Abstract
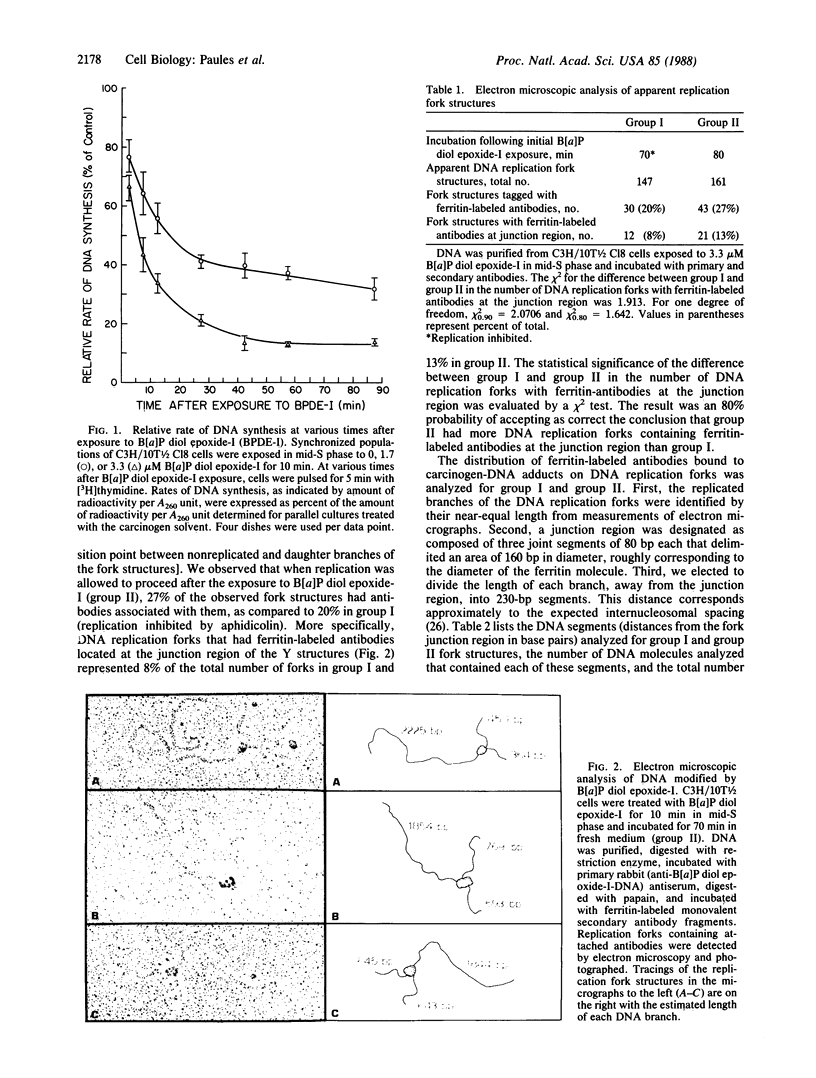
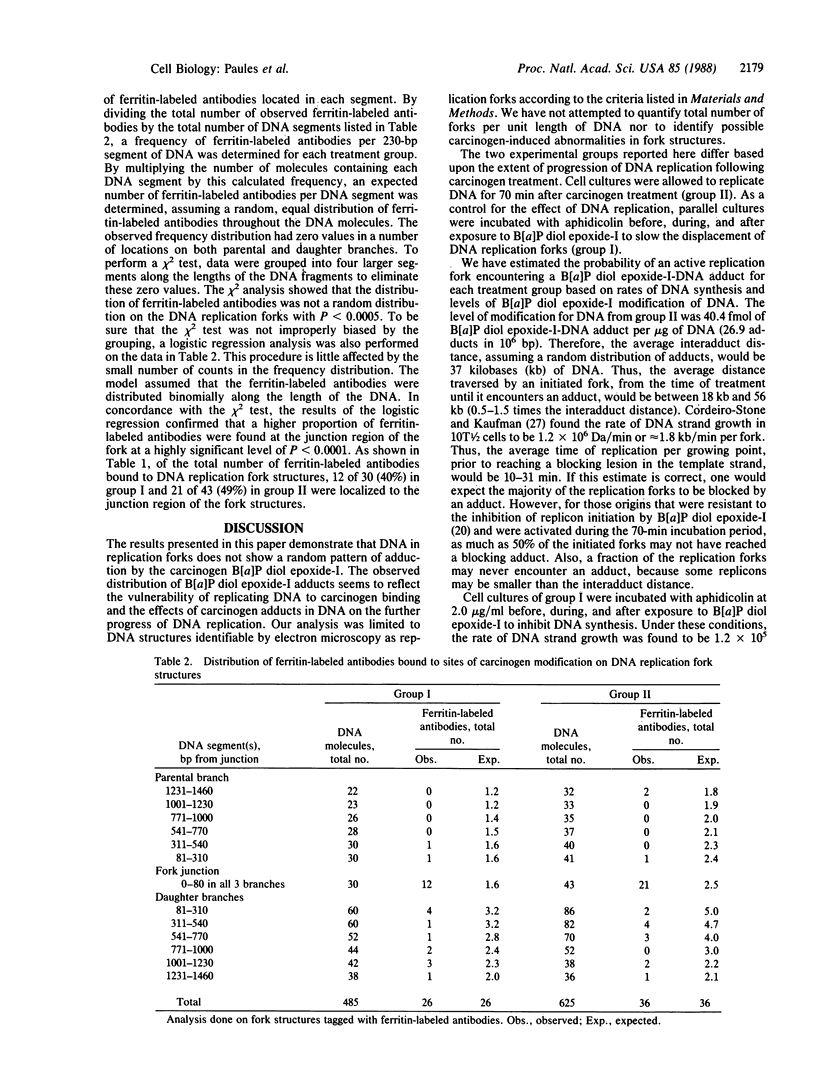
The distribution of lesions in DNA caused by (+/-)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo [alpha]pyrene (B[alpha]P diol epoxide-I) was studied in synchronized C3H/10T1/2 cells treated in S phase. Sites of carcinogen modification of DNA were identified by polyclonal rabbit antibodies elicited against DNA modified with B[alpha]P diol epoxide-I in vitro. This antigenic DNA contained trans-(7R)-N2-[10-(7 beta,8 alpha,9 alpha-trihydroxy-7,8,9,10-tetrahydrobenzo[alpha]pyrene)-yl]- deoxyguanosine; other adducts were not detected by liquid chromatography. In this study, DNA replication forks with antibodies bound to B[alpha]P diol epoxide-I adducts were detected by electron microscopy. The frequency of replication forks containing carcinogen adducts associated with the fork junction was found to be 8-fold higher than expected for an average distribution. The proportion of replication forks that were apparently blocked at the site of the DNA damage increased when replication was allowed to occur after carcinogen exposure. These results support the conclusions that the fork junction is particularly vulnerable to adduction by B[alpha]P diol epoxide-I and that B[alpha]P diol epoxide-I adducts block the displacement of replication forks during DNA synthesis in intact cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden G. T., Boutwell R. K. Studies on the role of stimulated epidermal DNA synthesis in the initiation of skin tumors in mice by N-methyl-N'-nitro-N-nitrosoguanidine. Cancer Res. 1974 Jul;34(7):1552–1563. [PubMed] [Google Scholar]
- Bowden G. T., McGovern V., Ossanna N., Rosenthal H. Concentration dependent alterations of DNA replication initiation and elongation by benzo[a]pyrene diol epoxide. Carcinogenesis. 1982;3(5):473–480. doi: 10.1093/carcin/3.5.473. [DOI] [PubMed] [Google Scholar]
- Bowden G. T., Yuspa S. H. Repair of daughter strand gaps in nascent DNA from mouse epidermal cells treated with dihydrodiol epoxide derivatives of benzo[a]pyrene. Biochim Biophys Acta. 1979 Nov 22;565(1):67–83. doi: 10.1016/0005-2787(79)90083-2. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Kaufmann W. K., Kapp L. N., Park S. D. Replicon size and excision repair as factors in the inhibition and recovery of DNA synthesis from ultraviolet damage. Biochim Biophys Acta. 1983 Mar 10;739(2):207–215. doi: 10.1016/0167-4781(83)90031-3. [DOI] [PubMed] [Google Scholar]
