Liver lymphocytes are enriched in natural killer (NK) cells, which play an important role in host defenses against microbial infection and tumor transformation in the liver.1 Generally, it is believed that the cytotoxicity of NK cells against target cells is controlled by the opposing signals from inhibitory and stimulatory receptors on NK cells interacting with their corresponding ligands expressed on target cells.2, 3 The NK cell inhibitory receptors include CD94/NKG2, Ly49A, and the Ig-like killer inhibitory receptor, which interact with inhibitory ligands (eg, self MHC class I molecules) expressed on target cells and inactivate NK cell function. The stimulatory receptors include NKp46, NKp30, NKp44, NKG2D, and DNAX accessory molecule 1 (CD226). Among these, the best-defined receptor is NKG2D (natural-killer group 2, member D), a highly conserved C-type lectin-like membrane glycoprotein that is also one of the major activating receptors on NK cells. 2, 3 Expressed on essentially all NK cells, as well as on γδ-TcR+ T cells and αβ-TcR+ CD8+ T cells, NKG2D is found in both humans and mice. In humans, known NKG2D ligands include major histocompatiblity complex (MHC) class I-related chain A and B (MICA/B) and UL16-binding protein 1, 2, 3, 4 (ULBP1, 2, 3, 4). In mice, known NKG2D ligands include retinoic acid early inducible gene-1 (RAE-1), minor histocompatibility H60, and murine UL16-binding protein-like transcript 1 (MULT1). The expression of these NKG2D ligands is usually upregulated on microbe-infected, transformed, or stressed cells. The interaction between these ligands and NKG2D on NK cells leads to NK cell activation, thereby playing an important role in host defenses against viral infection and tumor transformation. In addition, CD8+ T cells also express NKG2D, which serves as a co-stimulatory signal to activate CD8+ T cells. 2, 3
The important role of NK cells in the clearance of early HCV infection is suggested by the results of several genetic studies on the interaction between NK cell receptors and their ligands.4, 5 For instance, Khakoo et al4 reported that patients with the inhibitory NK cell receptor (KIR2DL3) and its ligand (HLA-C1: human leukocyte antigen C group1) had a better chance of spontaneous recovery from acute HCV infection. This is likely due to weak inhibitory KIR2DL3-HLA-C1 interaction, which results in the lack of strong NK cell inhibition and subsequent induction of strong NK cell functions that contribute to HCV clearance. However, the role of NK cell activating receptor NKG2D and its ligands in controlling HCV infection remains largely unknown. Recently, several studies have shown that NKG2D+NK cells are highly enriched in intrahepatic compartments in patients with chronic HCV infection, which correlates with hepatocellular damage.6 Although the expression of NKG2D ligands on HCV- or HBV-infected hepatocytes in humans has not yet been explored, it is expected to be elevated because in several murine models of liver injury, upregulated ligands have been detected on stressed hepatocytes (see below).
The expression of RAE-1, MULT-1, and H60 is not detected on normal mouse hepatocytes; however, it is detected at high levels on hepatocytes from bile duct-ligated mice,7 HBV transgenic mice,8, 9 and mice with drug-induced liver injury.10 Elevated levels of these ligands trigger activation of NK cells, as well as NKT cells, to kill hepatocytes, resulting in hepatocellular damage.7-10 Induction of RAE-1 expression has also been reported on Kupffer cells in mice treated with polyinosinic:polycytidylic acid (poly I:C) plus D-galactosamine (D-GalN).11 The interaction between NKG2D and RAE1 stimulates NK cells to produce IFN-γ, which then acts together with Kupffer cell-derived TNF-α to synergistically induce fulminant hepatitis.11 In addition to triggering hepatocyte damage, the interaction between NKG2D and corresponding ligands is also involved in NK cell-mediated cholangiocyte injury in a murine model of biliary atresia induced by rotavirus infection.12 In this model, NK cells accumulate in extrahepatic bile ducts and hepatic expression of RAE1, H60, MULT1 mRNAs is markedly upregulated. Blockade of NKGD2 prevents both epithelial cell injury and the development of the atresia phenotype. In vitro, NK cells lyse cholangiocytes in a contact- and NKG2D-dependent manner.
