Abstract
Background
Plasmalogens, which are key structural phospholipids in brain membranes, are decreased in the brain and serum of patients with Alzheimer disease (AD). We performed this pilot study to evaluate the relation between the levels of circulating plasmalogens and Alzheimer Disease Assessment Scale–Cognitive (ADAS-Cog) scores in patients with AD.
Methods
We evaluated participants’ ADAS-Cog scores and serum plasmalogen levels. For the 40 included AD patients with an ADAS-Cog score between 20 and 46, we retested their ADAS-Cog score 1 year later. The levels of docosahexaenoic acid plasmalogen were measured by use of liquid chromatography–tandem mass spectrometry.
Results
We found that the ADAS-Cog score increased significantly in AD patients with circulating plasmalogen levels that were ≤ 75% of that of age-matched controls at entry into the study. There was no change in score among participants with normal serum plasmalogen levels at baseline (> 75%).
Limitations
This was a pilot study with 40 patients, and the results require validation in a larger population.
Conclusion
Our study demonstrates that decreased levels of plasmalogen precursors in the central nervous system correlate with functional decline (as measured by ADAS-Cog scores) in AD patients. The use of both ADAS-Cog and serum plasmalogen data may be a more accurate way of predicting cognitive decline in AD patients, and may be used to decrease the risk of including patients with no cognitive decline in the placebo arm of a drug trial.
Introduction
Decreases in brain ethanolamine plasmalogens,1,2 one of the earliest biochemical changes detected in Alzheimer disease (AD), are specific to brain regions with AD pathology.1,2 These changes are disease specific; they are not present in Parkinson substantia nigra or Huntington caudate.2 Observations in transgenic mouse models of plasmalogen deficiency3,4 are characterized by a number of pathological features expressed in AD,5,6 including astrogliosis, microgliosis and demyelination. These data suggest that a decrease in the level of plasmalogen in the central nervous system (CNS) could be a focal point that leads to a number of the reported neuropathological features of AD.
Plasmalogens and plasmalogen precursors are supplied to the brain by the liver, bound to transport proteins.7 In the human CNS, ethanolamine plasmalogens constitute 30 mol% of total phospholipids and 90 mol% of ethanolamine glycerophospholipids in neuronal cell membranes.1 Decreases in these critical structural phospholipids, and the associated increases in membrane cholesterol, result in reduced membrane fluidity,8 ion channel function,9 membrane-bound enzyme activity,10,11 diffusion of signal-transduction molecules, such as nitric oxide,12 and vesicular fusion.13 The decreased neurotransmitter release associated with abnormal membrane phospholipid content can be corrected in aged rats with a diet enriched in docosahexaenoic acid (DHA) phospholipids.14 Both basal and KCl-stimulated acetylcholine release were augmented in the hippocampus of these DHA-treated aged rats.14
Plasmalogens are key regulators of cholesterol dynamics. Plasmalogen-deficient cells,15 transgenic mouse models of plasmalogen deficiency,3,4 Alzheimer brain membranes16 and tissues from patients with peroxisomal disorders (i.e., decreased plasmalogen synthesis)3 show elevated levels of membrane cholesterol and/or decreased amounts of membrane cholesterol esters. We previously reported that circulating levels of ethanolamine plasmalogens are decreased in patients with AD.17 To determine if these decreases predict a functional decline in AD patients, we monitored changes in patients’ scores on the Alzheimer Disease Assessment Scale–Cognitive (ADAS-Cog) instrument18 in 40 patients 1 year after their initial evaluation.
Methods
Participants
We recruited study participants by use of newspaper advertisements. We excluded people with potential neuropsychiatric disease. We included white people with probable AD (diagnosed based on criteria from the National Institute of Neurological and Communicative Diseases and Stroke –Alzheimer’s Disease and Related Disorders Association [NINCDS–ADRDA]). Brain imaging (computed tomography or magnetic resonance imaging) demonstrated cerebral atrophy and no evidence of ischemic stroke, brain tumours or hydrocephalus. We found no evidence of bipolar disorder, Parkinson disease, multi-infarct dementia, drug intoxication, thyroid disease, pernicious anemia, luetic brain disease, chronic infections of the nervous system, occult hydrocephalus, Huntington disease, Creutzfeldt–Jakob disease or brain tumours in any of the participants. We enrolled 40 people with AD with entry ADAS-Cog scores between 20 and 46 (Table 1).
