Abstract
Objectives
Emerging evidence suggests that mitochondrial damage-mediated neuronal apoptosis is a major contributor to neonatal hypoxic-ischemic (H-I) brain injury. This study was performed to determine whether targeted inhibition of the apoptotic protease activating factor-1 (Apaf-1) signaling pathway downstream of mitochondrial damage confers neuroprotection in rodent models of neonatal H-I.
Methods
H-I was induced in 7-day-old (P7) transgenic mice overexpressing the specific Apaf-1-inhibitory protein AIP. Apaf-1 inhibition was also achieved in P7 rats by protein transduction-enhanced delivery of recombinant AIP. Pups were euthanized 6 to 24 hours after H-I for assessing caspase activation and mitochondrial release of cytochrome c and AIF, and 7 days after H-I for analyzing brain tissue damage. Sensorimotor functions were assessed in rats up to 4 weeks after H-I.
Results
Transgenic overexpression of AIP protected against H-I brain injury, resulting in attenuated activation of caspase-9 and caspase-3, and attenuated brain tissue loss. In neonatal H-I rats, intraperitoneal injection of TAT-AIP, but not the control proteins TAT-GFP or AIP, decreased caspase activation and brain damage and improved neurological functions. Neuroprotection conferred by AIP was also associated with significantly reduced release of cytochrome c and AIF from mitochondria.
Conclusion
The Apaf-1 signaling pathway, which transmits cell death signals following mitochondrial damage to effector caspases, may be a legitimate therapeutic target for the treatment of neonatal H-I brain injury.
INTRODUCTION
Hypoxic-ischemic (H-I) injury in the developing brain contributes considerably to morbidity and mortality in children suffering from periventricular leukomalacia or cerebral palsy. In animal models, a mixture of apoptosis and necrosis constitutes the primary mode of cell death following neonatal H-I.1,2 Emerging evidence suggests that apoptosis has a more predominant role in neonatal HI than in adult brain ischemia,3,4 and indeed it may contribute to the prolonged progression of neurodegeneration and cerebral dysfunction after neonatal H-I.1 Thus, targeted inhibition of pro-apoptotic pathways represents an attractive and legitimate strategy for therapeutic intervention in neonatal H-I brain injury.
Apoptosis generally consists of two main signaling steps: the apoptosis-initiation cascade and the apoptosis-execution pathway. The death signals are initially induced by various pro-apoptotic stimuli such as oxidative stress, DNA damage and ER stress. These pathways eventually converge into a mitochondrial-dependent mechanism, the so-called intrinsic pathway, which activates the death-execution terminal caspases.5 An essential step in this pathway is the formation of a multimeric protein complex, the apoptosome,6 in which cytosolic cytochrome c facilitates the oligomerization of apoptotic protease activating factor-1 (Apaf-1). Once Apaf-1 is activated, procaspase-9 is recruited to the complex, which in turn activates terminal caspases such as caspase-3, -6, and -7. This mechanism of apoptosis is evolutionarily conserved, and likely plays an important role in mediating neuronal death after cerebral ischemia7, 8 and, possibly, in neonatal H-I as well.9
The present study aimed to test the hypothesis that targeted inhibition of the Apaf-1 signaling pathway may confer neuroprotection against neonatal H-I brain injury. Inhibition of the Apaf-1 pathway was achieved by transgenic overexpression or protein transduction-mediated delivery of Apaf-1 interacting protein (AIP). AIP is a recently cloned gene product that interrupts the formation of apoptosome by directly binding to Apaf-1.8
METHODS
Creation of AIP transgenic mice
The chimeric transgene used to create the transgenic mice contains rat AIP cDNA8 under the control of cytomegalovirus enhancer and a chicken β-actin promoter with the first intron. A hemagglutinin (HA) tag was added before the AIP coding region. The microinjection and ES screening were done using standard procedures. Founders were used to establish independent transgenic lines by breeding to wild-type F1 hybrid mice. In all experiments, wild-type mice backbred from the same transgenic colonies on the C57/B6 background were used as controls.
Construction, production and administration of TAT-AIP fusion protein
The AIP fusion protein containing the TAT protein transduction domain and the HA tag was produced as described previously.10 The purified proteins were verified by Coomassie Blue staining and Western blotting and then stored in 10% glycerol/PBS at −80°C until used. TAT-AIP or TAT-GFP in 180 μl of PBS containing 10% glycerol was injected intraperitoneally into the pups at the completion of H-I and again at 6 and 24 hr after H-I. The animals were randomly assigned to experimental groups.
Models of H-I
Animal protocols were approved by the institutional Animal Care and Use Committee. H-I brain injury was induced in postnatal day 7 (P7) mice or rats based on the Levine method with modifications.9 Pups were anesthetized with 2.0–2.5% isoflurane mixed with ambient air under spontaneous inhalation, and the left common carotid artery was permanently ligated. After a 1.0-hr recovery period, the pups were placed in a plastic chamber containing a humidified atmosphere of 8.0% oxygen/92% nitrogen, and submerged in a 37.5°C water bath to maintain normothermia. After 2.5 hr (for rats) or 0.5 hr (for mice) of hypoxia, the pups were returned to their dams for the time indicated in the experiments.