Although the contribution of NKG2D-ligands to hepatocyte and cholangiocyte injury is well documented in rodent models, little is known about their roles in the pathogenesis of human liver disease. In this issue of Hepatology, Kahraman et al13 report that hepatic NK cells, expression of NK cell-associated cytotoxic mediators (such as TRAIL), NKG2D, and MICA/B mRNAs in the liver are significantly elevated in nonalcoholic steatohepatitis (NASH) and, to a lesser extent, in nonalcoholic fatty liver (NAFL), when compared to normal healthy control livers. Immunohistochemical analyses reveal that MICA/B proteins are detected from hepatocytes in patients with NASH and NAFL. Moreover, the expression of MICA/B mRNAs are positively correlated with NAS score and hepatocyte apoptosis in NASH. These findings suggest that the MICA/B protein levels are upregulated in hepatocytes from NASH patients through mechanisms that have yet to be identified. This is then followed by activation of hepatic NK cells that release TRAIL as a means of killing hepatocytes, and thereby inducing hepatocelluar damage in these patients. It is not clear whether NK cells only kill MICA/B positive hepatocytes or if they also kill MICA/B negative cells. This could be confirmed by performing double-staining to determine whether MICA/B protein and TUNEL+ hepatocytes are co-localized. While the molecular mechanism underlying upregulation of hepatic MICA/B in NASH and NAFL patients was not explored in Kahraman et al study,13 it has been well documented that expression of NKG2D ligands are upregulated through a variety of stimuli that have been collectively termed “cellular stress,” such as cellular transformation, viral infection, and/or DNA damage.2, 3 It is known that fat accumulation can generate oxidative stress-induced DNA damage in hepatocytes,14 which may contribute to the upregulation of hepatic MICA/B in NASH and NAFL patients.
Interestingly, Kahraman et al13 also show that levels of MICA/B mRNAs correlate positively with stage of fibrosis, suggesting that MICA/B also contribute to the progression of liver fibrosis. However, recent studies using murine models of liver fibrosis suggest that the NKG2D-ligand interaction triggers the killing of activated hepatic stellate cells (HSCs) by NK cell through a TRAIL-dependent mechanism, thereby inhibiting liver fibrosis.15, 16 Liver fibrosis is a common scarring response to virtually all forms of chronic liver injury and is characterized by HSC activation and accumulation of collagen in the liver. In normal healthy livers, HSCs are quiescent, storing large amounts of vitamin A (retinol). During liver injury or culturing on plastic dishes, HSCs become activated and differentiate into myofiboblast cells that produce collagen. Activated HSCs can also become senescent during chronic liver injury or after 9-15 passages in vitro. Quiescent and fully activated HSCs do not express RAE1, while early-activated and senescent-activated HSCs express high levels of RAE1 and become sensitive to NK cell killing via NKG2D-RAE1 recognition.15, 16 Additionally, the expression of NK cell inhibitory ligand MHC I is downregulated,17 while TRAIL receptor expression is upregulated on activated HSCs,18, 19 which may be additional important mechanisms contributing to increased sensitivity of HCSs to NK cell/TRAIL killing. At present, it is not clear whether expression of NKG2D ligands (MICA/B) is also upregulated in activated human HSCs during chronic liver diseases. Although Kahraman et al13 performed immunohistochemical analyses of NASH liver samples with MICA/B antibodies, the expression of MICA/B on the HSCs was not well illustrated. Double-staining with MICA/B antibodies and HSC markers will be required in the future to identify whether MICA/B proteins are expressed on activated HSCs in patients with chronic liver disease.
In addition to having a beneficial effect on liver fibrosis, the interaction of NKG2D and corresponding ligands may also play an important role in immunosurveillance against cholangiocarcinoma and hepatocellular carcinoma. Genetic analyses of NKG2D polymorphisms strongly suggest that interaction of NKG2D-MICA protects against cholangiocarcinoma in patients with primary sclerosing cholangitis.20 The inhibitory effect of NKG2D on liver tumors is likely mediated via activation of NK cells and the subsequent production of TRAIL, which is a potent cytotoxic mediator that kills hepatocellular carcinoma and cholangiocarcinoma cells.21 However, MICA/B proteins are also cleaved proteolytically from hepatocellular carcinoma cells and appear as soluble particles in serum from these patients.22 These soluble MICA/B proteins can downregulate NKG2D expression and inhibit NKG2D-mediated NK cytotoxicity against liver tumor cells,23 allowing tumor cells to escape from immunity attacks mediated by NKG2D.
