Abstract
Background
Although cognition has been studied extensively among patients with schizophrenia, social cognition has only recently emerged as an area of interest. The objective of the current study was to use structural equation modelling to test the hypothesis that the relation between cognitive performance and social function is mediated by patients’ social cognitive abilities.
Methods
We assessed participants who met criteria for a schizophrenia-spectrum disorder, with equal distribution among first- and multi-episode participants, and nonpsychiatric controls on a range of measures within each of the domains of cognition, social cognition and social function.
Results
Using structural equation modelling, we derived a model that explained 79.7% of the variance in social function and demonstrated that the link between cognition and social function was fully mediated by social cognition.
Limitations
A limitation of this study is that the measures contributing to the structural equation modelling analysis were obtained at the same point in time. Thus, the temporal order of causation suggested by Model 2 remains theoretically specified.
Conclusion
This study provides some first steps in understanding the complex relation between cognition and social function. Such a relation has potential implications for the design of remediation strategies.
Introduction
In schizophrenia research, there has been a long history of studying cognition, which is nonsocial in nature. On the other hand, social cognition involves the cognitive processes involved in how individuals think about themselves, other people, social situations and interactions. Thus, social cognition involves the perception, interpretation and processing of social information that underlies social interactions and includes emotion perception, social perception, social knowledge and attributional bias.1 Although cognition has been studied extensively among patients with schizophrenia, social cognition has only recently emerged as an area of interest. This interest is partly motivated by ongoing questions regarding the causes of poor social function, which typically persist for many even in the face of symptomatic recovery.2
There are many published observations of important associations among cognition, social cognition and social function. However, these studies generally fail to utilize analytic techniques that allow for the direction and structure of these relations to be modelled. Moreover, although a handful of studies exist that explore the structure of these different relations, they focus on chronic samples, often use single measures of social function and are frequently underpowered. These issues can be addressed by using a range of measures of social cognition and social function; by examining these domains in samples of individuals with schizophrenia, both early in the course of the illness and chronic; and by examining the data with structural equation modelling, which allows the use of an aggregation of measures to yield latent constructs of interest.
There is some recent work in the area using modelling techniques. Brekke and colleagues3 demonstrated a role for affect perception in the pathway to functional outcome. Vauth and colleagues,4 using structural equation modelling, demonstrated a combined impact of cognition and social cognition (assessed by social perception) on vocational outcome. However, they did not test whether social cognition acts as a mediator via appropriate analytic means (for an example, see Baron and colleagues5). Third, Sergi and colleagues,6 also using structural equation modelling, demonstrated the mediating influence of a measure of social perception on the relation between visual perception and functional outcome. In 3 recent studies, with this current large sample of first- and multi-episode patients with psychosis, we demonstrated that the patients were clearly impaired relative to a sample of nonpsychiatric controls in all measures of cognition, social function and social cognition.7–9 Furthermore, in separate analyses testing mediation,5 there was evidence that facial affect recognition partially mediated the relation between cognitive and social function for the patient group but not for nonpsychiatric controls9 and that social knowledge and social perception were potential mediators between cognition and social problem-solving, particularly for the patient groups.8 However, in these earlier studies we did not use structural equation modelling, nor did we make use of latent constructs.
The central objective of the current study was to use structural equation modelling to test the hypothesis that the relation between cognitive performance and social function is mediated by patients’ social cognitive abilities. This analysis involved a large sample of well-characterized schizophrenia-spectrum disorder patients and non-psychiatric controls using, unlike the 2 earlier publications with this sample, a wide range of measures of each of the domains of cognition, social cognition and social function.
