Abstract
Background
Structural abnormality of the substantia nigra can be detected by transcranial sonography in neuropsychiatric disorders such as Parkinson disease and restless legs syndrome. We investigated echogenicity of the substantia nigra as a potential structural marker for dysfunction of the nigrostriatal dopamine system in children with attention-deficit hyperactivity disorder (ADHD).
Methods
We used a blinded design and determined echogenicity of the substantia nigra by use of transcranial sonography in 22 children with ADHD and 22 healthy controls matched for age and sex.
Results
The echogenic substantia nigra area was significantly larger in ADHD patients than in healthy controls (F1,42 = 9.298, p = 0.004, effect size = 0.92). We found no effects of age or sex.
Limitations
Owing to a lack of dimensional assessment, we could not analyze the correlation between echogenicity and clinical symptoms.
Conclusion
Our results support the hypothesis that the nigrostriatal dopaminergic system is abnormal in children with ADHD.
Introduction
Attention-deficit hyperactivity disorder (ADHD) is a highly prevalent neuropsychiatric disorder characterized by excessive motor activity, increased impulsivity and attention deficits.1 Hypotheses about its pathophysiology implicate various neurotransmitters including dopamine.2,3 Dopaminergic projections terminating in the striatum largely originate from the substantia nigra located in the midbrain. Because the structure of the substantia nigra is easily accessible by transcranial sonography (TCS), assessment of the substantia nigra by TCS may reveal important insights into the function of the dopaminergic system without ionizing radiation.4,5 Indeed, 2 prototypical neurologic disorders associated with dysfunction of dopaminergic neurotransmission have revealed abnormalities of the echogenic substantia nigra. In one study, the echogenic area of the substantia nigra was enlarged in a large proportion of patients with idiopathic Parkinson disease.4 In another study, a reduction of the echogenic area was found in patients with restless legs syndrome.5 To our knowledge, the echogenicity of the substantia nigra has previously not been investigated in patients with ADHD.
Methods
Participants
We included 22 clinically referred children with a DSM-IV diagnosis of ADHD combined subtype and 22 healthy control children matched for age and sex. Controls were recruited from local schools and via newspaper ads and flyers. The investigation was approved by the local ethics committee of the University of Wuerzburg. All participants and their legal guardians gave informed written consent.
All participants were fully assessed for psychiatric morbidity by use of a clinical interview (Kiddie–SADS)6 and a child behaviour checklist.7 We excluded patients with a history that suggested severe intellectual disability. All patients received the stimulant medication methylphenidate with good clinical response. For screening of controls, we retrieved German ADHD report forms (FBB-HKS)8 from both parents and teachers and a German depression inventory (DIKJ).9 We excluded controls with a psychiatric disorder according to Kiddie–SADS or any score above the clinical cut-offs of the applied instruments.
Transcranial sonography
We performed TCS using a commercial ultrasound device (Sonoline Elegra; Siemens) equipped with a 2.5 MHz transducer as previously described.10 An investigator experienced in TCS (M.S.), who was unaware of the clinical status of the participants, performed the TCS examinations in a random order. Axial TCS scans were obtained bilaterally at the level of the midbrain through the temporal acoustic bone window using a penetration depth of 16 cm and a dynamic range of 45 dB.11 For planimetric assessment of the echogenic substantia nigra area, we saved on the computer at least 3 ultrasound slides for each side with clearly visible and definable substantia nigra. The outer circumference of the echogenic substantia nigra area was manually circled off-line by an experienced sonographer (D.W.), who was also unaware of the clinical status of the participants.
The ultrasound image with the largest substantia nigra area was chosen for further analysis.11 The size of the echogenic area was quantified using the freely distributed computer software GIMP (version 2.2.13; GNU Image Manipulation Program; www.gimp.org) allowing off-line quantitative analysis of images in bitmap format. For the purpose of quality control, a second investigator (M.R.) also performed the quantification of the substantia nigra area. The Spearman correlation between the raters was r = 0.85 (p < 0.001). In cases of clear disagreement (difference between substantia nigra areas > 20% in 2 patients and 4 controls), the quantification was jointly performed in expert council.
Statistical analysis
We used SPSS 16.0.0 (SPSS Ltd.) to perform an analysis of covariance with side (left or right) as a repeated measure and group (patient or control) as an independent measures; substantia nigra area was the dependent variable. We tested for possible confounding effects of sex and age by use of the same analyses of covariance with sex and age as covariates in separate analyses. For group differences, we calculated sensitivity and specificity according to classification by Fisher linear discriminant. The effect size was calculated using the F test statistic and degrees of freedom for the group main effect in the analysis of variance.
