorry PD. The prodromal phase of first episode psychosis: Past and current conceptualisations. Schizophr Bull. 1996;22:353–70. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- 11.McGlashan TH. Early detection and intervention in schizophrenia: research. Schizophr Bull. 1996;22:327–45. doi: 10.1093/schbul/22.2.327. [DOI] [PubMed] [Google Scholar]
- 12.Klosterkötter J, Hellmich M, Steinmeyer EM, et al. Diagnosing schizophrenia in the intitial prodromal phase. Arch Gen Psychiatry. 2001;58:158–64. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- 13.Pantelis C, Yücel M, Wood SJ, et al. Early and late neurodevelopmental disturbances in schizophrenia and their functional consequences. Aust N Z J Psychiatry. 2003;37:399–406. doi: 10.1046/j.1440-1614.2003.01193.x. [DOI] [PubMed] [Google Scholar]
- 14.Phillips LJ, Velakoulis D, Pantelis C, et al. Non-reduction in hippocampal volume is associated with higher risk of psychosis. Schizophr Res. 2002;58:145–58. doi: 10.1016/s0920-9964(01)00392-9. [DOI] [PubMed] [Google Scholar]
- 15.Wood SJ, Yucel M, Velakoulis D, et al. Hippocampal and anterior cingulate morphology in subjects at ultra-high-risk for psychosis: the role of family history of psychotic illness. Schizophr Res. 2005;75:295–301. doi: 10.1016/j.schres.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Velakoulis D, Wood SJ, Wong MT, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–49. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 17.McGlashan TH, Miller TJ, Woods SW. A scale for the assassment of prodromal symptoms and states in early intervention in psychotic disorders. In: Miller TJ, Mednick SA, McGlashan TH, et al., editors. Early intervention in psychotic disorders. New York (NY): Kluwer Academic Publishers; 2001. pp. 135–49. [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994. [Google Scholar]
- 19.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 20.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 21.Lehrl S. Mehrfachauswahl-Wortschatz-Intelligenztest (MWT-B) Dr. Med. Erlangen (Germany): Straube Verlag; 1978. [Google Scholar]
- 22.Statistical Parametric Mapping Version 2. London (UK): Wellcome Department of Cognitive Neurology; [(accessed 2009 Dec. 9)]. Available: www.fil.ion.ucl.ac.uk/spm/ [Google Scholar]
- 23.Convit A, McHugh P, Wolf OT, et al. MRI volume of the amygdala: a reliable method allowing separation from the hippocampal formation. Psychiatry Res. 1999;90:113–23. doi: 10.1016/s0925-4927(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 24.Pantel J, O’Leary DS, Cretsinger K, et al. A new method for the in vivo volumetric measurement of the human hippocampus with high neuroanatomical accuracy. Hippocampus. 2000;10:752–8. doi: 10.1002/1098-1063(2000)10:6<752::AID-HIPO1012>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Pruessner JC, Li LM, Serles W, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–42. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- 26.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 27.Csernansky JG, Wang L, Jones D, et al. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry. 2002;159:2000–6. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- 28.Tepest R, Wang L, Miller MI, et al. Hippocampal deformities in the unaffected siblings of schizophrenia subjects. Biol Psychiatry. 2003;54:1234–40. doi: 10.1016/s0006-3223(03)00702-9. [DOI] [PubMed] [Google Scholar]
- 29.Gloor P. The temporal lobe and limbic system. Oxford (UK): Oxford University Press; 1997. [Google Scholar]
- 30.Jakob H, Beckmann H. Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm. 1986;65:303–26. doi: 10.1007/BF01249090. [DOI] [PubMed] [Google Scholar]
- 31.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 32.Bogerts B, Falkai P, Haupts M, et al. Post-mortem volume measurements of limbic system and basal ganglia structures in chronic schizophrenics. Initial results from a new brain collection. Schizophr Res. 1990;3:295–301. doi: 10.1016/0920-9964(90)90013-w. [DOI] [PubMed] [Google Scholar]
- 33.Wright IC, Rabe-Hesketh S, Woodruff PW, et al. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 34.Woods BT, Ward KE, Johnson EH. Meta-analysis of the time-course of brain volume reduction in schizophrenia: implications for pathogenesis and early treatment. Schizophr Res. 2005;73:221–8. doi: 10.1016/j.schres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–8. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 36.Pantelis C, Yucel M, Wood SJ, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–96. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 37.Douaud G, Mackay C, Andersson J, et al. Schizophrenia delays and alters maturation of the brain in adolescence. Brain. 2009;132(Pt 9):2437–48. doi: 10.1093/brain/awp126. [DOI] [PubMed] [Google Scholar]
- 38.Whitworth AB, Kemmler G, Honeder M, et al. Longitudinal volumetric MRI study in first-and multiple-episode male schizophrenia patients. Psychiatry Res. 2005;140:225–37. doi: 10.1016/j.pscychresns.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Morris JS, Frith CD, Perrett DI, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–5. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 40.Bremner JD. Effects of traumatic stress on brain structure and function: relevance to early responses to trauma. J Trauma Dissociation. 2005;6:51–68. doi: 10.1300/J229v06n02_06. [DOI] [PubMed] [Google Scholar]
- 41.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, et al. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–35. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawasaki Y, Suzuki M, Nohara S, et al. Structural brain differences in patients with schizophrenia and schizotypal disorder demonstrated by voxel-based morphometry. Eur Arch Psychiatry Clin Neurosci. 2004;254:406–14. doi: 10.1007/s00406-004-0522-1. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi T, Suzuki M, Zhou SY, et al. Morphologic alterations of the parcellated superior temporal gyrus in schizophrenia spectrum. Schizophr Res. 2006;83:131–43. doi: 10.1016/j.schres.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 44.Downhill JE, Buchsbaum MS, Hazlett EA, et al. Temporal lobe volume determined by magnetic resonance imaging in schizotypal personality disorder and schizophrenia. Schizophr Res. 2001;48:187–99. doi: 10.1016/s0920-9964(00)00131-6. [DOI] [PubMed] [Google Scholar]
- 45.Seidman LJ, Pantelis C, Keshavan MS, et al. A review and new report of medial temporal lobe dysfunction as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric family study of the parahippocampal gyrus. Schizophr Bull. 2003;29:803–30. doi: 10.1093/oxfordjournals.schbul.a007048. [DOI] [PubMed] [Google Scholar]
- 46.Dickey CC, McCarley RW, Xu ML, et al. MRI abnormalities of the hippocampus and cavum septi pellucidi in females with schizotypal personality disorder. Schizophr Res. 2007;89:49–58. doi: 10.1016/j.schres.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Häfner H, an der Heiden W. The course of schizophrenia in the light of modern follow-up studies: the ABC and WHO studies. Eur Arch Psychiatry Clin Neurosci. 1999;249(Suppl 4):14–26. doi: 10.1007/pl00014180. [DOI] [PubMed] [Google Scholar]
- 48.Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 1. Review of methodologies currently employed. Mol Psychiatry. 2005;10:147–59. doi: 10.1038/sj.mp.4001580. [DOI] [PubMed] [Google Scholar]