Methods
Participants
In this 1-year longitudinal study we included 3 groups of participants, which have been well described elsewhere.7–9 The first comprised patients with first-episode schizophrenia who were consecutively admitted to the Calgary Early Psychosis Program (EPP), which most likely included most potential incidence cases.10 We excluded patients if they had a history of neurologic disorders, head injury or epilepsy or did not speak English well enough to adequately complete the assessments. Diagnoses were completed at baseline and then repeated at 1 year. The second group comprised individuals in a specialized outpatient program for schizophrenia in a psychiatry department in a general hospital. These individuals all had received a diagnosis of schizophrenia at least 3 years previously and had a chronic course of multiple episodes of schizophrenia. They all met criteria for schizophrenia. The final group was a sample of local nonpsychiatric controls matched to the first-episode group for sex, age and education. We used the Structured Clinical Interview for DSM-IV axis 1 disorders (SCID) criteria to confirm no current or past psychiatric disorder.
Some participants dropped out at the 1-year follow-up; these participants were not included in any of the analyses. For the structural equation modelling, we excluded participants who met criteria for other psychotic disorders. We also excluded participants who insufficiently completed cognitive tasks at the 1-year assessment.
The bioethics committee at the University of Calgary approved our study. After complete description of the study to all participants, we obtained written informed consent. Only participants who were deemed capabale of giving informed consent were referred to the study by their physician. All participants were outpatients.
Measures
The measures of social cognition, social function and cognition that we used have been described in detail in earlier studies.7–9 Briefly, social cognition comprised the 2 domains of emotion perception and social perception. We assessed emotion perception with 2 facial affect recognition tests: the Facial Emotion Identification Test (FEIT) and the Facial Emotion Discrimination Test (FEDT).11 In both of these tests, participants are required to view photographs that are presented on video and either identify specific emotions or determine if 2 simultaneously presented emotions are the same or different. The FEIT and the FEDT are scored by summing the total number of correct emotion identifications (range 0–19) and the total number of correct discriminations (range 0–30), respectively. Based on our previous findings of similar profiles with these 2 tasks, we combined z scores to form a composite measure of facial affect recognition.9
Social perception is the ability to understand and appraise social roles, rules and context. We assessed social perception with the Social Cue Recognition Test (SCRT),12,13 which requires individuals to use social cues to make inferences about situational events that generated specific social cues or to identify interpersonal features in a given situation, and with the Situational Features Recognition Test (SFRT),13,14 which requires participants to identify features from a list of descriptors that describe 5 familiar situations (e.g., driving a car) and 4 unfamiliar situations (e.g., building an igloo). Both have been shown to have good psychometric properties.12–14 As in a previous study,8 we used transformed z scores to derive a composite measure of social perception from the SCRT and the SFRT.
We assessed social function using 3 different measures: the Quality of Life Scale (QLS), a semistructured interview;15 the Social Functioning Scale (SFS), a self-report questionnaire developed for outpatients with schizophrenia that has excellent psychometric properties;16 and the Assessment of Interpersonal Problem Solving (AIPPS), a measure of social problem-solving.17 The AIPPS is a videotaped vignette test used to assess the social skills of patients with schizophrenia and measures a patient’s ability to describe an interpersonal social problem, derive a solution to the problem and enact a solution in a role-played simulation test. The AIPSS has been shown to have adequate psychometric properties.17
We chose cognitive tests to assess a wide range of cognitive domains and used a battery of tests that was consistent with batteries generally used in the schizophrenia literature.18 Assessment of cognitive function included letter fluency (Controlled Oral Word Association Test; COWAT),19 category fluency (category instances),20 verbal memory (logical memory subtests of the Wechsler Memory Scale-Revised: LMI, LMII),21 Rey Auditory Verbal Learning Test (RAVLT),22 visual memory (Rey Complex figure),23 working memory (Letter–Number Span),24 Wisconsin Card Sorting Test (WCST),25 attention (Degraded Stimulus Continuous Performance Test; DS-CPT),26 early information-processing (Span of Apprehension; SPAN),27 visual–constructional ability (copy of the Rey Complex figure), visuomotor sequencing (Trails A and Trails B),28 psychomotor speed (Grooved Pegboard)29 and the Stroop.30
We transformed cognitive performance variables to adjust for violations of normality. Next, we replaced missing data for 0.4% of the cognitive data using regression imputation in Amos 7.0 software (SPSS Inc.) based on maximum likelihood estimates. Last, we reduced the cognitive measures using principal component factor analysis, which was deemed appropriate for the data (Bartlett test p < 0.001, Kaiser–Meyer–Olkin index 0.80). This analysis generated 6 factors with eigenvalues greater than 1. However, the tests all loaded on 1 factor with most of the variance being accounted for by the first factor (47%); examination of the scree plots indicated that only 1 factor was worth retaining. This finding of 1 factor replicates an earlier study with a large first-episode sample,18 our previous study with a first-episode population31 and with the present sample.9 Thus, we performed the factor analysis forcing 1 factor, and individuals’ factor scores formed the measure of cognition that we used for subsequent analyses.