Assessment of brain damage
At 7 days after H-I, the brains were removed, paraffin-embedded and cut into 4-μm-thick coronal sections. Sections through the corpus callosum and dorsal hippocampus were stained with hematoxylin and eosin (HE). The extent of tissue damage was determined as described previously.11 For mouse pups, brain damage was also assessed in eight pre-determined brain regions using the brain damage scoring system described previously.12 Briefly, each region of interest was scored 0 to 3, in which 0=no detectable cell damage; 1=small focal area of neuronal cell damage; 2=columnar damage; 3=cystic infarction and gliosis. The score of each region was summed to create a full-range score of 0 to 24 for each animal. All measurements were made by an investigator who was blinded to the experimental conditions.
Neurological evaluation
To evaluate sensorimotor neurological function, foot fault tests were performed at 1, 2, 3, and 4 weeks after H/I as described previously.11 Overall motor behavior was evaluated using grid walking to determinetotal steps taken over 1 minute. Gait testing was performed 2, 4, 6, and 7 days after H/I, and data were expressed as the first daythat the animal successfully moved off a circle within 30 seconds.11
Statistical analysis
All data are reported as mean ± SEM. Significant differences between means were assessed by Student’s t test or by ANOVA and post hoc Scheffe’s tests for multiple comparisons. A p ≤ 0.05 was considered statistically significant.
RESULTS
Activation of the Apaf-1 pathway after HI
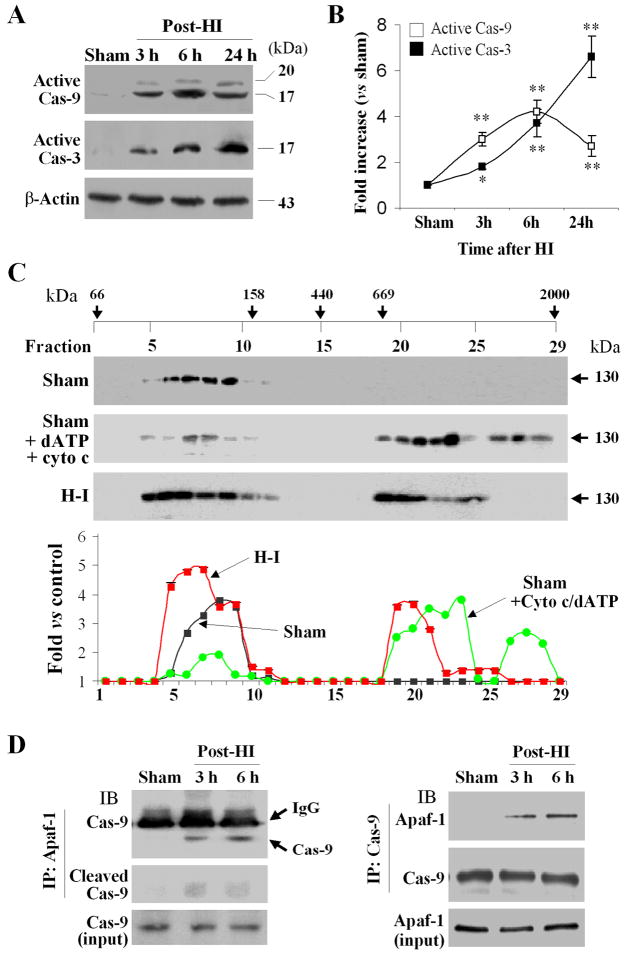
To test the hypothesis that the Apaf-1-dependent intrinsic pathway is activated after H-I, we examined Apaf-1 oligomerization at 6 hr after H-I, at the time caspase-9 activation reached its peak (Fig. 1A–B). Following H-I, a portion of Apaf-1 shifted to large protein complexes of ~700kDa (Fig. 1C), consistent with the sizes of active apoptosome.5 Moreover, co-immunoprecipitation revealed increased interaction between Apaf-1 and caspase-9 after H-I (Fig. 1D). These results confirmed that Apaf-1 entered an active state following H-I.
Fig. 1. The Apaf-1/caspase-9 pathway is activated after H-I in P7 mice.
(A) Western blots detecting active caspase-9 and caspase-3 in brain extracts at 3, 6, and 24 hr after H-I (30 min). (B) Semi-quantitative results for active caspase-9 and caspase-3, respectively. *p<0.05; **p<0.01 vs. sham controls (n=6/group). (C) Gel filtration analysis followed by immunoblotting against Apaf-1 using cytosolic extracts at 6 hr after H-I or sham controls. The positive control was done by adding purified cytochrome c and dATP to cytosolic extracts from sham control brains, which resulted in formation of protein complexes at sizes between 669 and 2000 kDa. (D) Co-immunoprecipitation revealed increased interactions between Apaf-1 and caspase-9 at 3 and 6 hr after H-I. The data in panels C and D are representative of 3–4 independent experiments with similar results.