- Columbano A., Rajalakshmi S., Sarma D. S. Requirement of cell proliferation for the initiation of liver carcinogenesis as assayed by three different procedures. Cancer Res. 1981 Jun;41(6):2079–2083. [PubMed] [Google Scholar]
- Connell J. R. The relationship between sister chromatid exchange, chromosome aberration and gene mutation induction by several reactive polycyclic hydrocarbon metabolites in cultured mammalian cells. Int J Cancer. 1979 Oct 15;24(4):485–489. doi: 10.1002/ijc.2910240417. [DOI] [PubMed] [Google Scholar]
- Cordeiro-Stone M., Boyer J. C., Smith B. A., Kaufmann W. K. Effect of benzo[a]pyrene-diol-epoxide-I on growth of nascent DNA in synchronized human fibroblasts. Carcinogenesis. 1986 Oct;7(10):1775–1781. doi: 10.1093/carcin/7.10.1775. [DOI] [PubMed] [Google Scholar]
- Cordeiro-Stone M., Kaufman D. G. Kinetics of DNA replication in C3H 10T1/2 cells synchronized by aphidicolin. Biochemistry. 1985 Aug 27;24(18):4815–4822. doi: 10.1021/bi00339a015. [DOI] [PubMed] [Google Scholar]
- Cordeiro-Stone M., Schumacher R. I., Meneghini R. Structure of the replication fork in ultraviolet light-irradiated human cells. Biophys J. 1979 Aug;27(2):287–300. doi: 10.1016/S0006-3495(79)85218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro-Stone M., Topal M. D., Kaufman D. G. DNA in proximity to the site of replication is preferentially alkylated in S phase 10T1/2 cells treated with N-methyl-N-nitroso-urea. Carcinogenesis. 1982;3(10):1119–1127. doi: 10.1093/carcin/3.10.1119. [DOI] [PubMed] [Google Scholar]
- Frei J. V., Harsono T. Increased susceptibility to low doses of a carcinogen of epidermal cells in stimulated DNA synthesis. Cancer Res. 1967 Aug;27(8):1482–1484. [PubMed] [Google Scholar]
- Gehly E. B., Landolph J. R., Heidelberger C., Nagasawa H., Little J. B. Induction of cytotoxicity, mutation, cytogenetic changes, and neoplastic transformation by benzo(a)pyrene and derivatives in C3H/10T1/2 clone 8 mouse fibroblasts. Cancer Res. 1982 May;42(5):1866–1875. [PubMed] [Google Scholar]