In summary, interaction between NKG2D and ligands can trigger NK cell activation, playing an important role in host defenses against hepatitis viral infection and liver cancer development, as well as the inhibition of liver fibrosis. Such activation may also contribute to hepatocellular damage in patients with HCV infection and NAFL/NASH. Additionally, the downregulation of this activation (eg, by alcohol drinking) may accelerate the progression of liver disease, including viral infection and liver fibrosis,24, 25 while upregulation of NKG2D expression on NK cells by poly I:C and IFN-γ can markedly enhance NK cell cytotoxicity against hepatocytes and HSCs, leading to hepatocellular damage and the reduction of liver fibrosis, respectively.11, 15 Interestingly, treating mice with IFN-α also markedly increased expression of NKG2D on liver NK cells (our unpublished data). It will be very interesting to determine whether current treatment protocol using IFN-α in patients with chronic HCV infection also induces NKG2D expression on hepatic and peripheral NK cells. This may be an important mechanism contributing to the well-documented anti-viral, anti-fibrotic, and anti-tumor effects of IFN-α in patients with chronic liver disease.
Genetic variations in NKG2D and it ligands (such as MICA/B) are known to affect the binding affinity of NKG2D-ligands, which can subsequently alter NK cell function. Therefore, genetic variations may be important in explaining spontaneous recovery of acute HCV infection,4 the susceptibility of primary sclerosing cholangitis,26 and cholangiocarcinoma development.20 The Kahraman et al13 study highlights an unappreciated mechanism by which the interaction of NKG2D-MICA plays an important role in the pathogenesis of NASH. Therefore, future studies evaluating the association of genetic variants in the NKGD2 and MICA genes with NASH will certainly generate interesting data that could be helpful in the diagnosis and therapeutic treatment of NASH patients.
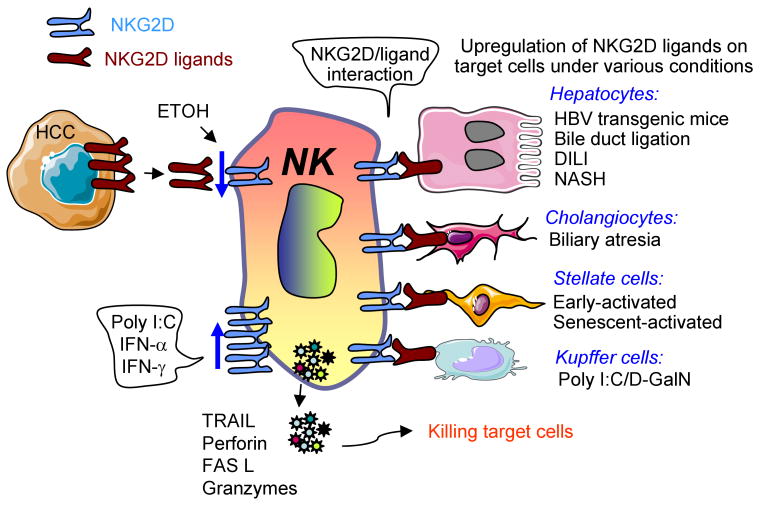
Fig. 1. Divergent roles of NKG2D-ligands in the pathogenesis of liver disease.
Expression of NKG2D ligands is upregulated in hepatocytes, cholangiocytes, stellate cells, and Kupffer cells by a variety of stimuli. These ligands can bind NKG2D on NK cells and subsequently trigger NK cell activation to produce a variety of cytotoxic mediators. These mediators can kill hepatocytes and cholangiocytes to induce liver damage and kill activated HSCs to reduce liver fibrosis. Elevated NKG2D ligands on Kupffer cells can also trigger NK cell activation and induce hepatocellular damage. Hepatocelluar carcinoma and cholangiocarcinoma cells also express NKG2D ligands, which can trigger NKG2D-dependent anti-tumor immune responses to kill these tumor cells. However, NKG2D ligands (MICA/B) can also be cleaved proteolytically from hepatocellular carcinoma cells to generate soluble forms of MICA/B that downregulate NKG2D expression and inhibit NKG2D-mediated antitumor effects. Chronic alcohol drinking downregulates NKG2D expression while treatment with poly I:C and type I IFN induces its expression. DILI: drug induced liver injury.