Transgenic overexpression of AIP attenuates brain injury
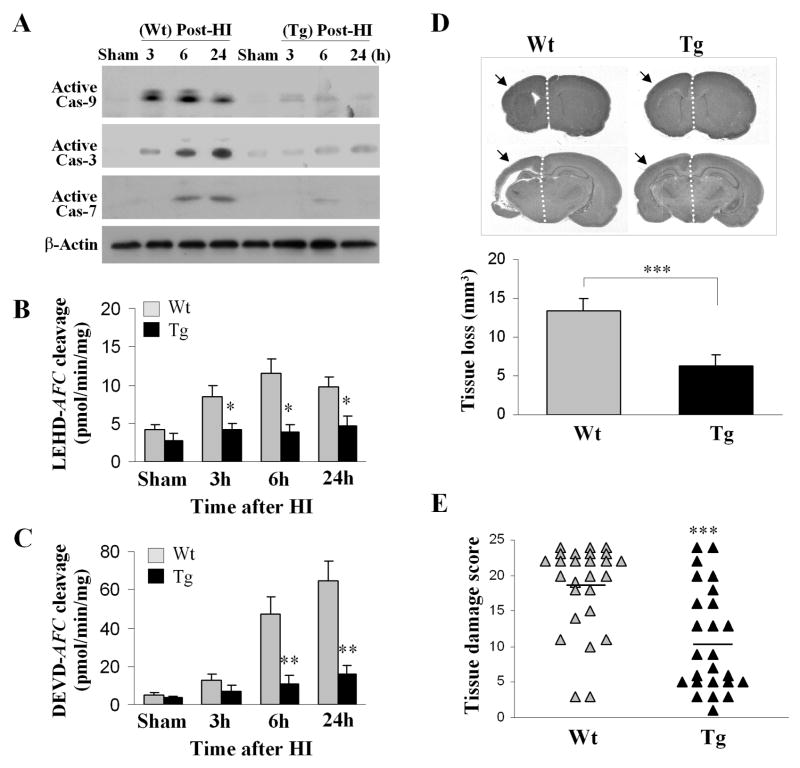
We created transgenic mice overexpressing the Apaf-1 inhibitory protein AIP. Tg-AIP mice express ~10-fold AIP in brain over their wild-type (Wt) littermates (Online Fig. 1). Double-label immunofluorescence revealed that AIP transgenic expression is localized primarily in neurons and, to a lesser extent, in astrocytes. In the in vitro assays, brain extracts from Tg-AIP mice were incompetent for cytochrome c/dATP-induced caspase-9 or caspase-3 activation (Online Fig. 1). Determined 3–24 hr after H-I, caspase-9 and caspase-3 activation was markedly attenuated in Tg-AIP mice compared to Wt mice (Fig. 2A–C). Tg-AIP mice exhibited significantly decreased brain damage compared to Wt mice 7 days after HI (Fig. 2D–E).
Fig. 2. Transgenic AIP overexpression attenuates H-I brain injury.
(A) Western blots show decreased formation of active caspase-9, caspase-3, and caspase-7 after H-I in Tg-AIP mice compared to Wt mice. (B–C) Substrate-based assays performed using the same sets of samples from panel A show that caspase-9-like (LEHD-AFC) and caspase-3-like (DEVD-AFC) activities are decreased in Tg-AIP mice compared to Wt mice after H-I. *p<0.05; **p<0.01 vs. Wt (n=6/group). (D) Cerebral tissue loss is attenuated in Tg-AIP mice (n=25) compared to Wt mice (n=26) 7 days after H-I ***p<0.001. (E) Brain damage scores (sum of eight pre-determined brain regions) based on the same sets of samples from panel D. The mean value for each group is indicated by a line.
Brain surface vascular anatomy appeared unaffected by AIP transgenic overexpression. Furthermore, regional blood flow in H-I brains, as determined in 5 Wt and 6 Tg-AIP mice using laser Doppler flowmetry (probe location: 2 mm posterior to the bregma and 2 mm to the midline), was also not significantly different between groups (data not shown). These results suggest that the reduced brain injury observed in AIP transgenic mice was not the result of a diminished H-I insult.
Administration of TAT-AIP attenuates brain injury
To explore the possibility that exogenous delivery of AIP can be a therapeutic strategy protecting against HI, we generated recombinant AIP containing the TAT sequence.9 The TAT-AIP fusion protein was purified to near homogeneity and was first tested for its effects in primary neuron cultures. TAT-AIP conferred significant neuroprotection against cell death induced by bleomycin or oxygen-glucose deprivation (Online Fig. 2).