Procedures
Testing for nonpsychiatric controls took place in 2 sessions, usually in the same day. For the first-episode and multi-episode participants, we completed the assessments in 3 sessions, all within a 7-day period. We repeated all assessments 1 year later.
Raters were experienced research clinicians who routinely used all of these measures and who demonstrated adequate reliability at routine reliability checks as part of the ongoing Early Psychosis Program evaluation. Criteria for reliability were that the scoring of each item on the QLS was within 1 point and that there was at least 80% agreement on total scores and subscale scores for all measures. We calculated agreement as the number of ratings within 1 point divided by the total number of ratings. Two of us (J.A. and D.A.) made the DSM-IV diagnoses using the SCID-I. We determined inter-rater reliability by 100% agreement on the diagnosis and at least 80% agreement for symptom presence. Trained and experienced psychometricians, under the supervision of J.A, administered the cognitive and social cognitive tests. Independent raters completed the cognitive battery, the social cognition tasks and the social function measures. All raters remained blind to the results of the other assessments.
Design
We previously reported that in the domains of cognition, social cognition and social function the nonpsychiatric controls demonstrated superior performance to the patient groups; that the 2 patient groups did not differ on any social cognition, social function or cognitive measures; and that performance on cognition, social cognition and social function was stable over time.7–9 Therefore, for the structural equation modelling, we combined 1-year assessment data for the first-episode and multiepisode participants to create 1 patient group in which we assessed the role of illness in comparison to nonpsychiatric controls using the models.
Statistical analyses
We used structural equation modelling to examine our hypothesis among the 3 constructs of cognitive function, social cognition and social function at the 1-year assessment. Structural equation modelling consists of a combination of confirmatory factor analysis and multiple regressions to determine the relations among latent constructs. In the factor analysis, the constructs are considered to be unobserved or latent variables and are estimated by factor analysis of data from theoretically related measures, the observed or indicator variables. Factor loadings reflect the relations between the indicators’ latent variables. The regression analyses determine the relations among the latent variables. Each association reported between 2 latent variables is a partial correlation with the other latent variables of the model held constant.
We evaluated the mediation hypothesis9 using Amos 7.0 software (SPSS Inc.),32 beginning with a test of the fit of the data to a model with the relation between group and social function being mediated first by cognition and then by social cognition along with 3 additional direct paths: group to social function, group to social cognition and cognition to social function. We used a model-trimming approach by testing the difference in fit for nested models with path coefficients constrained to zero. To achieve broad conceptual and statistical coverage in evaluating model fit, we used multiple goodness-of-fit indices. We tested 4 aspects of fit using a total of 8 indices:
χ2 and the ratio of the χ2 to degrees of freedom (χ2/df) assessed absolute fit
Bentler comparative fit index (CFI) and non-normed fit index (NNFI) assessed relative fit (i.e., v. independence model)
parsimonious CFI and NFI (PCFI and PNFI, respectively) were relative indices adjusted for model complexity and
root mean square error of approximation (RMSEA) was the non–centrality based estimate of error, along with its 90% confidence interval (CI) and significance test probability of close fit (PCLOSE).