Results
The mean age of children in the ADHD group was 10.7 (standard deviation [SD] 2.5, range 7–16 yr), and the mean age in the control group was 11.1 (SD 2.4, range 7–16 yr; t42 = 0.447, p = 0.66). In both groups, the male:female ratio was 15:7. Comorbid disorders were frequent in the patient sample (oppositional–defiant disorder 4, conduct disorder 5, dyslexia 5, dyscalculia 2, adjustment disorder 6, enuresis 3, encopresis 1, tic disorder 1). Scores on the child behaviour checklist were significantly higher among ADHD patients than among controls for externalizing and internalizing subscales and total score (data not shown).
The 2-factor analysis of variance showed a significant main effect of group (F1,42 = 9.298, p = 0.004) but no significant main effect of side (F1,42 = 1.750, p = 0.19) and no significant interaction effect of side by group (F1,42 = 0.937, p = 0.34), indicating that ADHD patients had a larger substantia nigra area (left: mean 0.20, SD 0.06 cm2; right: mean 0.20, SD 0.07 cm2) than did controls (left: mean 0.16, SD 0.06 cm2; right: mean 0.14, SD 0.04 cm2) (Fig. 1). The effect size was 0.92, specificity was 0.73 and sensitivity was 0.82. The inclusion of sex as a covariate did not change the results (group main effect: F1,42 = 9.150, p = 0.004) and revealed no main or interaction effects of sex. Including age as a covariate gave similar results (group main effect: F1,42 = 8.840, p = 0.005), and no main or interaction effects of age were found (Fig. 2).
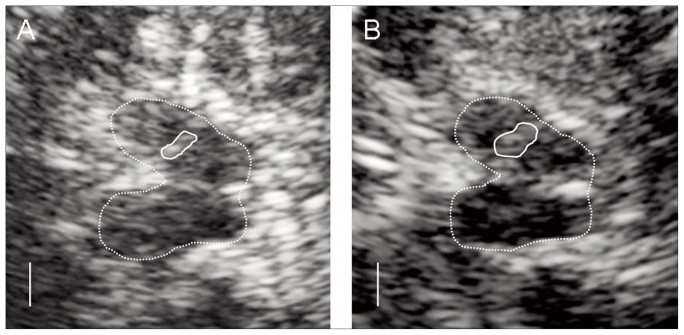
Fig. 1.
Typical transcranial sonography findings. In the butterfly shaped mesencephalic brainstem (circled by the dotted line), the ipsilateral echogenic substantia nigra can be seen (full line). The echogenic area indicated by the substantia nigra area was regular in control participants (A) but was enlarged in patients with attention-deficit hyperactivity disorder (B).
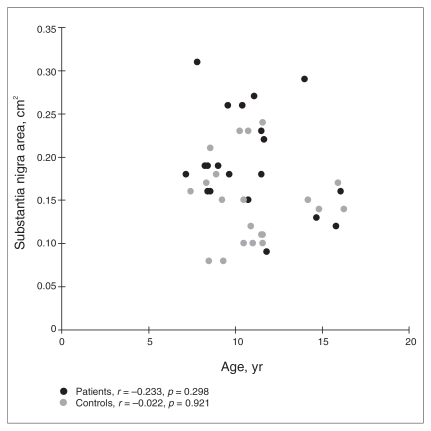
Fig. 2.
Scatterplot of substantia nigra echogenicity (left and right side combined) versus age. A visual inspection indicates no relevant effect of age on the findings. There are no outliers that would have caused the group differences.