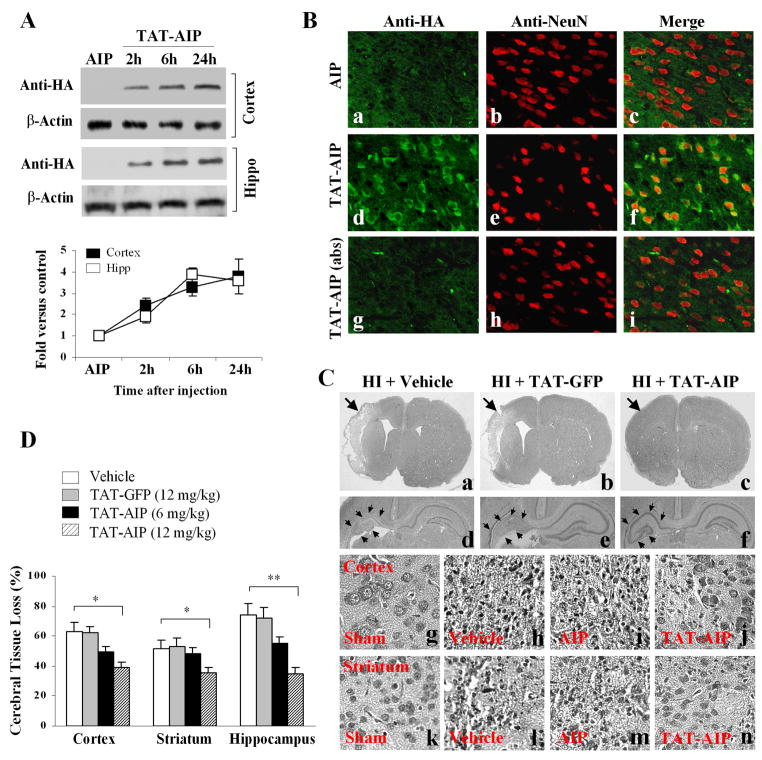
For in vivo studies, TAT-AIP was injected intraperitoneally into the pups at the completion of H-I. Protein transduction into brain was detected at 2–24 hr after injections (Fig. 3A–B). TAT-AIP administration ameliorated brain damage dose-dependently at 7 days after H-I (Fig. 3C–D). At the dose of 12 mg/kg (× 3 times), TAT-AIP decreased cerebral tissue loss by ~38% (p<0.05 vs. controls), ~30% (p<0.05 vs. controls), and ~52% (p<0.01 vs. controls) in the cortex, striatum, and hippocampus, respectively.
Fig. 3. TAT-AIP treatment confers neuroprotection against H-I in P7 rats.
(A) Western blotting with the anti-HA antibody shows that intraperitoneal injection of TAT-AIP (12 mg/kg, 0 and 6 hr after H-I) results in transduction of the protein (molecular size: ~21 kDa) into the cerebral cortex and hippocampus. Injection of AIP without TAT serves as a negative control. The graph (lower panel) summarizes the results from 4 sets of samples. (B) Double-label immunofluorescent staining of TAT-AIP (anti-HA) and NeuN in the cortex at 3 hr after protein injection. Immunoreactivity is detected in a large number of neurons (d-f) after TAT-AIP injection; the signals are abolished in sections (g-i) pre-absorbed with the recombinant TAT-AIP (10 μg/ml). Immunoreactivity is also not detected in brain receiving AIP injection (a-c). (C) H-E staining 7 days after H-I show improved histology in TAT-AIP-treated rat brains. Panels a-c, the arrows point to the injured hemisphere; panels d-f, low-power images of the dorsal hippocampus; panels g-j, high-power images (magnification ×200) of cortex after sham surgery (g) or after H-I (h-j); panels k-n, high-power images of striatum after sham surgery (k) or after H-I (l-n). (B) Brain tissue loss was measured 7 days after H-I. *p<0.05; **p<0.01 vs. vehicle treatment (n=12–15/group).
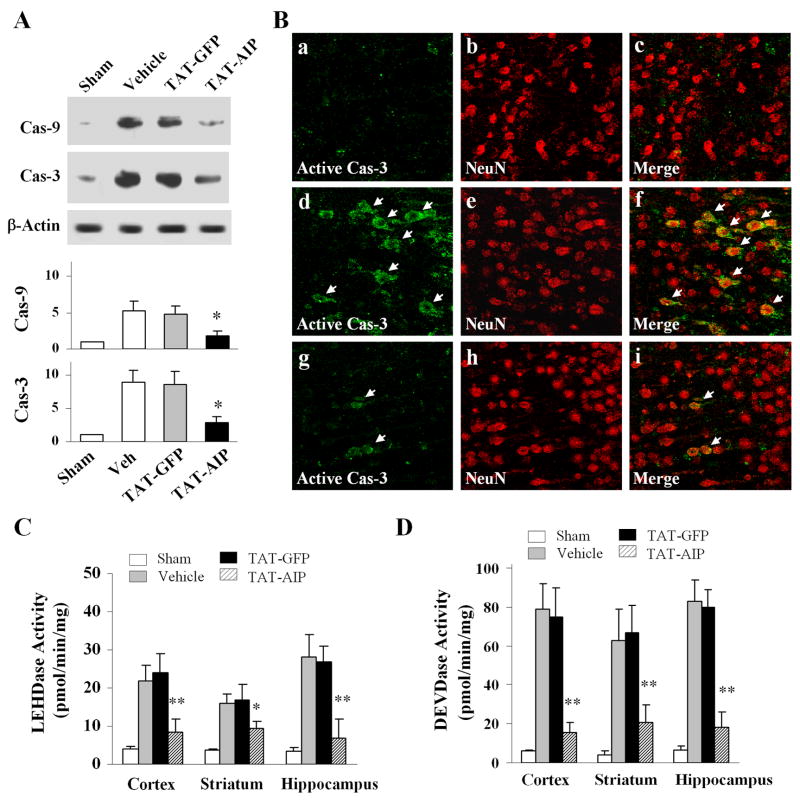
We investigated the effect of TAT-AIP administration on activation of caspase-9 and caspase-3 after H-I. TAT-AIP treatment (12 mg/kg × 3 times) robustly decreased the activation of both caspase-3 and caspase-9 after H-I, as determined using Western blot analysis, caspase activity assays, and immunohistochemistry (Fig. 4).