A nonsignificant χ2, a χ2/df ratio less than 2 (< 3 acceptable), NNFI and CFI greater than 0.95 (> 0 0.90 good), PCFI and PNFI less than 0.60, RMSEA less than 0.05 (< 0.08 reasonable, < 0.10 acceptable) and PCLOSE greater than 0.05 each indicate a close fit between the data and the hypothesized model, as collectively recommended by several sources.32–35 These sources, among others, also indicate that our sample size (n = 147) can be considered “medium” size (n > 100) with respect to the structural equation modelling literature. Although a minimum sample of 100 has been recommended by some (e.g., Kline33 and Hoyle34), model complexity is often deemed more relevant. Here our sample exceeds some minimum rules-of-thumb for our most complex Model 1 (i.e., 15 × 7 observed variables = 105; 5 × 18 parameter estimates = 90), although larger samples are always preferable.
Results
Participants
The first-episode group comprised 43 patients (26 men, 17 women) with a mean age of 25.1 years (standard deviation [SD] 8.01, range 16–42 yr). Most were single (88.0%) and lived at home (78.0%). Sixty-six percent had completed grade 12. At the time of the 2 assessments (baseline and 1 year), 82% and 87.5%, respectively, were reportedly taking second-generation antipsychotics (mean dose in chlorpromazine equivalents of 307 and 380 mg/d, respectively). Diagnoses were completed at baseline and then repeated at 1 year; at 1 year, 74% had a diagnosis of schizophrenia and 26% of schizophreniform disorders.
The multiple-episode group comprised 53 individuals (38 men, 15 women) in a specialized outpatient program for schizophrenia in a psychiatry department in a general hospital. They all had received a diagnosis of schizophrenia at least 3 years previously and had a chronic course of schizophrenia having had multiple episodes. On average, they had had 5 hospital admissions. The mean age was 35.5 years (SD 7.17, range 22–46 yr). Most were single (77.4%), had completed grade 12 (71.7%) and lived at home (45.2%). At the time of both assessments, 98.1% were reportedly taking second-generation antipsychotics (mean dose in chlorpromazine equivalents of 715 and 665 mg/d, respectively). They all met criteria for schizophrenia.
We included a sample of 55 nonpsychiatric controls matched for sex (33 men, 22 women), age (mean 21.7, SD 6.05, range 15–40 yr) and education to the first-episode sample. For education, 27% had some high school, 22% had high school, 36% had some postgraduate training and 15% had postgraduate degrees. We used SCID-1 criteria to confirm that they had no current or past psychiatric disorders.
The original samples recruited consisted of 55 participants in the first-episode group, 59 participants in the multiple episode group and 61 controls; however, at 1-year follow-up 5, 6 and 6 participants from those groups, respectively, had dropped out of the study. At baseline, the dropouts did not differ from those who remained in terms of demographic characteristics, symptoms, cognition or social function. For the structural equation modelling, we excluded the 7 participants who met criteria for other psychotic disorders, and we excluded an additional 3 patients in the first-episode group and 1 control owing to insufficient completion of cognitive tasks at 1-year follow-up.
We combined the 1-year follow-up data for the first-episode and multiple-episode groups to create 1 patient group (schizophrenia group, n = 93) with which we assessed the role of illness in comparison to nonpsychiatric controls (n = 54) in the models. The correlation matrix for the variables employed in the structural equation modelling analyses is shown in Table 1.
Table 1.
Pearson product-moment correlations* among measures of cognition, social cognition and social function and their point-biserial correlation with group†
| Measure | Group‡ | Cognition | FAR | Social perception | AIPPS | SFS | QLS |
|---|---|---|---|---|---|---|---|
| Group‡ | 0.97§ | 0.65 | 0.43 | 0.37 | 0.45 | 0.50 | 0.76 |
| Cognition | 1.00§ | 0.64 | 0.59 | 0.51 | 0.44 | 0.68 | |
| FAR | 1.75§ | 0.59 | 0.47 | 0.36 | 0.54 | ||
| Social perception | 2.68§ | 0.56 | 0.34 | 0.51 | |||
| AIPPS | 38.33§ | 0.44 | 0.52 | ||||
| SFS | 26.65§ | 0.65 | |||||
| QLS | 25.20§ |
AIPPS = Assessment of Interpersonal Problem Solving;17 FAR = facial affect recognition; QLS = Quality of Life Scale;15SFS = Social Functioning Scale.16
Above diagonal.