Discussion
We found that the echogenic substantia nigra area was enlarged in children with ADHD compared with age- and sex-matched controls. There is little knowledge about the physiologic development of substantia nigra echogenicity during life. To date, we are only aware of one study that investigated the substantia nigra of children by use of TCS. In a correlational study, a gradual postnatal decline of substantia nigra echogenicity in 109 children aged 0–192 months was reported, suggesting an age-dependent modification during the first decade of life.12 Therefore, the increased substantia nigra echogenicity in our sample of ADHD patients relative to healthy controls might be explained by a developmental delay. This assumption is supported by findings in ADHD pointing to a maturational delay in relevant neuroanatomic brain regions.13 However, in our study, with participants aged between 7 and 16 years, we were unable to detect any significant correlation between age and echogenicity, suggesting that maturational changes in substantia nigra echogenicity might predominantly occur in the first years of life.13 Although most findings with regard to a presumptive developmental delay in ADHD relate to diminished growth of cortical thickness, recent studies have reported structural alterations in the basal ganglia of patients with ADHD.14,15
In adults, enlargement of the echogenic substantia nigra area is considered to be a structural marker of dysfunction of the nigrostriatal dopaminergic system. Enlarged echogenicity in healthy individuals was associated with impaired uptake of [18F]-dopa in the basal ganglia (assessed by positron emission tomography).10 Substantia nigra echogenicity was correlated to presynaptic dopaminergic dysfunction elicited by single photon emission computed tomography in patients with Parkinson disease16 and with the severity of parkinsonism induced by neuroleptics in patients with schizophrenia.17 However, there is still disagreement about how abnormal extension of substantia nigra echogenicity is related to the pathogenic substrate of Parkinson disease. Furthermore, the functional significance of increased substantia nigra echogenicity in children with ADHD is yet unknown.
Several studies suggest that enlarged echogenic substantia nigra area may be related to deposition of iron compounds (but no other trace metals).18 Our results may then suggest that the developmental maturation of iron metabolism is altered in patients with ADHD. Because iron is an essential cofactor of tyrosine hydroxylase, disturbance of its function might result in alterations of dopamine synthesis. Thus, this mechanism might deliver a potential basis for an etiological model linking neuropsychiatric disorders like Parkinson disease, schizophrenia and ADHD to iron metabolism and TCS findings of enlarged substantia nigra echogenicity.19,20 However, the well-established association between iron content and echogenicity of the substantia nigra has not yet been demonstrated in children. Nevertheless, recent findings suggesting an association between decreased ferritin levels and severity of ADHD symptoms, supporting the notion that iron metabolism might be involved in the pathophysiology of ADHD.21
An enlarged echogenic substantia nigra area has also been reported in individuals with Parkinson disease and depression, who concomitantly showed altered echogenicity of the raphe nuclei as a presumptive structural trait marker of a disturbed serotonergic system.22 Because we did not determine the structure of the raphe nuclei in this study, we cannot draw any conclusions about what further neurotransmitter systems may be altered in children with ADHD.
Limitations
Both comorbidity and central nervous medication in our patient sample might have confounded our results; however, neither methylphenidate nor the comorbid disorders present in our sample are known to influence substantia nigra echogenicity. The large effect size and both its high sensitivity and specificity render it unlikely that our findings are because of chance. Nevertheless, residual confounding cannot be completely ruled out.
Conclusion
Our results suggest that assessment of substantia nigra echogenicity might be useful in diagnosing ADHD. To clarify its clinical significance, future studies should involve dimensional assessment of neuropsychiatric symptoms and investigations of executive function while accounting for possible confounders such as comorbidity and the use of central nervous system medications. To date, it remains unclear whether an enlarged echogenic substantia nigra area in ADHD patients can be attributed to a primary disturbance of nigral iron metabolism, whether it is related to a primary developmental delay of brain structure, or whether it indicates a general structural marker for dysfunction of the dopaminergic system. Longitudinal study designs may determine the physiologic course of substantia nigra echogenicity and its relevance in the pathophysiology of neuropsychiatric disorders.
Acknowledgments
The authors thank all families for their participation and support. This study was performed within the framework of the Clinical Research Program ADHD (KFO 125/1-1 & 1-2) supported by the Deutsche Forschungsgesellschaft. The research of the authors is also supported by a grant from the Münchner Medizinische Wochenschrift and the “Verein zur Durchführung neurowissenschaftlicher Kongresse e.V.”
Footnotes
Competing interests: None declared.
Contributors: Drs. Romanos, Weise, Warnke, Gerlach, Classen and Mehler-Wex designed the study. Drs. Romanos and Weise and Mrs. Schliesser and Mrs. Löffler acquired the data, which Drs. Romanos, Weise, Schecklmann, Warnke, Gerlach, Classen and Mehler-Wex analyzed. Drs. Romanos, Weise and Schecklmann wrote the article. All authors reviewed the article and approved its publication.
