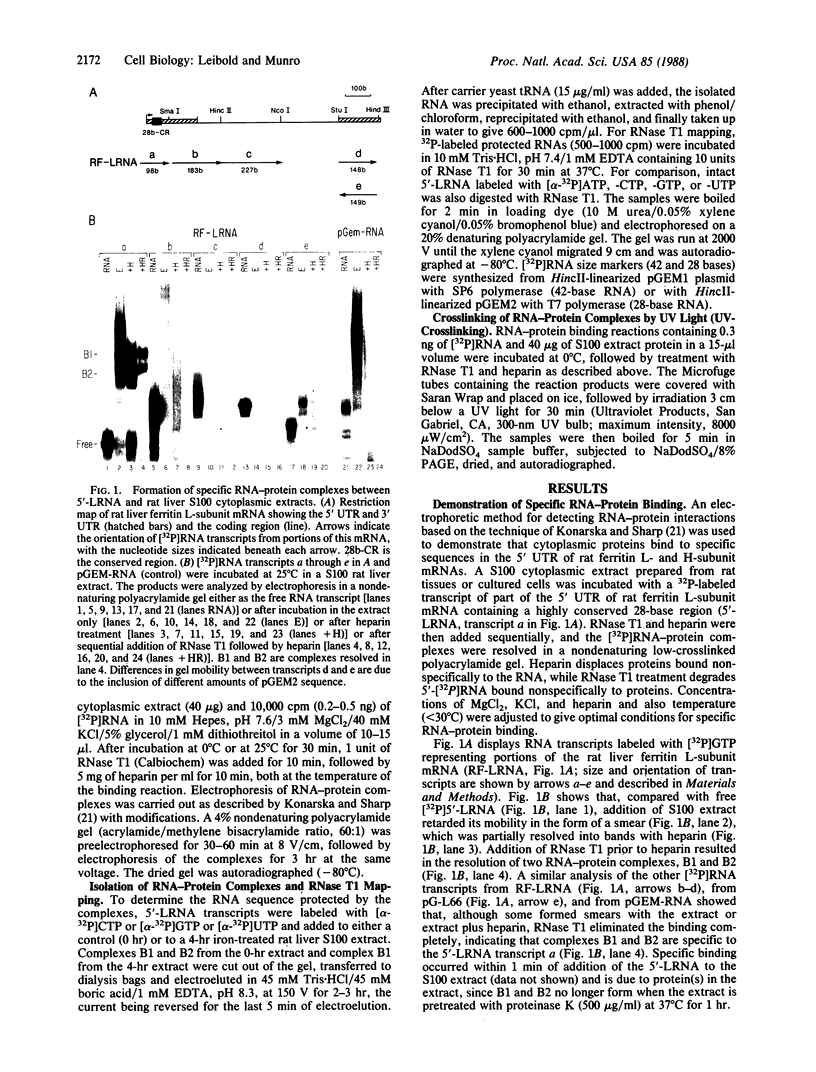
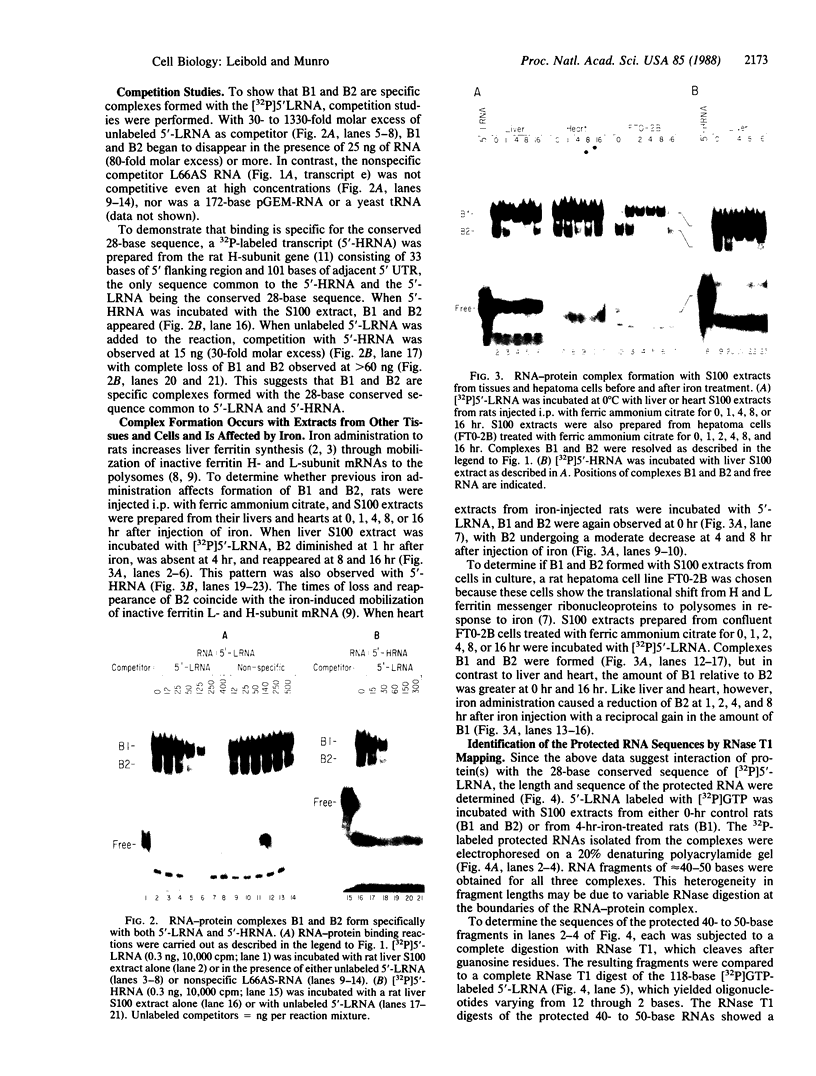
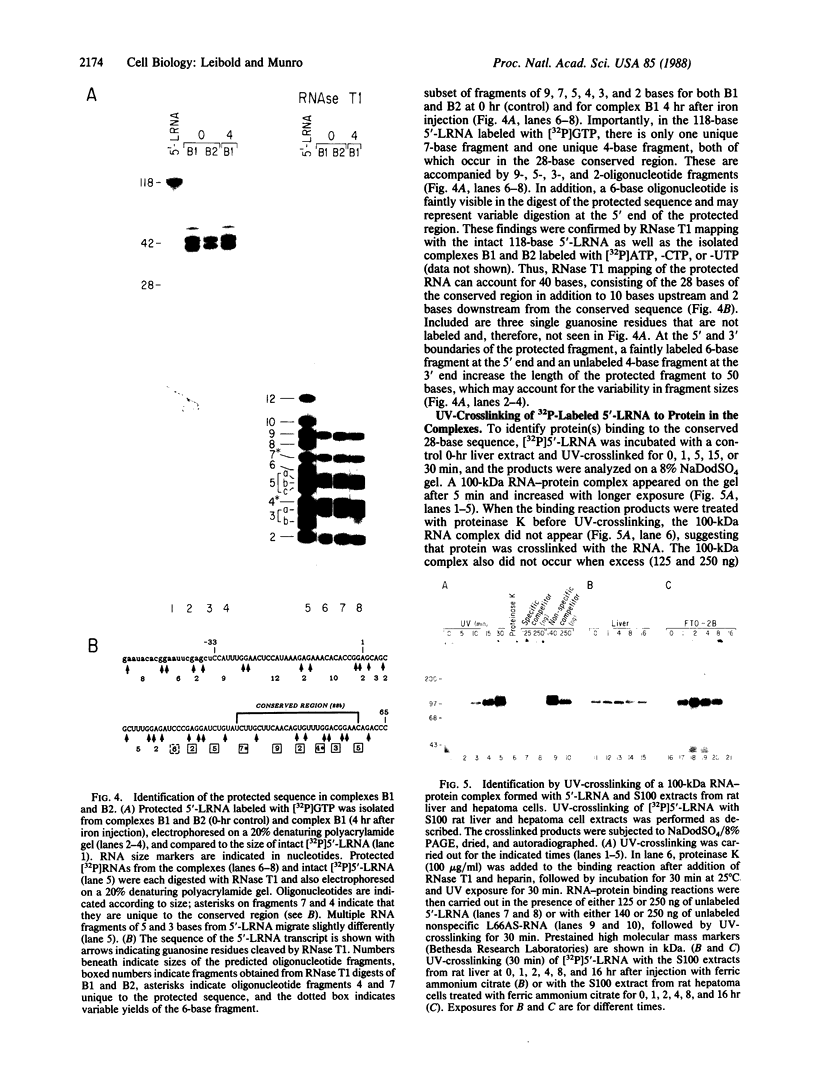
Abstract
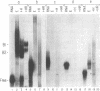
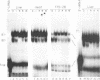
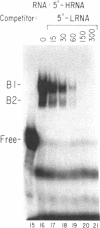
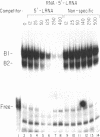
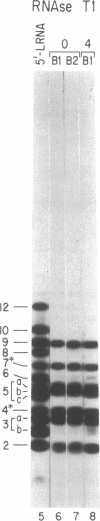
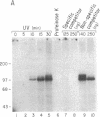
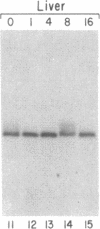
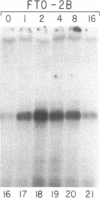
The mRNAs for the heavy and light subunits of the iron-storage protein ferritin occur in cells largely as inactive ribonucleoprotein particles, which are recruited for translation when iron enters the cell. Cytoplasmic extracts from rat tissues and hepatoma cells were shown by an electrophoretic separation procedure to form RNA-protein complexes involving a highly conserved sequence in the 5' untranslated region of both ferritin heavy- and light-subunit mRNAs. The pattern of complex formation was affected by pretreatment of rats or cells with iron. Crosslinking by UV irradiation showed that the complexes contained an 87-kDa protein interacting with the conserved sequence of the ferritin mRNA. We propose that intracellular iron levels regulate ferritin synthesis by causing changes in specific protein binding to the conserved sequence in the ferritin heavy- and light-subunit mRNAs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Both subunits of rat liver ferritin are regulated at a translational level by iron induction. Nucleic Acids Res. 1986 Jan 24;14(2):915–927. doi: 10.1093/nar/14.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomford A., Conlon-Hollingshead C., Munro H. N. Adaptive responses of rat tissue isoferritins to iron administration. Changes in subunit synthesis, isoferritin abundance, and capacity for iron storage. J Biol Chem. 1981 Jan 25;256(2):948–955. [PubMed] [Google Scholar]