All correlations are significant at p < 0.001, n = 147.
Group is a binary variable defined by (multiple-episode and first-episode) patients versus controls.
Standard deviations along diagonal.
Analyses
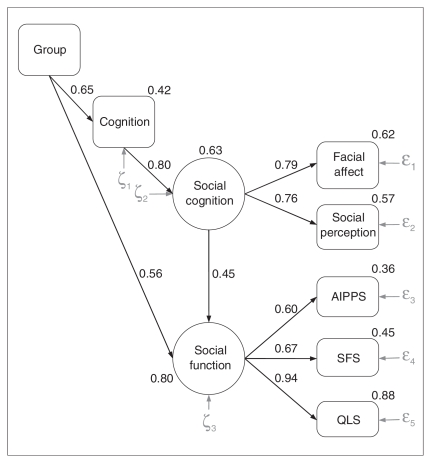
The confirmatory factor analysis showed that all measured variables made significant contributions to their respective latent variables of social cognition and social function. The model demonstrates that the measures for social cognition and social function load onto their respective latent variables (loadings range 0.60–0.94; Fig. 1); this finding supports that the latent constructs are reliable and sound, as defined by the chosen test measures.
Fig. 1.
Structural equation model of the direct and indirect relation mediated through cognition and social cognition between group status and social function. Rectangles represent observed variables and ovals represent latent variables (see text for further descriptions). Epsilons (ɛ) and zetas (ζ) reflect residual error terms. AIPPS = Assessment of Interpersonal Problem Solving;17 QLS = Quality of Life Scale;15 SFS = Social Functioning Scale.16
Model 1 provided some good indices of fit: χ2/df = 2.67, CFI = 0.97, NNFI = 0.91, PCFI = 0.34 and PNFI = 0.35. The χ2 test result was significant (χ210 = 26.65, p = 0.003), but this test is known to be overly sensitive to sample size, and convergence across other indices is often preferred.32,33 In this context, the RMSEA and associated measures were unacceptable: RMSEA = 0.11 (95% confidence interval [CI] 0.06–0.16), PCLOSE = 0.03. Results revealed that the path coefficients for the direct relations from group to social cognition (γ = 0.01) and from cognition to social function (β = −0.01) were not significant and near zero; all other paths were significant (coefficients > 0.45, p < 0.001). Thus, we tested a second model (Model 2), eliminating these 2 paths. Although the χ2 test result was significant (χ212 = 26.65, p = 0.009), all other measures indicated good fit: χ2/df = 2.22, CFI = 0.97, NNFI = 0.93, PCFI = 0.41, PNFI = 0.42, RMSEA = 0.09 (95% CI 0.04–0.14) and PCLOSE = 0.07. Moreover, Model 2 represented a nonsignificant improvement from Model 1 (χ2Δ2 = 0.01, p = 0.99, NNFIΔ = −0.02), indicating that inclusion of the 2 direct paths in Model 1 did not improve the model. Trimming of any additional paths resulted in significantly worse fit indices. To confirm our assumption regarding the combination of the first-episode and multiple-episode groups into 1 schizophrenia group, we ran a parallel analysis such that group reflected the comparison of these 2 schizophrenia samples. This model revealed nonsignificant direct effects of group on cognition (γ = −0.14, p = 0.19) and on social function (γ = 0.07, p = 0.48). All other coefficients remained significant. Thus, there was no differential contribution to the model between patient groups.
We considered Model 2 to be the final model. As shown in Figure 1, this model explained 79.7% of the variance in social function. This model indicates at least 4 other notable findings:
illness-related effects on social cognition are fully mediated by cognitive abilities
the link between cognition and social function is fully mediated by social cognition
a significant portion of the relation between group status (schizophrenia-spectrum v. nonpsychiatric) and social function was mediated via cognitive and social-cognitive skills (R2 = 0.23, or 29.2% of 79.7%) and
a substantial portion of illness-related associations with social function remains to be determined (γ = 0.56, or 70.8% of the total effect).