- Grisham J. W., Greenberg D. S., Kaufman D. G., Smith G. J. Cycle-related toxicity and transformation in 10T1/2 cells treated with N-methyl-N'-nitro-N-nitrosoguanidine. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4813–4817. doi: 10.1073/pnas.77.8.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman T. M., DePamphilis M. L., Wassarman P. M. Structure of chromatin at deoxyribonucleic acid replication forks: location of the first nucleosomes on newly synthesized simian virus 40 deoxyribonucleic acid. Biochemistry. 1981 Feb 3;20(3):621–630. doi: 10.1021/bi00506a027. [DOI] [PubMed] [Google Scholar]
- Hsu I. C., Bowden G. T., Harris C. C. A comparison of cytotoxicity, ouabain-resistant mutation, sister-chromatid exchanges, and nascent DNA synthesis in Chinese hamster cells treated with dihydrodiol epoxide derivatives of benzo[a]pyrene. Mutat Res. 1979 Dec;63(2):351–359. doi: 10.1016/0027-5107(79)90066-6. [DOI] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Kadlubar F. F. A transversion mutation hypothesis for chemical carcinogenesis by N2-substitution of guanine in DNA. Chem Biol Interact. 1980 Sep;31(3):255–263. doi: 10.1016/0009-2797(80)90014-9. [DOI] [PubMed] [Google Scholar]
- Kakunaga T. Requirement for cell replication in the fixation and expression of the transformed state in mouse cells treated with 4-nitroquinoline-1-oxide. Int J Cancer. 1974 Dec 15;14(6):736–742. doi: 10.1002/ijc.2910140607. [DOI] [PubMed] [Google Scholar]
- Kapitulnik J., Wislocki P. G., Levin W., Yagi H., Jerina D. M., Conney A. H. Tumorigenicity studies with diol-epoxides of benzo(a)pyrene which indicate that (+/-)-trans-7beta,8alpha-dihydroxy-9alpha,10alpha-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene is an ultimate carcinogen in newborn mice. Cancer Res. 1978 Feb;38(2):354–358. [PubMed] [Google Scholar]
- Kaufmann W. K., Boyer J. C., Smith B. A., Cordeiro-Stone M. DNA repair and replication in human fibroblasts treated with (+/-)-r-7,t-8-dihydroxy-t-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene . Biochim Biophys Acta. 1985 Feb 20;824(2):146–151. doi: 10.1016/0167-4781(85)90091-0. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. K., Cleaver J. E. Mechanisms of inhibition of DNA replication by ultraviolet light in normal human and xeroderma pigmentosum fibroblasts. J Mol Biol. 1981 Jun 25;149(2):171–187. doi: 10.1016/0022-2836(81)90297-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. K., Rahija R. J., MacKenzie S. A., Kaufman D. G. Cell cycle-dependent initiation of hepatocarcinogenesis in rats by (+/-)-7r,8t-dihydroxy-9t,10t-epoxy-7,8,9,10-tetrahydrobenzo(a)py rene. Cancer Res. 1987 Jul 15;47(14):3771–3775. [PubMed] [Google Scholar]