Fig. 4. TAT-AIP treatment attenuates caspase activation after H-I.
(A) Representative Western blots (of three sets) show that TAT-AIP treatment (12 mg/kg, 0 and 6 hr after H-I) decreased formation of active caspase-9 and caspase-3 in cerebral cortex 24 hr after H-I. n=6/group. (B) Immunofluorescent images show that the numbers of neurons (NeuN positive) containing active caspase-3 are decreased in cortex after H-I following TAT-AIP treatment (g-i) compared to vehicle treatment (d-f). Panels a-c are sham control brain. (C–D) Determined 24 hr after H-I, caspase-9-like (LEHDase) and caspase-3-like (DEVDase) activities are decreased in rats treated with TAT-AIP (12 mg/kg, 0 and 6 hr after H-I) compared to rats treated with vehicle or TAT-GFP. *p<0.05; **p<0.01 vs. vehicle or TAT-GFP (n=6/group).
Effects of Apaf-1 inhibition on cytochrome c and AIF release after H-I
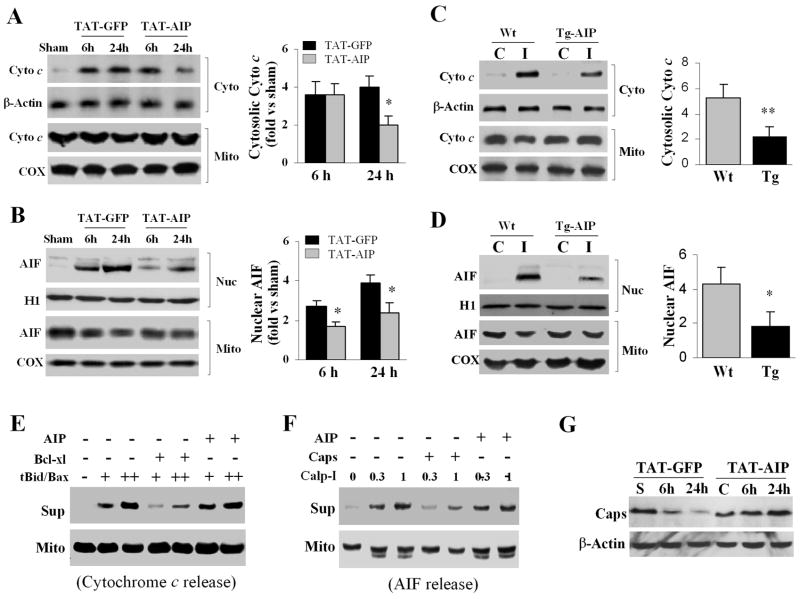
Two recent studies suggest that under certain conditions, caspase activation may cause secondary release of cytochrome c or AIF from mitochondria.13, 14 In this study we examined the effect of TAT-AIP on cytochrome c and AIF release after H-I. Compared to TAT-GFP-treated brains, TAT-AIP treatment significantly attenuated cytochrome c release at 24 hr after H-I (Fig. 5A) and significantly decreased AIF nuclear translocation at 6 and 24 hr after H-I (Fig. 5B).
Fig. 5. TAT-AIP treatment attenuates HI-induced cytochrome c and AIF release in rats.
(A–B) Western blots of subcellular fractions from cerebral cortices show that cytosolic (cyto) cytochrome c and nuclear (nuc) AIF accumulation is attenuated by TAT-AIP treatment after HI. The graphs in the right panel illustrate the semi-quantitative results (n=8/group). *p<0.05 vs. TAT-GFP. (C–D) Western blots show attenuated cytochrome c and AIF release in Tg-AIP mice at 24 hr after H-I. *p<0.05; **p<0.01 vs. Wt (n=6/group). (E–F) Cell-free assay using isolated brain mitochondria (50 μg). Recombinant tBid (+3; ++30 ng)/Bax (+10; ++100 ng) proteins induce cytochrome c release; calpain I (0.3 or 1.0 unit) induces AIF release. Immunoblotting was done using both pellets (mitochondria) and supernatant fractions. The addition of bcl-xL (500 ng) or calpastatin (300 ng) inhibited cytochrome c and AIF release, respectively, whereas the addition of TAT-AIP (1 μg) had no effect on either cytochrome c or AIF release. (G) Western blots (representative of three sets) show that caspase-dependent loss of calpastatin after H-I was markedly reduced in rat brains treated with TAT-AIP.
Cytochrome c and AIF were also examined in Tg-AIP and Wt mice at 24 hr after H-I. H-I-induced cytochrome c release and AIF translocation were significantly reduced in Tg-AIP mice compared to Wt mice (Fig. 5C–D).
Previous studies identified tBid and Bax as inducers of cytochrome c release from brain mitochondria after ischemia,15 while activated calpain I was found to be a direct trigger for AIF release.16,17 In this study, we investigated the potential direct effect of TAT-AIP on cytochrome c or AIF release from isolated mitochondria. As shown (Fig. 5E–F), TAT-AIP failed to inhibit either tBid/Bax-induced cytochrome c release or calpain I-induced AIF release in isolated brain mitochondria, suggesting that the inhibitory effect of TAT-AIP on cytochrome c and AIF release after H-I is secondary instead of primary. This notion was supported by the observation that TAT-AIP treatment prevented H-I-induced degradation of the endogenous calpain inhibitor calpastatin (Fig. 5G), which is a substrate for caspase-3.
