Abstract
Objective
To determine the relationship of HIV infection, demographic and cardiovascular disease (CVD) risk factors with mortality in the recent HAART era.
Methods
Vital status was ascertained from 2004–2007 in 922 HIV-infected and 280 controls in the Study of Fat Redistribution and Metabolic Change in HIV infection; 469 HIV-infected were included in analysis comparing HIV to similar age controls. Multivariable exponential survival regression (adjusting for demographic and CVD factors) estimated hazard ratios (HR) for death.
Results
After 5 years of follow-up, the overall adjusted mortality HR was 3.4[95% confidence interval (CI):1.35,8.5]; HR was 6.3 among HIV-infected with CD4<200(95% CI:2.2,18.2), 4.3 with CD4 200–350(95% CI:1.14,16.0), and 2.3 with CD4>350(95% CI:0.78,6.9). Among HIV-infected, current smoking (HR=2.73 vs. never smokers, 95% CI:1.64,4.5) and older age (HR=1.61 per decade, 95% CI:1.27,2.1) were independent risk factors for death; higher baseline CD4 count was associated with lower risk (HR=0.65 per CD4 doubling, 95% CI:0.58,0.73).
Conclusion
HIV infection was associated with a 3-fold mortality risk compared to controls after adjustment for demographic and CVD risk factors. In addition to low baseline CD4 count, older age and current smoking were strong and independent predictors of mortality in a US cohort of HIV-infected participants in clinical care.
Keywords: Cardiovascular disease, Mortality, HIV infection, FRAM
INTRODUCTION
The introduction of highly active antiretroviral therapy (HAART) has led to marked reduction in HIV-related mortality [1]. The decrease in HIV-related mortality, however has been accompanied by increases in non-HIV-related mortality including cardiovascular, hepatic and pulmonary diseases, and non-AIDS malignancies [2–5]. Furthermore, studies in the HAART era show that mortality in HIV-infected persons remains higher than in the general population [6–8]. We evaluated the association of HIV infection with mortality risk relative to controls of similar age over a 5-year follow up period in the recent HAART era. We studied a geographically and ethnically diverse U.S. cohort of HIV-infected individuals in clinical care and controls with detailed information on demographic and cardiovascular disease (CVD) risk factors, allowing us to adjust for these factors. We also identified the HIV-related factors associated with mortality.
METHODS
From June 2000 to September 2002, 1183 HIV-infected and 297 control participants were enrolled into the study of Fat Redistribution and Metabolic Change in HIV infection (FRAM). HIV-infected were recruited from 16 HIV or infectious disease clinics or cohorts throughout the US. Control participants were recruited from 2 centers of the Coronary Artery Risk Development in Young Adults (CARDIA) study [9, 10], a population-based cohort that recruited a sample of healthy 18- to 30-year old white and African American men and women from 4 US cities in 1985-6 for a longitudinal study of cardiovascular risk factors. The FRAM study design, recruitment, and data collection procedures have been described elsewhere [11]. A follow-up exam (FRAM2) was conducted from 2004 to 2007. The institutional review boards at all sites approved the protocols. Figure 1 shows FRAM2 retention outcomes. Available for analysis were 922 HIV-infected and 280 control participants known to be alive or dead.
FIGURE 1.