Table 1.
Demographic characteristics of participants with Alzheimer disease (n = 40), grouped based on their initial serum plasmalogen levels relative to those of healthy controls (n = 66)
| PlsEtn level | Sex | Age, yr | ApoE | PlsEtn level | Sex | Age, yr | ApoE |
|---|---|---|---|---|---|---|---|
| ≤ 25% | F | 89 | E3/4 | F | 89 | E4/4 | |
| M | 89 | E2/3 | F | 81 | E3/4 | ||
| M | 81 | E3/4 | F | 80 | E3/3 | ||
| M | 72 | E3/4 | 51%–75% | M | 78 | E4/4 | |
| F | 80 | E3/4 | F | 82 | E3/4 | ||
| 26%–50% | F | 82 | E3/4 | F | 86 | E3/4 | |
| F | 78 | E2/3 | M | 89 | E3/3 | ||
| F | 92 | E2/3 | M | 89 | E4/4 | ||
| F | 91 | E3/4 | M | 74 | E3/3 | ||
| F | 82 | E2/3 | M | 78 | E3/4 | ||
| F | 83 | E3/4 | M | 95 | E3/3 | ||
| F | 85 | E2/3 | F | 81 | E3/4 | ||
| F | 74 | E3/4 | M | 80 | E4/4 | ||
| M | 71 | E4/4 | F | 71 | E4/4 | ||
| F | 84 | E3/4 | M | 92 | E3/3 | ||
| M | 80 | E2/3 | > 75% | M | 81 | E4/4 | |
| F | 81 | E4/4 | M | 90 | E3/4 | ||
| F | 88 | E3/4 | F | 83 | E3/3 | ||
| F | 82 | E3/4 | F | 68 | E4/4 | ||
| M | 89 | E3/3 | F | 87 | E2/3 |
ApoE = apolipoprotein; F = female; M = male; PlsEtn = ethanolamine plasmalogen.
As age-matched controls, we included white people whose cognitive status was normal and who had no family history of AD and a Mini Mental State examination19 score greater than 28. We included 66 controls (40 women and 26 men) with a mean age of 77 (standard deviation [SD] 6, range 67–89) years.
The protocol received ethics approval (Western internal review board), and all participants or caregivers signed informed consent forms for blood sample collection, cognitive testing and medical evaluation.
Plasmalogen measurements
Blood samples were collected from all participants by Precision Medica (San Diego, California). Serum ethanolamine plasmalogen levels were measured by liquid chromatography–mass spectrometry, as reported previously.17
Data analysis
We analyzed the data using paired t tests (one-tail) with p < 0.05 used for significance.
Results
The rate of cognitive decline over 12 months in 40 participants with AD was determined by use of the ADAS-Cog instrument. Overall, participants experienced a significant decline in cognition (increased ADAS-Cog from 30.4 [SD 7.4] to 44.6 [SD 16.7]).
To determine if there was a relation between serum ethanolamine plasmalogen levels and the rate of cognitive decline, the level of ethanolamine plasmalogens was compared among AD patients and controls (Table 1). The patients with AD were grouped based on the degree of depletion of ethanolamine plasmalogens relative to controls: normal (> 75% of control), mild depletion (50%–75% of control), moderate depletion (25%–50% of control) or severe depletion (< 25% of control).
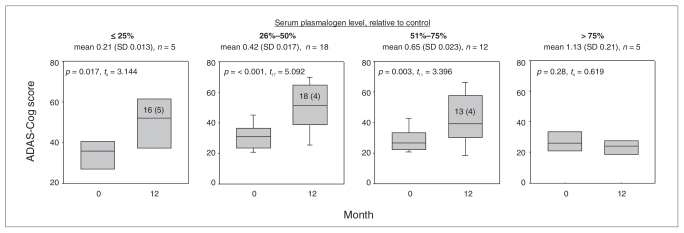
We found no cognitive decline among AD participants with normal plasmalogen levels (Fig. 1). Participants with mild, moderate or severe ethanolamine plasmalogen depletion had significant cognitive decline (Fig. 1). There was no correlation between apolipoprotein genotype and circulating plasmalogen levels.