- Costanzo F., Colombo M., Staempfli S., Santoro C., Marone M., Frank R., Delius H., Cortese R. Structure of gene and pseudogenes of human apoferritin H. Nucleic Acids Res. 1986 Jan 24;14(2):721–736. doi: 10.1093/nar/14.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didsbury J. R., Theil E. C., Kaufman R. E., Dickey L. F. Multiple red cell ferritin mRNAs, which code for an abundant protein in the embryonic cell type, analyzed by cDNA sequence and by primer extension of the 5'-untranslated regions. J Biol Chem. 1986 Jan 15;261(2):949–955. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Paterson M., Listowsky I. Iron uptake and regulation of ferritin synthesis by hepatoma cells in hormone-supplemented serum-free media. J Biol Chem. 1983 Apr 25;258(8):5248–5255. [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Caughman S. W., Dancis A., Harford J. B., Klausner R. D. A cis-acting element is necessary and sufficient for translational regulation of human ferritin expression in response to iron. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6730–6734. doi: 10.1073/pnas.84.19.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohgo Y., Yokota M., Drysdale J. W. Differential turnover of rat liver isoferritins. J Biol Chem. 1980 Jun 10;255(11):5195–5200. [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Electrophoretic separation of complexes involved in the splicing of precursors to mRNAs. Cell. 1986 Sep 12;46(6):845–855. doi: 10.1016/0092-8674(86)90066-8. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell. 1987 Jun 19;49(6):763–774. doi: 10.1016/0092-8674(87)90614-3. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Aziz N., Brown A. J., Munro H. N. Conservation in rat liver of light and heavy subunit sequences of mammalian ferritin. Presence of unique octopeptide in the light subunit. J Biol Chem. 1984 Apr 10;259(7):4327–4334. [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Characterization and evolution of the expressed rat ferritin light subunit gene and its pseudogene family. Conservation of sequences within noncoding regions of ferritin genes. J Biol Chem. 1987 May 25;262(15):7335–7341. [PubMed] [Google Scholar]
- Murray M. T., White K., Munro H. N. Conservation of ferritin heavy subunit gene structure: implications for the regulation of ferritin gene expression. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7438–7442. doi: 10.1073/pnas.84.21.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Pikielny C. W., Rosbash M. Specific small nuclear RNAs are associated with yeast spliceosomes. Cell. 1986 Jun 20;45(6):869–877. doi: 10.1016/0092-8674(86)90561-1. [DOI] [PubMed] [Google Scholar]
- Pikielny C. W., Rymond B. C., Rosbash M. Electrophoresis of ribonucleoproteins reveals an ordered assembly pathway of yeast splicing complexes. 1986 Nov 27-Dec 3Nature. 324(6095):341–345. doi: 10.1038/324341a0. [DOI] [PubMed] [Google Scholar]
- Rittling S. R., Woodworth R. C. The synthesis and turnover of ferritin in rat L-6 cells. Rates and response to iron, actinomycin D, and desferrioxamine. J Biol Chem. 1984 May 10;259(9):5561–5566. [PubMed] [Google Scholar]