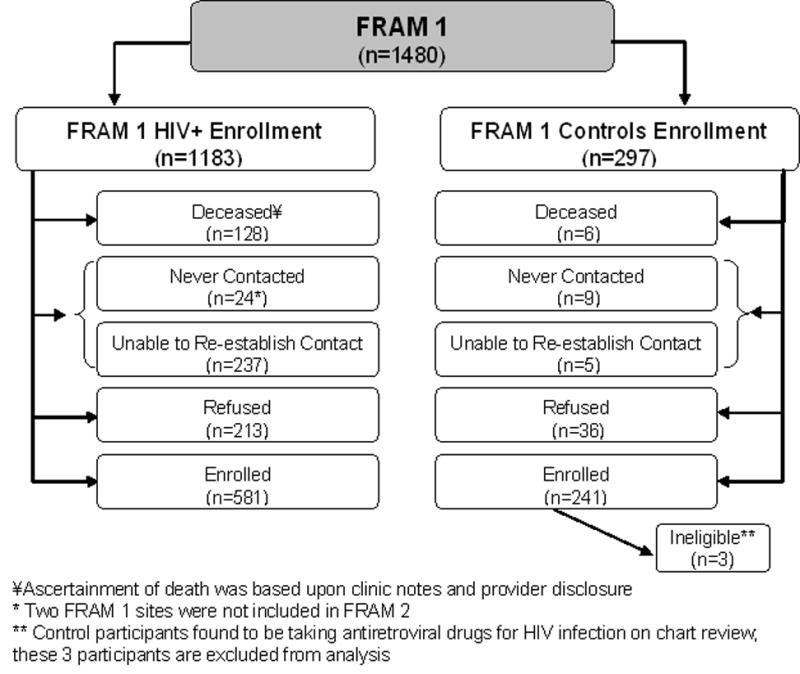
Schema of results of FRAM 2 Retention
After exclusion of HIV-infected participants with opportunistic infections or malignancies (OI/OM) in the same or previous calendar month as the baseline examination, and with an age outside of the 33 to 45 year age range of the controls, a total of 469 HIV-infected participants were included in multivariable exponential regression analysis comparing HIV-infected to controls. Among the 469, there were a total of 54 deaths. Follow-up time was defined as elapsed time from baseline to follow-up exam or last contact. For deceased subjects, the left-censoring time was defined using the median follow-up time for HIV-infected or control participants, since death could have occurred at any time during this interval. Similar analyses were conducted categorizing HIV-infected participants by their CD4 count (>350, 200 to ≤350, and <200) compared with controls. Subsequent analyses of all 922 HIV-infected participants determined the demographic, CVD, and HIV-related factors independently predictive of mortality. We conducted sensitivity analyses using multiple imputation [12] to estimate mortality status for participants with unknown vital status. In addition, sensitivity analysis using an IPCW (inverse probability of censoring weight) approach was performed to adjust estimates [13, 14]. All analyses were conducted using the SAS system, version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Table 1 shows the baseline characteristics of the 469 HIV-infected and 280 control participants of similar age (as well as the 922 HIV-infected) with known vital status. Current smoking and diabetes were more common in HIV-infected than controls; total and HDL cholesterol was lower. Average elapsed time between exams was shorter in HIV-infected than control participants; elapsed time ranged from 2.5 to 6.9 years. Among the 922 HIV-infected, 76% were on HAART, 12% were HAART-naive, and 13% received HAART in the past.
TABLE 1.
Baseline Characteristics of HIV+ and Control Participants
| HIV+ (age-restricted*) | Controls (age-restricted*) | HIV+ (all) | |
|---|---|---|---|
| n | 469 | 280 | 922 |
| Age (y)** | 40.0 (36.0–42.0) | 41.0 (37.0–43.0) | 43.0 (37.0–48.0) |
| Gender | |||
| Female | 152 (32%) | 135 (48%) | 279 (30%) |
| Male | 315 (67%) | 145 (52%) | 639 (69%) |
| Transgender | 2 (1%) | 4 (1%) | |
| Race | |||
| African-American | 191 (41%) | 133 (48%) | 377 (41%) |
| Caucasian | 225 (48%) | 147 (53%) | 449 (49%) |
| Other | 53 (11%) | 96 (10%) | |
| Smoking Status | |||
| Current | 206 (46%) | 44 (16%) | 366 (41%) |
| Past | 88 (20%) | 34 (13%) | 212 (24%) |
| Never | 152 (34%) | 192 (71%) | 307 (35%) |
| Diabetic | 30 (7%) | 8 (3%) | 81 (9%) |
| Systolic BP (mmHg) | 113 (104–122) | 116 (108–125) | 116 (106–124) |
| Diastolic BP (mmHg) | 78 (70–84) | 79 (71–85) | 78 (71–84) |
| Total Cholesterol (mg/dL) | 190 (156–226) | 196 (177–224) | 191 (159–229) |
| HDL Cholesterol (mg/dL) | 41 (34–52) | 51 (42–61) | 41 (33–53) |
| HIV RNA (/1000) | 0.4 (0.4–12.0) | 0.4 (0.4–10.0) | |
| Detectable HIV RNA | 230 (50%) | 442 (49%) | |
| Current CD4 | 373 (222–548) | 366 (215–547) | |
| HCV infection | 84 (18%) | 192 (21%) | |
| AIDS (by CD4 or OI/OM) | 343 (74%) | 657 (72%) | |
| Elapsed Time (y) | 4.6 (4.2–5.1) | 5.7 (5.6–5.9) | 4.6 (4.1–5.1) |
BP, blood pressure; OI, opportunistic infection; OM, opportunistic malignancy
Participants were restricted to those between the ages of 33 and 45 y at baseline
Data are presented as Median (Interquartile range) for all continuous measures.