Discussion
We have demonstrated that if an individual has schizophrenia, then the impact of cognitive deficits on his or her functional outcome is mediated through social cognition. The model that we tested was based on earlier work that suggested that social cognition, albeit measured via different instruments, mediated the effect of cognition on social function. To test our model we reduced our data to aggregate measures of latent constructs, including cognition; social cognition, which reflected domains of emotion and social perception; and social function, which reflected community function and interpersonal problem-solving. The confirmatory factor analysis showed that all measured variables made significant contributions to their respective latent variables of social cognition and social function. These results are consistent with other studies in the literature that we reviewed earlier.4,6
Model 2 indicates that both cognition and, in particular, social cognition are relevant targets for intervention. Interventions at the cognitive level36–38 could have an impact on both cognition and social cognition. Additionally, this model confirms that social cognition has unique relations to functional outcome over and above those explained by cognition. Moreover, because it is more proximal to social function, social cognition represents a particularly valuable target for intervention. Several treatment studies that have successfully used some form of training in social cognition are appearing in the literature. For example, one group using a program for the remediation of deficits in facial affect recognition demonstrated significant improvements.39,40 Penn and colleagues41 have also recently developed a targeted treatment called Social Cognitive and Interpersonal Treatment (SCIT) that is a 3-phase, 18-session group intervention to address difficulties in emotion perception, attributional bias and theory of mind with promising early results.42,43 Although exciting, this area of treatment is in its infancy, and there is much work required to develop a consensus on how to define social cognition, a wider focus on targeting social cognition deficits, which techniques may be most effective and the best assessment instruments for such clinical trials.
Limitations
Although a strength of the present study is that we used a wide range of measures, which are commonly used with this population, to build the latent constructs of interest, the constructs are, nonetheless, constrained by these measures. A limitation of this study is that we obtained the measures contributing to the structural equation modelling analysis at the same point in time. Thus, the temporal order of causation suggested by Model 2 remains theoretically specified. However, given the observed stability of the measures across the 2 assessments, a similar outcome would be expected if each step in the model were determined across 4 sequential separate time periods. Moreover, this mediational model converges well with several other reports. Our sample was in the “medium” range for structural equation modelling,33 thus it would be important to replicate such results in larger samples. A further limitation is that the measure of cognition and the measure of social cognition are both defined by the specific tasks used. However, it should be noted that both of these variables comprise various aspects of their constructs. Our findings do in fact converge with other findings based on different tests of social cognition. A future direction would be to confirm similar findings across other batteries.
In conclusion, social cognition appears to mediate the association between cognition and social function. Such a result provides some first steps in understanding this complex relation between cognition and outcome, which has potential implications for the design of a range of remediation strategies involving social cognition as well as cognition.
Footnotes
This study was conducted at the University of Calgary, Alberta, Canada, and was supported by a grant to Jean Addington from The Canadian Institutes of Health Research.
Competing interests: None declared for Drs. Girard and Christensen. Dr. J. Addington has received investigator-initiated funding support from not-for-profit entities, including the National Institute of Mental Health and the Schizophrenia Society of Ontario. She has also received consultant fees from AstraZeneca and speaker fees from AstraZeneca and Pfizer. Dr. D. Addington has served as a consultant to Pfizer and Eli Lilly.
Contributors: Drs. J. and D. Addington designed the study and acquired the data, which Drs. J. Addington, Girard and Christensen analyzed. Drs. J. Addington and Girard wrote the article. All authors reviewed the article and approved the final version for publication.