TAT-AIP treatment improves neurological outcomes after HI
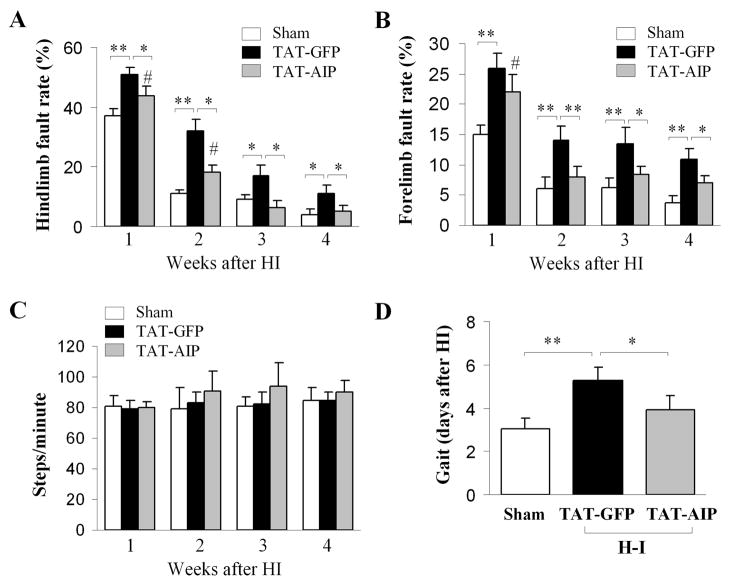
Contralateral limb faults were significantly increased in H-I animals compared to sham controls (Fig. 6A–B). There was no significant difference in total number of steps between groups, indicating that the differences in fault steps were not due to alterations in overall motor activity (Fig. 6C). Similarly, directed movement (as evidenced by gait testing) was significantly delayed in H-I animals (Fig. 6D). The neurological deficiencies were attenuated by TAT-AIP treatment (Fig. 6A, B, D). Thus, TAT-AIP treatment is effective at improving neurological outcomes after H-I.
Fig. 6. TAT-AIP improves neurological outcomes after H/I.
(A–B) Foot fault testing from contralateral forelimb and hindlimb of sham control (n=12) or H-I rats treated with TAT-GFP (n=14) or TAT-AIP (n=15). (C) Quantification of total steps among groups. (D) Assessment of gait determined by the first day that pups successfully moved off of a circle during the testing period. *p<0.05; **p<0.01 vs. the indicated group. #p<0.05 vs. sham controls.
DISCUSSION
Emerging evidence suggests that molecular inhibition of neuronal apoptosis is a legitimate therapeutic strategy against neonatal H-I brain injury. Cytochrome c-initiated activation of apoptotic protease activating factor-1 (Apaf-1) is believed to be a key step in the intrinsic signaling pathway for the activation of death-executing caspases in neuronal apoptosis. The current study provides novel evidence that directly targeting this signaling pathway by transgenic overexpression or protein delivery of AIP, a specific Apaf-1 inhibitor, confers robust neuroprotection in models of neonatal H-I injury.
Recently cloned from a rat brain cDNA library, AIP exhibits potent anti-apoptotic effects by interfering with the Apaf-1-dependent apoptotic cascade.8 AIP contains an N-terminal caspase-recruiting domain (CARD), allowing direct binding to Apaf-1 upon its activation by cytosolic cytochrome c and disrupting the formation of apoptosome and subsequent activation of caspase-9 and caspase-3.8 AIP has potent cell death-suppressing activity against various apoptosis-inducing stimuli.8 In this study, we created transgenic mice overexpressing AIP and found that the Tg-AIP mice were highly protected against H-I brain injury. Moreover, we successfully delivered the recombinant AIP (TAT-AIP) in a rat model of neonatal H-I, and this treatment attenuated H-I-induced activation of caspase-9 and caspase-3. Correlating to this caspase-suppressing effect, administration of TAT-AIP conferred significant neuroprotection against H-I brain injury. These results thus highlight the essential role of the Apaf-1-dependent intrinsic pathway in neonatal ischemic cell death.
Several approaches that target caspase-dependent apoptosis have shown neuroprotective effects in models of neonatal H-I.9,18 The magnitude of neuroprotection demonstrated by TAT-AIP in the current study is compatible to that conferred by small-peptide caspase inhibitors in a similar H-I model.9,18 However, the use of AIP may be advantageous over the conventional caspase inhibitors, as AIP inhibits specifically the cytochrome c-activated Apaf-1 signaling pathway without interfering with the basal caspase activities in neurons. Recent studies suggest that caspase proteases may have important physiological roles in a variety of normal non-death-related cellular functions in the nervous system.19,20 Interestingly, the Tg-AIP mice are viable and do not show developmental brain abnormality, contrasting to the Apaf-1-null mice which are embryonic lethal and exhibit severe brain malformations. These observations are consistent with the notion that AIP binds to Apaf-1 and inhibits the caspase-9 pathway only when Apaf-1 is activated by cytochrome c 8, thus AIP is not an inhibitor for developmental neuronal apoptosis.