Over the five-year period, the mortality rate was higher in HIV-infected than control participants (12% vs. 2%, p<0.0001). In multivariable analysis controlling for demographic and traditional CVD risk factors, HIV infection remained associated with a higher risk of death (HR=3.4, 95%CI: 1.35, 8.5, p=0.0094). Compared with controls, mortality risk was highest for HIV-infected with CD4<200 (HR=6.3, 95% CI: 2.2, 18.2, p=0.0007), followed by CD4 200–350 (HR=4.3, 95% CI: 1.14, 16.0, p=0.031), and CD4>350 (HR=2.3, 95% CI: 0.78, 6.9, p=0.13) (Figure 2). Mortality risk among HIV-infected participants with CD4>500 remained higher than controls, although the association did not reach statistical significance (HR: 2.1; 95% CI: 0.60, 7.6, p=0.24). In sensitivity analyses to evaluate the potential effect of those with unknown vital status, HIV-infection remained associated with higher risk of death (Multiple Imputation: HR=4.2, 95%CI: 1.57–11.0, p=0.0046; IPCW: HR=3.6, 95%CI: 1.50–8.5, p=0.0040).
FIGURE 2.
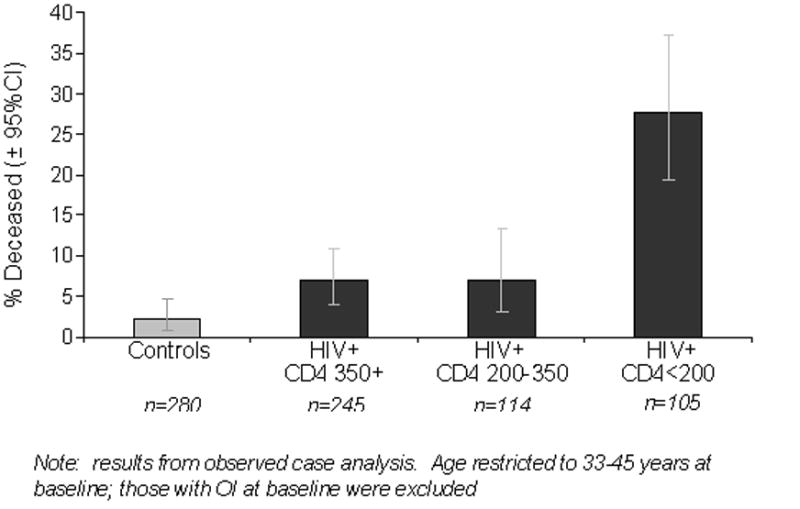
Association of HIV status and baseline CD4 count with mortality after adjustment for demographic and CVD risk factors
In multivariable analysis of HIV-infected participants alone (Table 2), higher baseline CD4 count was strongly associated with lower risk of death. Factors associated with higher risk of death included current smoking and older age. Mortality was highest among those with both low CD4 counts and current smoking status (40%) compared with the lowest risk group of high CD4 count non-smokers (5.3%). Higher non-HDL cholesterol was associated with lower risk of death. There was little association between HAART use (i.e. current use, total duration of use, and duration categorized by number of years used) and mortality risk (data not shown). Sensitivity analyses showed similar results (data not shown).
TABLE 2.