References
- 1.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 3.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–27. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 4.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 5.Zuniga J, Romero V, Azocar J, Terreros D, Vargas-Rojas MI, Torres-Garcia D, et al. Protective KIR-HLA interactions for HCV infection in intravenous drug users. Mol Immunol. 2009;46:2723–2727. doi: 10.1016/j.molimm.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151–1160. doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 7.Kahraman A, Barreyro FJ, Bronk SF, Werneburg NW, Mott JL, Akazawa Y, et al. TRAIL mediates liver injury by the innate immune system in the bile duct-ligated mouse. Hepatology. 2008;47:1317–1330. doi: 10.1002/hep.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilarinho S, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc Natl Acad Sci U S A. 2007;104:18187–18192. doi: 10.1073/pnas.0708968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Wei H, Sun R, Dong Z, Zhang J, Tian Z. Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2D-ligand interaction and natural killer cells. Hepatology. 2007;46:706–715. doi: 10.1002/hep.21872. [DOI] [PubMed] [Google Scholar]
- 10.Cheng L, You Q, Yin H, Holt M, Franklin C, Ju C. Effect of polyI:C cotreatment on halothane-induced liver injury in mice. Hepatology. 2009;49:215–226. doi: 10.1002/hep.22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou X, Zhou R, Wei H, Sun R, Tian Z. NKG2D-retinoic acid early inducible-1 recognition between natural killer cells and Kupffer cells in a novel murine natural killer cell-dependent fulminant hepatitis. Hepatology. 2009;49:940–949. doi: 10.1002/hep.22725. [DOI] [PubMed] [Google Scholar]
- 12.Shivakumar P, Sabla GE, Whitington P, Chougnet CA, Bezerra JA. Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia. J Clin Invest. 2009;119:2281–2290. doi: 10.1172/JCI38879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahraman ASM, Kocabayoglu P, Yildiz-Meziletoglu, Schlensak M, Fingas C, Wedemeyer I, Marquitan G, Gieseler R, Baba H, Gerken G, Canbay A. Major histocompatibility complex class I-related chains A and B (MICA/B): a novel role in non-alcoholic steatohepatitis. Hepatology. 2009 doi: 10.1002/hep.23253. in press. [DOI] [PubMed] [Google Scholar]
- 14.Fujita K, Nozaki Y, Wada K, Yoneda M, Fujimoto Y, Fujitake M, et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772–780. doi: 10.1002/hep.23094. [DOI] [PubMed] [Google Scholar]
- 15.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 16.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melhem A, Muhanna N, Bishara A, Alvarez CE, Ilan Y, Bishara T, et al. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol. 2006;45:60–71. doi: 10.1016/j.jhep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Taimr P, Higuchi H, Kocova E, Rippe RA, Friedman S, Gores GJ. Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology. 2003;37:87–95. doi: 10.1053/jhep.2003.50002. [DOI] [PubMed] [Google Scholar]
- 19.Fischer R, Cariers A, Reinehr R, Haussinger D. Caspase 9-dependent killing of hepatic stellate cells by activated Kupffer cells. Gastroenterology. 2002;123:845–861. doi: 10.1053/gast.2002.35384. [DOI] [PubMed] [Google Scholar]
- 20.Melum E, Karlsen TH, Schrumpf E, Bergquist A, Thorsby E, Boberg KM, et al. Cholangiocarcinoma in primary sclerosing cholangitis is associated with NKG2D polymorphisms. Hepatology. 2008;47:90–96. doi: 10.1002/hep.21964. [DOI] [PubMed] [Google Scholar]
- 21.Akazawa Y, Mott JL, Bronk SF, Werneburg NW, Kahraman A, Guicciardi ME, et al. Death receptor 5 internalization is required for lysosomal permeabilization by TRAIL in malignant liver cell lines. Gastroenterology. 2009;136:2365–2376. e2361–2367. doi: 10.1053/j.gastro.2009.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohga K, Takehara T, Tatsumi T, Ohkawa K, Miyagi T, Hiramatsu N, et al. Serum levels of soluble major histocompatibility complex (MHC) class I-related chain A in patients with chronic liver diseases and changes during transcatheter arterial embolization for hepatocellular carcinoma. Cancer Sci. 2008;99:1643–1649. doi: 10.1111/j.1349-7006.2008.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao W, Xi X, Hao Z, Li W, Kong Y, Cui L, et al. RAET1E2, a soluble isoform of the UL16-binding protein RAET1E produced by tumor cells, inhibits NKG2D-mediated NK cytotoxicity. J Biol Chem. 2007;282:18922–18928. doi: 10.1074/jbc.M702504200. [DOI] [PubMed] [Google Scholar]
- 24.Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan HN, Sun R, Jaruga B, Hong F, Kim WH, Gao B. Chronic ethanol consumption inhibits hepatic natural killer cell activity and accelerates murine cytomegalovirus-induced hepatitis. Alcohol Clin Exp Res. 2006;30:1615–1623. doi: 10.1111/j.1530-0277.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- 26.Norris S, Kondeatis E, Collins R, Satsangi J, Clare M, Chapman R, et al. Mapping MHC-encoded susceptibility and resistance in primary sclerosing cholangitis: the role of MICA polymorphism. Gastroenterology. 2001;120:1475–1482. doi: 10.1053/gast.2001.24041. [DOI] [PubMed] [Google Scholar]