Fig. 1.
Box plots of the progression of Alzheimer Disease Assessment Scale–Cognitive (ADAS-Cog) scores over a 1-year period among 40 patients with Alzheimer disease. Patients were grouped based on their initial serum plasmalogen level (month 0) relative to that of age-matched controls (> 75%, < 25%, 26%–50% or 51%–75%). The boxes represent the 25th and 75th percentiles, and the bars represent the 10th and 90th percentiles. Serum plasmalogen levels are given in brackets; control serum plasmalogen levels were 1.0, standard deviation 0.45 (n = 66). The 1-year change in ADAS-Cog scores (mean [SD]) are included in the boxes. SD = standard deviation.
Discussion
It has been established that DHA17,20 and DHA-containing ethanolamine plasmalogens1,2,17 are decreased in the serum and brains of AD patients. These decreases in key structural phospholipids result in many detrimental effects on neuronal function8–13 that presumably underlie the cognitive decline in AD patients. Although the specificity of the decrease in cortical plasmalogens in AD has been shown,2 we continue to explore the specificity of the decrease in circulating plasmalogen levels noted in AD patients.
The current gold standard for monitoring cognitive decline in AD patients is the ADAS-Cog instrument.18 The expected 1-year increase in ADAS-Cog score for an AD patient with a baseline score of 25–35 would be 10–15 units,21,22 which was observed in this study. All patients with this level of cognitive decline had serum plasmalogen levels less than 75% of the average for age-matched controls. These data suggest that, as we move ahead in the evaluation of dosing with plasmalogen precursors in AD patients, the focus should be on AD patients with this biochemical deficit. Those patients with no decrease in serum plasmalogens may be a different disease subset with another cause.
These data also suggest that replacing deficient plasmalogen precursors in the serum may restore brain plasmalogens and improve neuronal and, hence, cognitive function in AD patients. The advantages of augmenting brain plasmalogen levels, in addition to normalizing neuronal membrane function, potentially include augmentation of cholinergic neurotransmission14 and normalization of myelin dysfunction. Decreased myelin volumes in the brain of AD patients are more consistent than atrophy of the substantia innominata (nucleus basalis)23 and extend even to the optic nerve, which is also enriched in DHA-containing plasmalogens.24
The structural alterations in the brains of AD patients, as a result of decreased membrane plasmalogens, increased membrane cholesterol and hypomyelination, probably underlie the decline in cognitive function in this complex disease. Restoring normal neuronal membranes and brain connectivity should offer a potential disease-modifying therapy for AD. In this regard, DHA ethyl ester has been shown to improve myelination and cognition in some patients with peroxisomal disorders,25 and liver transplants have been reported to improve myelination and cognition in patients with viral hepatitis.26 Liver transplant corrects many metabolic imbalances and leads to normalization of the liver’s ability to supply plasmalogen precursors to the brain.
The synthesis of plasmalogens is critically dependent on peroxisomes to generate 1-alkyl-glyceryl ether lipid precursors; however, peroxisomal function decreases during aging.27 To bypass this potentially rate-limiting step, investigators have shown that 1-alkyl-glyceryl ether lipid precursors (Fig. 2) can be processed by the endoplasmic reticulum of rodents and humans to generate tissue plasmalogens.28–31 We are currently developing a more stable and bioavailable alkyl-glyceryl ether lipid analog for evaluation in AD. Monitoring serum plasmalogens will provide initial indices of the pharmacokinetic and pharmacodynamic efficacy of this approach and the ADAS-Cog will provide evidence of clinical efficacy.
Fig. 2.
Plasmalogen biosynthesis and the point of entry of 1-alkyl-2-acyl-glycerol ethers (underlined) into the pathway. DHAP = dihydroxyacetone phosphate; G = glycerol; GPE = glycerylphospho-ethanolamine; P = phosphate.
Limitations
This was a pilot study with 40 AD patients, and the results require validation in a larger population.