Risk factors for death in all HIV-infected participants†
| Unadjusted (n=922*) |
Adjusted (n = 922*) |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Demographic Factors | ||||||
| Female vs. Male | 1.13 | (0.77, 1.66) | 0.53 | 1.23 | (0.79, 1.91) | 0.36 |
| African-American vs. Caucasian | 1.72 | (1.17, 2.52) | 0.006 | 1.27 | (0.81, 1.97) | 0.30 |
| Other vs. Caucasian | 1.47 | (0.81, 2.68) | 0.21 | 1.80 | (0.95, 3.43) | 0.074 |
| Age (per decade) | 1.25 | (1.03, 1.52) | 0.024 | 1.61 | (1.27, 2.05) | <.0001 |
| HIV-related Factors | ||||||
| Current CD4 (log2) | 0.63 | (0.58, 0.69) | <.0001 | 0.65 | (0.58, 0.73) | <.0001 |
| Detectable HIV RNA | 1.92 | (1.32, 2.78) | 0.001 | 0.88 | (0.56, 1.37) | 0.56 |
| AIDS (by CD4 or OI/OM) | 3.00 | (1.75, 5.16) | <.0001 | 1.66 | (0.90, 3.08) | 0.11 |
| HCV infection | 2.04 | (1.40, 2.97) | 0.0002 | 1.16 | (0.74, 1.83) | 0.52 |
| Traditional Risk Factors | ||||||
| Smoking: current vs. never | 2.81 | (1.77, 4.46) | <.0001 | 2.73 | (1.64, 4.53) | 0.0001 |
| Smoking: past vs. never | 1.40 | (0.79, 2.49) | 0.24 | 1.46 | (0.81, 2.64) | 0.21 |
| Diabetic | 0.93 | (0.49, 1.78) | 0.83 | 1.01 | (0.50, 2.03) | 0.99 |
| Systolic BP (per 10 mmHg) | 0.93 | (0.82, 1.05) | 0.25 | 0.93 | (0.76, 1.13) | 0.45 |
| Diastolic BP (per 10 mmHg) | 0.98 | (0.83, 1.16) | 0.82 | 1.15 | (0.88, 1.52) | 0.31 |
| Non HDL Cholesterol (per 10 mg/dL) | 0.92 | (0.88, 0.95) | <.0001 | 0.96 | (0.92, 1.00) | 0.050 |
| HDL Cholesterol (per 10 mg/dL) | 0.91 | (0.81, 1.03) | 0.13 | 0.88 | (0.78, 1.01) | 0.063 |
HR, hazard ratio; CI, confidence interval; BP, blood pressure; OI, opportunistic infection; OM, opportunistic malignancy;
Observed case exponential regression mortality analysis
N includes all HIV-infected participants known to be dead (N=128) or alive (N=794) and excludes those who were lost to follow-up
DISCUSSION
In our cohort of HIV-infected individuals in clinical care in the US, we found that mortality risk was three-fold higher among HIV-infected individuals compared to controls of a similar age during an approximate 5-year period beginning from 2000 to 2002. As expected, HIV-infected participants with advanced immunosuppression at the baseline exam (CD4 <200) had a 6-fold risk of death compared to controls. Nonetheless, HIV-infected participants with less advanced immunosuppression (CD4 >350) also appeared to have a higher risk of death than controls. Among HIV-infected participants, in addition to a low baseline CD4 count, current smoking and older age were strong independent predictors of mortality.
The findings of our study are important for several reasons. First, we observed a higher mortality risk in a representative US population of HIV-infected persons compared to controls of similar age in the recent HAART era. Our findings are consistent with European studies [7, 8, 15, 16] that have examined age-specific mortality risk in HIV-infected persons compared to controls in the recent HAART era. The excess risk in these studies appeared somewhat higher than our study, likely because they did not control for CVD risk factors such as smoking.
Second, we were able to estimate mortality risk stratified by the baseline CD4 count relative to controls. We found a higher risk of death in HIV-infected participants compared to controls in each CD4 stratum studied (CD4 <200, 200 to 350, >350); as expected, those in the lowest CD4 strata had the highest risk of death. The findings of a 2-fold risk of death in those with a CD4 >350 are notable as our HIV-infected participants were relatively young, were receiving clinical care and most were receiving antiretroviral therapy. When we further stratified to a CD4 >500, a 2-fold higher risk of death remained. In contrast, another study found that among HIV-infected patients with a CD4 >500, the mortality rate after 6 years of follow-up was similar to that of the general population [16]. We followed patients for 5 years, so may not have observed this trend. Some have proposed that inflammatory changes related to HIV infection may contribute to a premature increase in non-AIDS related deaths irrespective of CD4 count [17]. HIV infection may also be associated with specific behaviors that lead to an increased risk of death, although we were able to control for smoking, which is a major such behavior.