- McCormick P. J., Bertram J. S. Differential cell cycle phase specificity for neoplastic transformation and mutation to ouabain resistance induced by N-methyl-N'-nitro-N-nitrosoguanidine in synchronized C3H10T 1/2 C18 cells. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4342–4346. doi: 10.1073/pnas.79.14.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa H., Kakefuda T. Inhibition of DNA synthesis in vitro by binding of benzo(a) pyrene metabolite diol-epoxide I to DNA. Nature. 1979 May 3;279(5708):75–78. doi: 10.1038/279075a0. [DOI] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Paules R. S., Poirier M. C., Mass M. J., Yuspa S. H., Kaufman D. G. Quantitation by electron microscopy of the binding of highly specific antibodies to benzo[a]pyrene-DNA adducts. Carcinogenesis. 1985 Feb;6(2):193–198. doi: 10.1093/carcin/6.2.193. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Ribovich M. L., Kurian P., Milo G. E. Specific BPDE I modification of replicating and parental DNA from early S phase human foreskin fibroblasts. Carcinogenesis. 1986 May;7(5):737–744. doi: 10.1093/carcin/7.5.737. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B., Jeffrey A. M., Jennette K. W., Blobstein S. H., Harvey R. G., Harris C., Autrup H., Kasai H., Nakanishi K. Benzo(a)pyrene diol epoxides as intermediates in nucleic acid binding in vitro and in vivo. Science. 1976 Aug 13;193(4253):592–595. doi: 10.1126/science.959820. [DOI] [PubMed] [Google Scholar]
- Wood A. W., Wislocki P. G., Chang R. L., Levin W., Lu A. Y., Yagi J., Hernandez O., Herina D. M., Conney A. H. Mutagenicity and cytotoxicity of benzo(a)pyrene benzo-ring epoxides. Cancer Res. 1976 Sep;36(9 PT1):3358–3366. [PubMed] [Google Scholar]
- Yamaura I., Rosenberg B. H., Cavalieri L. F. The major adducts of cis and trans benzo[a]pyrene diol epoxides cause chain termination during DNA synthesis in vitro. Chem Biol Interact. 1981 Oct;37(1-2):171–180. doi: 10.1016/0009-2797(81)90174-5. [DOI] [PubMed] [Google Scholar]