References
- 1.Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–8. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 2.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–48. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- 3.Mehler-Wex C, Riederer P, Gerlach M. Dopaminergic dysbalance in distinct basal ganglia neurocircuits: implications for the pathophysiology of Parkinson’s disease, schizophrenia and attention deficit hyperactivity disorder. Neurotox Res. 2006;10:167–79. doi: 10.1007/BF03033354. [DOI] [PubMed] [Google Scholar]
- 4.Becker G, Seufert J, Bogdahn U, et al. Degeneration of substantia nigra in chronic Parkinson’s disease visualized by transcranial color-coded real-time sonography. Neurology. 1995;45:182–4. doi: 10.1212/wnl.45.1.182. [DOI] [PubMed] [Google Scholar]
- 5.Berg D, Godau J, Walter U. Transcranial sonography in movement disorders. Lancet Neurol. 2008;7:1044–55. doi: 10.1016/S1474-4422(08)70239-4. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children –— present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Achenbach TM. Manual for the child behavior checklist/4–18 and 1991 profile. Burlington (VT): University of Vermont; 1991. [Google Scholar]
- 8.Döpfner M, Lehmkuhl J. Diagnostik-System für psychische Störungen im Kindes- und Jugendalter nach ICD-10 und DSM-IV. Bern: Huber Verlag; 1998. [Google Scholar]
- 9.Stiensmeier-Pelster J, Schürmann M, Duda K. Depressions-Inventar für Kinder und Jugendliche (DIKJ) Göttingen: Hogrefe; 1989. [Google Scholar]
- 10.Berg D, Becker G, Zeiler B, et al. Vulnerability of the nigrostriatal system as detected by transcranial ultrasound. Neurology. 1999;53:1026–31. doi: 10.1212/wnl.53.5.1026. [DOI] [PubMed] [Google Scholar]
- 11.Walter U, Behnke S, Eyding J, et al. Transcranial brain parenchyma sonography in movement disorders: state of the art. Ultrasound Med Biol. 2007;33:15–25. doi: 10.1016/j.ultrasmedbio.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Iova A, Garmashov A, Androuchtchenko N, et al. Postnatal decrease in substantia nigra echogenicity. Implications for the pathogenesis of Parkinson’s disease. J Neurol. 2004;251:1451–4. doi: 10.1007/s00415-004-0556-3. [DOI] [PubMed] [Google Scholar]
- 13.Rubia K. Neuro-anatomic evidence for the maturational delay hypothesis of ADHD. Proc Natl Acad Sci U S A. 2007;104:19663–4. doi: 10.1073/pnas.0710329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu A, Crocetti D, Adler M, et al. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166:74–82. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellison-Wright I, Ellison-Wright Z, Bullmore E. Structural brain change in attention deficit hyperactivity disorder identified by meta-analysis. BMC Psychiatry. 2008;8:51. doi: 10.1186/1471-244X-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weise D, Lorenz R, Schliesser M, et al. Substantia nigra echogenicity: a structural correlate of functional impairment of the dopaminergic striatal projection in Parkinson’s disease. Mov Disord. 2009;24:1669–75. doi: 10.1002/mds.22665. [DOI] [PubMed] [Google Scholar]
- 17.Berg D, Jabs B, Merschdorf U, et al. Echogenicity of substantia nigra determined by transcranial ultrasound correlates with severity of parkinsonian symptoms induced by neuroleptic therapy. Biol Psychiatry. 2001;50:463–7. doi: 10.1016/s0006-3223(01)01190-8. [DOI] [PubMed] [Google Scholar]
- 18.Berg D, Roggendorf W, Schröder U, et al. Echogenicity of the substantia nigra: association with increased iron content and marker for susceptibility to nigrostriatal injury. Arch Neurol. 2002;59:999–1005. doi: 10.1001/archneur.59.6.999. [DOI] [PubMed] [Google Scholar]
- 19.Berg D, Gerlach M, Youdim MB, et al. Brain iron pathways and their relevance to Parkinson’s disease. J Neurochem. 2001;79:225–36. doi: 10.1046/j.1471-4159.2001.00608.x. [DOI] [PubMed] [Google Scholar]
- 20.Berg D. Disturbance of iron metabolism as a contributing factor to SN hyperechogenicity in Parkinson’s disease: implications for idiopathic and monogenetic forms. Neurochem Res. 2007;32:1646–54. doi: 10.1007/s11064-007-9346-5. [DOI] [PubMed] [Google Scholar]
- 21.Oner P, Oner O. Relationship of ferritin to symptom ratings children with attention deficit hyperactivity disorder: effect of comorbidity. Child Psychiatry Hum Dev. 2008;39:323–30. doi: 10.1007/s10578-007-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walter U, Hoeppner J, Prudente-Morrissey L, et al. Parkinson’s disease-like midbrain sonography abnormalities are frequent in depressive disorders. Brain. 2007;130:1799–807. doi: 10.1093/brain/awm017. [DOI] [PubMed] [Google Scholar]