Among the factors associated with death in HIV-infected participants in our study, we found an independent association with cigarette smoking, older age and lower CD4 count. Smoking is highly prevalent in HIV-infected individuals and is a known risk factor for CVD and pulmonary diseases. A recent study found that cigarette smoking (current or past) (as well as older age and lower CD4 count) were associated with an increased risk of death from non-AIDS defining malignancy [18] ; lung cancer being the most common fatal non-AIDS defining malignancy. While smoking could also indicate a less healthy lifestyle in general, the findings of our study and others suggest that smoking cessation may be an important target for intervention in individuals with HIV infection. Estimates from our multivariable model suggest that those who quit smoking may see a lowered risk of death equivalent to a 2.7-fold increase in CD4 count. Although the risk of death was 2-fold higher in those with HIV/HCV coinfection compared to those with HIV monoinfection in unadjusted analysis, HCV infection did not appear to be a strong independent risk factor for death, after controlling for HIV-related, demographic and traditional CVD risk factors.
Our study has limitations. A large percentage of participants could not be contacted (23% of HIV-infected and 5% of controls), which may have led to an underestimation in the mortality rate; those not contacted, especially the HIV-infected, may have died during the five-year follow-up period. We therefore performed sensitivity analyses to address missing death status in those not contacted; all sensitivity analyses produced results that were similar to our primary observed case approach. Cause of death was also unknown for our HIV-infected participants and the sample size was relatively small with few deaths in controls.
In summary, mortality risk remains 3 times as high among HIV-infected persons in the recent HAART era compared to controls of similar age. Cigarette smoking, older age and lower CD4 count were independent predictors of mortality in those with HIV infection. Furthermore, our observation that HIV-infected patients were at greater risk of death compared to controls even at higher CD4 levels could suggest a possible role of chronic inflammatory changes (from HIV infection) leading to increased mortality risk, an association that needs further investigation.
Acknowledgments
Supported by NIH grants R01- DK57508, HL74814, and HL53359; K23 AI66943 and NIH center grants M01- RR00036, RR00051, RR00052, RR00054, RR00083, RR0636, RR00865, and UL1 RR024131. The funding agency had no role in the conduct of the study, collection of the data, management of the study, analysis of data, interpretation of the data or preparation of the manuscript. A representative of the funding agent participated in planning the protocol. As part of the standard operating procedures of CARDIA, the manuscript was reviewed at the NHLBI, but no revisions were requested.
Footnotes
Conflicts of Interest: None
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 3.Lewden C, May T, Rosenthal E, Burty C, Bonnet F, Costagliola D, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: The “Mortalite 2000 and 2005” surveys (ANRS EN19 and Mortavic) J Acquir Immune Defic Syndr. 2008;48:590–598. doi: 10.1097/QAI.0b013e31817efb54. [DOI] [PubMed] [Google Scholar]
- 4.Mocroft A, Brettle R, Kirk O, Blaxhult A, Parkin JM, Antunes F, et al. Changes in the cause of death among HIV positive subjects across Europe: results from the EuroSIDA study. Aids. 2002;16:1663–1671. doi: 10.1097/00002030-200208160-00012. [DOI] [PubMed] [Google Scholar]
- 5.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 6.Jaggy C, von Overbeck J, Ledergerber B, Schwarz C, Egger M, Rickenbach M, et al. Mortality in the Swiss HIV Cohort Study (SHCS) and the Swiss general population. Lancet. 2003;362:877–878. doi: 10.1016/S0140-6736(03)14307-3. [DOI] [PubMed] [Google Scholar]
- 7.Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, Porter K. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. Jama. 2008;300:51–59. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- 8.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 9.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 10.Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) Study. Control Clin Trials. 1987;8:68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 11.Tien PC, Benson C, Zolopa AR, Sidney S, Osmond D, Grunfeld C. The study of fat redistribution and metabolic change in HIV infection (FRAM): methods, design, and sample characteristics. Am J Epidemiol. 2006;163:860–869. doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 13.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 15.Martinez E, Milinkovic A, Buira E, de Lazzari E, Leon A, Larrousse M, et al. Incidence and causes of death in HIV-infected persons receiving highly active antiretroviral therapy compared with estimates for the general population of similar age and from the same geographical area. HIV Med. 2007;8:251–258. doi: 10.1111/j.1468-1293.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 16.Lewden C, Chene G, Morlat P, Raffi F, Dupon M, Dellamonica P, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr. 2007;46:72–77. doi: 10.1097/QAI.0b013e318134257a. [DOI] [PubMed] [Google Scholar]
- 17.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 18.Monforte A, Abrams D, Pradier C, Weber R, Reiss P, Bonnet F, et al. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. Aids. 2008;22:2143–2153. doi: 10.1097/QAD.0b013e3283112b77. [DOI] [PMC free article] [PubMed] [Google Scholar]