Conclusion
The data from these studies allow for 2 conclusions that require validation in a larger population. First, AD patients with ADAS-cog scores in the 25–35 range, as recruited in most drug studies, and serum plasmalogen levels less than 75% of the level of controls reliably progress into cognitive decline. This biochemical parameter may be used to reduce the risk of including in clinical trials participants whose cognitive function is unlikely to deteriorate, making the results from trials of AD drugs much more reliable. Second, the re-supply of plasmalogen precursors to AD patients has the potential to correct a major structural defect in AD brain membranes. This may have positive effects on other aspects of AD pathology and function.
Footnotes
Competing interests: Dr. P. Wood is CEO of and owns stock in Phreedom Pharma. Drs. Mankidy, Ritchie, Heath and J. Wood are employed by Phenomenome Discoveries. Dr. Goodenowe is CEO of and owns stock in Phenomenome Discoveries. Dr. Flax is employed by and owns stock in PrecisionMed, Inc. None declared for Dr. Mankidy.
Contributors: Drs. P. Wood and Goodenowe designed the study and wrote the article. Drs. P. Wood, Mankidy, Ritchie, Heath and Flax acquired the data, which Drs. P. Wood, Ritchie, Heath, J. Wood and Goodenowe analyzed. Drs. Mankidy, Richie, Heath, J. Wood and Flax reviewed the article. All authors approved its publication.
References
- 1.Han X, Holtzman DM, McKeel DW., Jr Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J Neurochem. 2001;77:1168–80. doi: 10.1046/j.1471-4159.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg L, Rafique S, Xuereb JH, et al. Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer’s disease brain. Brain Res. 1995;698:223–6. doi: 10.1016/0006-8993(95)00931-f. [DOI] [PubMed] [Google Scholar]
- 3.Gorgas K, Teigler A, Komljenovic D, Just WW. The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim Biophys Acta. 2006;1763:1511–26. doi: 10.1016/j.bbamcr.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 4.Brites P, Mooyer PA, El Mrabet L, et al. Plasmalogens participate in very-long-chain fatty acid-induced pathology. Brain. 2009;132:482–92. doi: 10.1093/brain/awn295. [DOI] [PubMed] [Google Scholar]
- 5.Kavcic V, Ni H, Zhu T, et al. White matter integrity linked to functional impairments in aging and early Alzheimer’s disease. Alzheimers Dement. 2008;4:381–9. doi: 10.1016/j.jalz.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood JA, Wood PL, Ryan R, et al. Cytokine indices in Alzheimer’s temporal cortex: no changes in mature IL-1 beta or IL-1RA but increases in the associated acute phase proteins IL-6, alpha-2-macroglobulin and C-reactive protein. Brain Res. 1993;629:245–52. doi: 10.1016/0006-8993(93)91327-o. [DOI] [PubMed] [Google Scholar]
- 7.Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci U S A. 1989;86:2903–7. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto M, Hossain S, Masumura S. Effect of aging on plasma membrane fluidity of rat aortic endothelial cells. Exp Gerontol. 1999;34:687–98. doi: 10.1016/s0531-5565(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto M, Hossain S, Tanabe Y, et al. Effects of aging on the relation of adenyl purine release with plasma membrane fluidity of arterial endothelial cells. Prostaglandins Leukot Essent Fatty Acids. 2005;73:475–83. doi: 10.1016/j.plefa.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Xiu J, Nordberg A, Qi X, et al. Influence of cholesterol and lovastatin on alpha-form of secreted amyloid precursor protein and expression of alpha7 nicotinic receptor on astrocytes. Neurochem Int. 2006;49:459–65. doi: 10.1016/j.neuint.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Miersch S, Espey MG, Chaube R, et al. Plasma membrane cholesterol content affects nitric oxide diffusion dynamics and signaling. J Biol Chem. 2008;283:18513–21. doi: 10.1074/jbc.M800440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo J, Chi S, Xu H, et al. Effects of cholesterol levels on the excitability of rat hippocampal neurons. Mol Membr Biol. 2008;25:216–23. doi: 10.1080/09687680701805541. [DOI] [PubMed] [Google Scholar]