References
- 1.Penn DL, Addington J, Pinkham A. Social cognitive impairments. In: Lieberman JA, Stroup TS, Perkins DO, editors. American Psychiatric Association textbook of schizophrenia. Washington (DC): American Psychiatric Publishing Press, Inc; 2006. [Google Scholar]
- 2.Addington J, Young J, Addington D. Social outcome in early psychosis. Psychol Med. 2003;33:1119–24. doi: 10.1017/s0033291703007815. [DOI] [PubMed] [Google Scholar]
- 3.Brekke J, Kay DD, Lee KS, et al. Biosocial pathways to functional outcome in schizophrenia. Schizophr Res. 2005;80:213–25. doi: 10.1016/j.schres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Vauth R, Rusch N, Wirtz M, et al. Does social cognition influence the relation between neurocognitive deficits and vocational functioning in schizophrenia? Psychiatry Res. 2004;128:155–65. doi: 10.1016/j.psychres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 6.Sergi MJ, Rassovsky Y, Nuechterlein K, et al. Social perception as a mediator of early visual processing on functional status in schizophrenia. Am J Psychiatry. 2006;163:448–54. doi: 10.1176/appi.ajp.163.3.448. [DOI] [PubMed] [Google Scholar]
- 7.Addington J, Addington D. Social and cognitive functioning in psychosis. Schizophr Res. 2008;99:176–81. doi: 10.1016/j.schres.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Addington J, Saeedi H, Addington D. Influence of social perception and social knowledge on cognitive and social functioning in early psychosis. Br J Psychiatry. 2006;189:373–8. doi: 10.1192/bjp.bp.105.021022. [DOI] [PubMed] [Google Scholar]
- 9.Addington J, Saeedi H, Addington D. Facial affect recognition: A mediator between cognitive and social functioning in psychosis? Schizophr Res. 2006;85:142–50. doi: 10.1016/j.schres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Addington J, Addington D. Early intervention for psychosis: the Calgary Early Psychosis Treatment and Prevention Program. [(accessed 2009 Sep. 4)];Can Psychiatr Assoc Bull. 2001 33:11–6. Available: https://ww1.cpa-apc.org/Publications/Archives/Bulletin/2001/November/Nov2001.asp. [Google Scholar]
- 11.Kerr SL, Neale JM. Emotional perception in schizophrenia: Specific deficit or futher evidence of generalized poor performance? J Abnorm Psychol. 1993;102:312–8. doi: 10.1037//0021-843x.102.2.312. [DOI] [PubMed] [Google Scholar]
- 12.Corrigan PW. The social perceptual deficits of schizophrenia. Psychiatry. 1997;60:309–26. doi: 10.1080/00332747.1997.11024809. [DOI] [PubMed] [Google Scholar]
- 13.Corrigan PW, Buican B, Toomey R. Construct validity of two tests of social cognition in schizophrenia. Psychiatry Res. 1996;63:77–82. doi: 10.1016/0165-1781(96)02897-1. [DOI] [PubMed] [Google Scholar]
- 14.Corrigan PW, Garman A, Nelson D. Situational feature recognition in schizophrenic outpatients. Psychiatry Res. 1996;62:251–7. doi: 10.1016/0165-1781(96)02798-9. [DOI] [PubMed] [Google Scholar]
- 15.Heinrichs DW, Hanlon T, Carpenter WT. The quality of life scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10:388–98. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- 16.Birchwood M, Smith J, Cochrane R, et al. The Social Functioning Scale: the development and validation of a new scale adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry. 1990;157:853–9. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- 17.Donahoe CP, Carter MJ, Bloem WD, et al. Assessment of interpersonal problem-solving skills. Psychiatry. 1990;53:329–39. doi: 10.1080/00332747.1990.11024517. [DOI] [PubMed] [Google Scholar]
- 18.Keefe RS, Seidman LJ, Christensen BK, et al. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry. 2004;161:985–95. doi: 10.1176/appi.ajp.161.6.985. [DOI] [PubMed] [Google Scholar]
- 19.Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City (IA): AJA Associates; 1983. [Google Scholar]
- 20.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–77. [PubMed] [Google Scholar]
- 21.Wechsler D. Wechsler Memory Scale revised. New York (NT): The Psychological Corporation; 1987. [Google Scholar]
- 22.Lezak M. Neuropsychological assessment. New York (NY): Oxford University Press; 1995. [Google Scholar]