The mitochondrial signaling pathway induced by ischemia activates both caspase-dependent and caspase-independent death-execution cascades. While cytochrome c release is essential for caspase-dependent apoptosis via activating Apaf-1, AIF release is thought to be independent of caspase activities.16,21 In this study, we investigated the effect of Apaf-1 inhibition on cytochrome c and on AIF release after H-I. Interestingly, H-I-induced cytochrome c and AIF release were attenuated in brains overexpressing AIP or receiving TAT-AIP treatment. While TAT-AIP was effective in reducing cytochrome c release only at 24 hr after H-I, it inhibited AIF release at both 6 and 24 hr after H-I. These results suggest that the release of cytochrome c and AIF after H-I may be mediated by different pathways, but that both may involve secondary mechanisms requiring caspase activation. Considering that the Apaf-1/caspase-9 signaling pathway is downstream of cytochrome c release, it can be speculated that caspase-3 and caspase-7 activation through the Apaf-1-signaling pathway causes secondary mitochondrial damage, thus facilitating cytochrome c release. A recent study also reported that caspase inhibition abrogated cytochrome c release in a model of traumatic brain injury.22 This action of terminal caspases may not be due to the direct attacks of caspase-3 or caspase-7 on mitochondrial membranes, but via the cleavage-mediated activation of another intermediate pro-apoptotic molecule, such as an initiator caspase (caspase-2, caspase-8 or caspase-10) or Bid,15, 23 which in turn targets the mitochondria. Further studies examining these potential intermediate proteins may reveal a secondary mechanism underlying cytochrome c release after H-I.
Recently, calpain I has been found to trigger AIF release from mitochondria in ischemic neurons by truncating AIF at its N-terminus.16,17 Calpain I is normally scavenged by the endogenous calpain inhibitor, calpastatin, which determines the threshold for calpain I activation by calcium.24 However, calpastatin is a specific substrate for caspase-3 and caspase-7; it has been suggested that the proteolytic degradation of calpastatin by caspase-3 facilitates calpain activation during neuronal apoptosis.25 Thus, Apaf-1-mediated activation of caspase-3 may enhance calpain-mediated AIF release by degrading calpastatin. This notion is supported by our data showing that H-I-induced calpastatin degradation was attenuated in TAT-AIP treated brains. An alternative potential mechanism is that caspase-3 may facilitate AIF release after H-I injury by activating other intermediate pro-apoptotic proteins, such as caspase-8 and Bid.23 Taken together, these results suggest that overexpression of AIP confers neuroprotection against H-I not only by preventing the Apaf-1-dependent activation of terminal caspases, but also by interrupting a caspase-mediated feed-forward loop that causes secondary release of cytochrome c and AIF.
In summary, the results suggest that the Apaf-1 apoptotic pathway is a potential therapeutic target for neonatal H-I brain injury. It should be noted, however, that the neuroprotection conferred by targeting Apaf-1 is incomplete. Other mechanisms, including necrosis, inflammation, and autophagy all may contribute to H-I-induced neurodegeneration.26,27 Thus, future studies should evaluate therapeutic strategies that would simultaneously alleviate the multi-injurious factors involved in H-I. Targeting the Apaf-1 pathway by AIP may be a legitimate component of such strategies.
Supplementary Material
Acknowledgments
YG, WL and XH contributed equally to this work. This project was supported by NIH/NINDS grants NS36736, NS43802, and NS45048 (to JC), and an AHA fellowship grant (RAS). YG and WL were supported by the Chinese Natural Science Foundation (Grants 30470592, 30670642 and 30772079). We thank Carol Culver for excellent editorial assistance.
References
- 1.Nakajima W, Ishida A, Lange MS, Gabrielson KL, Wilson MA, Martin LJ, Blue ME, Johnston MV. Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat. J Neurosci. 2000;20:7994–8004. doi: 10.1523/JNEUROSCI.20-21-07994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Northington FJ, Ferriero DM, Flock DL, Martin LJ. Delayed neurodegeneration in neonatal rat thalamus after hypoxia-ischemia is apoptosis. J Neurosci. 2001;21:1931–1938. doi: 10.1523/JNEUROSCI.21-06-01931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y, Gidday JM, Yan Q, Shah AR, Holtzman DM. Marked age-dependent neuroprotection by brain-derived neurotrophic factor against neonatal hypoxic-ischemic brain injury. Ann Neurol. 1997;41:521–529. doi: 10.1002/ana.410410416. [DOI] [PubMed] [Google Scholar]