- 13.Amatore C, Arbault S, Guille M, et al. The nature and efficiency of neurotransmitter exocytosis also depend on physicochemical parameters. ChemPhysChem. 2007;8:1597–605. doi: 10.1002/cphc.200700225. [DOI] [PubMed] [Google Scholar]
- 14.Favrelière S, Perault MC, Huguet F, et al. DHA-enriched phospholipid diets modulate age-related alterations in rat hippocampus. Neurobiol Aging. 2003;24:233–43. doi: 10.1016/s0197-4580(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 15.Nagan N, Hajra AK, Larkins LK, et al. Isolation of a Chinese hamster fibroblast variant defective in dihydroxyacetonephosphate acyltransferase activity and plasmalogen biosynthesis: use of a novel two-step selection protocol. Biochem J. 1998;332:273–9. doi: 10.1042/bj3320273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutler RG, Kelly J, Storie K, et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:2070–5. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodenowe DB, Cook LL, Liu J, et al. Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in Alzheimer’s disease and dementia. J Lipid Res. 2007;48:2485–98. doi: 10.1194/jlr.P700023-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Mohs RC, Davis BM, Johns CA, et al. Oral physostigmine treatment of patients with Alzheimer’s disease. Am J Psychiatry. 1985;142:28–33. doi: 10.1176/ajp.142.1.28. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Conquer JA, Tierney MC, Zecevic J, et al. Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids. 2000;35:1305–12. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 21.Stern RG, Mohs RC, Davidson M, et al. A longitudinal study of Alzheimer’s disease: measurement, rate, and predictors of cognitive deterioration. Am J Psychiatry. 1994;151:390–6. doi: 10.1176/ajp.151.3.390. [DOI] [PubMed] [Google Scholar]
- 22.Suh GH, Ju YS, Yeon BK, et al. A longitudinal study of Alzheimer’s disease: rates of cognitive and functional decline. Int J Geriatr Psychiatry. 2004;19:817–24. doi: 10.1002/gps.1168. [DOI] [PubMed] [Google Scholar]
- 23.Moon WJ, Kim HJ, Roh HG, et al. Atrophy measurement of the anterior commissure and substantia innominata with 3T high-resolution MR imaging: Does the measurement differ for patients with frontotemporal lobar degeneration and Alzheimer disease and for healthy subjects? AJNR Am J Neuroradiol. 2008;29:1308–13. doi: 10.3174/ajnr.A1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danesh-Meyer HV, Birch H, Ku JY, et al. Reduction of optic nerve fibers in patients with Alzheimer disease identified by laser imaging. Neurology. 2006;67:1852–4. doi: 10.1212/01.wnl.0000244490.07925.8b. [DOI] [PubMed] [Google Scholar]
- 25.Martínez M, Vázquez E, García-Silva MT, et al. Therapeutic effects of docosahexaenoic acid ethyl ester in patients with generalized peroxisomal disorders. Am J Clin Nutr. 2000;71(Suppl):376S–85S. doi: 10.1093/ajcn/71.1.376s. [DOI] [PubMed] [Google Scholar]
- 26.Rovira A, Mínguez B, Aymerich FX, et al. Decreased white matter lesion volume and improved cognitive function after liver transplantation. Hepatology. 2007;46:1485–90. doi: 10.1002/hep.21911. [DOI] [PubMed] [Google Scholar]
- 27.Périchon R, Bourre JM, Kelly JF, et al. The role of peroxisomes in aging. Cell Mol Life Sci. 1998;54:641–52. doi: 10.1007/s000180050192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das AK, Hajra AK. High incorporation of dietary 1-O-heptadecyl glycerol into tissue plasmalogens of young rats. FEBS Lett. 1988;227:187–90. doi: 10.1016/0014-5793(88)80895-0. [DOI] [PubMed] [Google Scholar]
- 29.Oswald EO, Anderson CE, Piantadosi C, et al. Metabolism of alkyl glyceryl ethers in the rat. Lipids. 1968;3:51–8. doi: 10.1007/BF02530969. [DOI] [PubMed] [Google Scholar]
- 30.Bickerstaffe R, Mead JF. Metabolism of chimyl alcohol and phosphatidyl ethanolamine in the rat brain. Lipids. 1968;3:317–20. doi: 10.1007/BF02530931. [DOI] [PubMed] [Google Scholar]
- 31.Das AK, Holmes RD, Wilson GN, et al. Dietary ether lipid incorporation into tissue plasmalogens of humans and rodents. Lipids. 1992;27:401–5. doi: 10.1007/BF02536379. [DOI] [PubMed] [Google Scholar]