- 23.Rey A. L’examin clinique en psychologie. Paris (France): Presse Universitaire de France; 1958. [Google Scholar]
- 24.Gold JM, Carpenter C, Randolph C, et al. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–65. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- 25.Heaton R. Wisconsin Card testing manual. Odessa (TX): Psychological Assessment Resources; 1981. [Google Scholar]
- 26.Nuechterlein K, Dawson M, Ventura J, et al. Testing vulnerability models: stability of potential vulnerability indicators across clinical state. In: Hafner H, Gattaz W, editors. Search for the causes of schizophrenia. New York (NY): Springer–Verlag; 1991. pp. 178–91. [Google Scholar]
- 27.Asarnow R, Granholm E, Sherman T. Span of apprehension in schizophrenia. In: Steinhauer S, Gruzelier J, Zubin J, editors. Handbook of shizophrenia. Amsterdam (The Netherlands): Elsevier Science Publishers; 1991. pp. 335–70. [Google Scholar]
- 28.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test battery. Tucson (AZ): Neuropsychology Press; 1985. [Google Scholar]
- 29.Mathews CG, Klove N. Instruction Manual for the Adult Neuropsychological Test Battery. Madison (WI): University of Madison Medical School; 1964. [Google Scholar]
- 30.Golden C. Stroop color and word test manual. Chicago (IL): Stoelting Co; 1978. [Google Scholar]
- 31.Addington J, Saeedi H, Addington D. The course of cognitive functioning in first episode psychosis: changes over time and impact on outcome. Schizophr Res. 2005;78:35–43. doi: 10.1016/j.schres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Arbuckle JL. Amos 7.0 user’s guide. Spring House (PA): AMOS Development Corporation; 2006. [Google Scholar]
- 33.Kline RB. Principles and practice of structural equation modeling. New York (NY): The Guilford Press; 1998. [Google Scholar]
- 34.Hoyle RH. Structural equation modeling: concepts, issues and applications. Thousand Oaks (CA): Sage; 1995. [Google Scholar]
- 35.Burton DB, Sepehri A, Hecht F, et al. A confirmatory factor analysis of the WISC-III in a clinical sample with cross-validation in the standardization sample. Child Neuropsychol. 2001;7:104–16. doi: 10.1076/chin.7.2.104.3130. [DOI] [PubMed] [Google Scholar]
- 36.Wykes T, Reeder C, Landau S, et al. Cognitive remediation therapy in schizophrenia: randomised controlled trial. Br J Psychiatry. 2007;190:421–7. doi: 10.1192/bjp.bp.106.026575. [DOI] [PubMed] [Google Scholar]
- 37.Wykes T, Newton E, Landau S, et al. Cognitive remediation therapy (CRT) for young early onset patients with schizophrenia: an exploratory randomized controlled trial. Schizophr Res. 2007;94:221–30. doi: 10.1016/j.schres.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 38.Medalia A, Richardson R. What predicts a good response to cognitive remediation interventions? Schizophr Bull. 2005;31:942–53. doi: 10.1093/schbul/sbi045. [DOI] [PubMed] [Google Scholar]
- 39.Frommann N, Streit M, Wolwer W. Remediation of facial affect recognition impairments in patients with schizophrenia: a new training program. Psychiatry Res. 2003;117:281–4. doi: 10.1016/s0165-1781(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 40.Wolwer W, Frommann N, Halfmann S, et al. Remediation of impairments in facial affect recognition in schizophrenia: efficacy and specificity of a new training program. Schizophr Res. 2005;80:295–303. doi: 10.1016/j.schres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Penn DL, Roberts DL, Combs D, et al. Best practices: the development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58:449–51. doi: 10.1176/ps.2007.58.4.449. [DOI] [PubMed] [Google Scholar]
- 42.Combs DR, Adams SD, Penn DL, et al. Social Cognition and Interaction Training (SCIT) for inpatients with schizophrenia spectrum disorders: preliminary findings. Schizophr Res. 2007;91:112–6. doi: 10.1016/j.schres.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Penn D, Roberts DL, Munt ED, et al. A pilot study of social cognition and interaction training (SCIT) for schizophrenia. Schizophr Res. 2005;80:357–9. doi: 10.1016/j.schres.2005.07.011. [DOI] [PubMed] [Google Scholar]