- 4.Hu BR, Liu CL, Ouyang Y, Blomgren K, Siesjo BK. Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. J Cereb Blood Flow Metab. 2000;20:1294–1300. doi: 10.1097/00004647-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and datp-dependent formation of apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Benedict MA, Ding L, Nunez G. Role of cytochrome c and datp/atp hydrolysis in apaf-1-mediated caspase- 9 activation and apoptosis. Embo J. 1999;18:3586–3595. doi: 10.1093/emboj/18.13.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noshita N, Sugawara T, Fujimura M, Morita-Fujimura Y, Chan PH. Manganese superoxide dismutase affects cytochrome c release and caspase- 9 activation after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21:557–567. doi: 10.1097/00004647-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Cao G, Xiao M, Sun F, Xiao X, Pei W, Li J, Graham SH, Simon RP, Chen J. Cloning of a novel apaf-1-interacting protein: A potent suppressor of apoptosis and ischemic neuronal cell death. J Neurosci. 2004;24:6189–6201. doi: 10.1523/JNEUROSCI.1426-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin W, Cao G, Johnnides MJ, Signore AP, Luo Y, Hickey RW, Chen J. Tat-mediated delivery of bcl-xl protein is neuroprotective against neonatal hypoxic-ischemic brain injury via inhibition of caspases and aif. Neurobiol Dis. 2006;21:358–371. doi: 10.1016/j.nbd.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, Lu A, Ran R, Graham SH, Chen J. In vivo delivery of a bcl-xl fusion protein containing the tat protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J Neurosci. 2002;22:5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38:2795–2803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- 12.Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Res. 1998;810:114–122. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- 13.Arnoult D, Parone P, Martinou JC, Antonsson B, Estaquier J, Ameisen JC. Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli. J Cell Biol. 2002;159:923–929. doi: 10.1083/jcb.200207071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu C, Wang X, Huang Z, Qiu L, Xu F, Vahsen N, Nilsson M, Eriksson PS, Hagberg H, Culmsee C, Plesnila N, Kroemer G, Blomgren K. Apoptosis-inducing factor is a major contributor to neuronal loss induced by neonatal cerebral hypoxia-ischemia. Cell Death Differ. 2007;14:775–784. doi: 10.1038/sj.cdd.4402053. [DOI] [PubMed] [Google Scholar]
- 15.Yin XM, Luo Y, Cao G, Bai L, Pei W, Kuharsky DK, Chen J. Bid-mediated mitochondrial pathway is critical to ischemic neuronal apoptosis and focal cerebral ischemia. J Biol Chem. 2002;277:42074–42081. doi: 10.1074/jbc.M204991200. [DOI] [PubMed] [Google Scholar]
- 16.Cao G, Xing J, Xiao X, Liou AK, Gao Y, Yin XM, Clark RS, Graham SH, Chen J. Critical role of calpain i in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci. 2007;27:9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polster BM, Basanez G, Etxebarria A, Hardwick JM, Nicholls DG. Calpain i induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem. 2005;280:6447–6454. doi: 10.1074/jbc.M413269200. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Y, Deshmukh M, D’Costa A, Demaro JA, Gidday JM, Shah A, Sun Y, Jacquin MF, Johnson EM, Holtzman DM. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest. 1998;101:1992–1999. doi: 10.1172/JCI2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohn TT, Cusack SM, Kessinger SR, Oxford JT. Caspase activation independent of cell death is required for proper cell dispersal and correct morphology in pc12 cells. Exp Cell Res. 2004;295:215–225. doi: 10.1016/j.yexcr.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 20.Fernando P, Brunette S, Megeney LA. Neural stem cell differentiation is dependent upon endogenous caspase 3 activity. Faseb J. 2005;19:1671–1673. doi: 10.1096/fj.04-2981fje. [DOI] [PubMed] [Google Scholar]
- 21.Zhu C, Qiu L, Wang X, Hallin U, Cande C, Kroemer G, Hagberg H, Blomgren K. Involvement of apoptosis-inducing factor in neuronal death after hypoxia-ischemia in the neonatal rat brain. J Neurochem. 2003;86:306–317. doi: 10.1046/j.1471-4159.2003.01832.x. [DOI] [PubMed] [Google Scholar]
- 22.Clark RS, Nathaniel PD, Zhang X, Dixon CE, Alber SM, Watkins SC, Melick JA, Kochanek PM, Graham SH. Boc-aspartyl(ome)-fluoromethylketone attenuates mitochondrial release of cytochrome c and delays brain tissue loss after traumatic brain injury in rats. J Cereb Blood Flow Metab. 2007;27:316–326. doi: 10.1038/sj.jcbfm.9600338. [DOI] [PubMed] [Google Scholar]
- 23.Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M, Blomgren K, Plesnila N. Apoptosis-inducing factor triggered by poly(adp-ribose) polymerase and bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci. 2005;25:10262–10272. doi: 10.1523/JNEUROSCI.2818-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vosler PS, Brennan CS, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol. 2008;38:78–100. doi: 10.1007/s12035-008-8036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumar RW, Xu YA, Gada H, Guttmann RP, Siman R. Cross-talk between calpain and caspase proteolytic systems during neuronal apoptosis. J Biol Chem. 2003;278:14162–14167. doi: 10.1074/jbc.M212255200. [DOI] [PubMed] [Google Scholar]
- 26.Benjelloun N, Renolleau S, Represa A, Ben-Ari Y, Charriaut-Marlangue C. Inflammatory responses in the cerebral cortex after ischemia in the p7 neonatal rat. Stroke. 1999;30:1916–1923. doi: 10.1161/01.str.30.9.1916. discussion 1923–1914. [DOI] [PubMed] [Google Scholar]
- 27.Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, Davis RJ, Mizushima N, Rakic P, Dardzinski BJ, Holland SK, Sharp FR, Kuan CY. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol. 2006;169:566–583. doi: 10.2353/ajpath.2006.051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.