- Rogers J., Munro H. Translation of ferritin light and heavy subunit mRNAs is regulated by intracellular chelatable iron levels in rat hepatoma cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2277–2281. doi: 10.1073/pnas.84.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk P. J., Lowary P., Wu H. N., Stormo G., Uhlenbeck O. C. RNA binding site of R17 coat protein. Biochemistry. 1987 Mar 24;26(6):1563–1568. doi: 10.1021/bi00380a011. [DOI] [PubMed] [Google Scholar]
- Santoro C., Marone M., Ferrone M., Costanzo F., Colombo M., Minganti C., Cortese R., Silengo L. Cloning of the gene coding for human L apoferritin. Nucleic Acids Res. 1986 Apr 11;14(7):2863–2876. doi: 10.1093/nar/14.7.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull G. E., Theil E. C. Translational control of ferritin synthesis by iron in embryonic reticulocytes of the bullfrog. J Biol Chem. 1982 Dec 10;257(23):14187–14191. [PubMed] [Google Scholar]
- Stevens P. W., Dodgson J. B., Engel J. D. Structure and expression of the chicken ferritin H-subunit gene. Mol Cell Biol. 1987 May;7(5):1751–1758. doi: 10.1128/mcb.7.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Thomas M. S., Nomura M. Translational regulation of the L11 ribosomal protein operon of Escherichia coli: mutations that define the target site for repression by L1. Nucleic Acids Res. 1987 Apr 10;15(7):3085–3096. doi: 10.1093/nar/15.7.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Cole C. N. Identification of a complex associated with processing and polyadenylation in vitro of herpes simplex virus type 1 thymidine kinase precursor RNA. Mol Cell Biol. 1987 Sep;7(9):3277–3286. doi: 10.1128/mcb.7.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]