Abstract
Objectives
Results from our previous trial revealed that infants with delayed cord clamping (DCC) had significantly less intraventricular hemorrhage (IVH) and late onset sepsis (LOS) than infants with immediate cord clamping (ICC). A priori, we hypothesized that infants with DCC would have better motor function by 7 months CA.
Study Design
Infants between 24 and 316 weeks were randomized to ICC or DCC and follow-up evaluation was completed at 7 months corrected age.
Results
We found no differences in the Bayley Scales of Infant Development (BSID) scores between the DCC and ICC groups. However, a regression model of effects of DCC on motor scores controlling for gestational age, IVH, bronchopulmonary dysplasia, sepsis, and male gender suggested higher motor scores of male infants with DCC.
Conclusions
Delayed cord clamping at birth appears to be protective of very low birth weight male infants against motor disability at 7 months corrected age.
Keywords: cord clamping, motor outcomes, very low birth weight infants, randomized controlled trial, gender
INTRODUCTION
A brief delay in cord clamping at birth results in approximately 10 to 15 mL/kg of additional whole cord blood for the very low birth weight (VLBW)1 without placing the infant at increased risk.2–3 Aladangady found that infants with delayed cord clamping (DCC) obtained variable amounts of additional blood with a mean of 12 mL/kg from a 30 to 90 second delay and lowering of the infant at vaginal and cesarean birth.1 Several beneficial effects of DCC have been demonstrated for preterm infants.1,4–13 In a meta-analysis of randomized controlled trials on DCC in preterm infants, Rabe et al found a decreased need for transfusion and lower rates of intraventricular hemorrhage (IVH) with no evidence of adverse effects.2–3 Our randomized controlled trial14 also showed that infants in the DCC group had significantly less IVH and late onset sepsis (LOS) with an advantage for male infants for both outcomes. IVH, sepsis and male gender have all been reported to be associated with adverse neurodevelopmental outcomes among preterm infants.15 A priori, we hypothesized that VLBW infants with DCC would have better motor function by seven months corrected age when compared with VLBW infants with ICC. The hypothesis was based on the concept that infants with DCC have more red blood cell flow to the brain (motor cortex) thus better oxygen delivery in the initial few days of life16 and that DCC is associated with decreased rates of neonatal morbidities.3 Also, the lower incidence of IVH and sepsis may be reflected in better motor performance at 7 months. No prior cord clamping studies have reported neurodevelopmental outcomes after discharge from the neonatal intensive care unit (NICU). The male advantage with reference to IVH and LOS with DCC also prompted examination of gender differences in neurodevelopmental outcomes.
PATIENTS AND METHODS
Patient Population
The study cohort was derived from the 72 infants in the Delayed Cord Clamping Trial.14 The study was conducted at Women and Infants’ Hospital (WIH), Providence, RI, with infants born between August 2004 and December 2005. The institutional review boards from WIH and the University of Rhode Island granted approval of the study. Informed consent, which included post-discharge follow-up, was obtained on all subjects before randomization.
Protocol at Birth
The neonatal protocols have been fully described.14 Briefly, mothers between 24 and 31.6 weeks gestation consented for their infants and were assigned via block stratified (24–27 wks, 28–31 wks) randomization through numbered cards in opaque envelopes to receive either immediate (ICC) or delayed (DCC) cord clamping. Research nurses enrolled the mothers and attended the births to assign randomization. The obstetrician either clamped the umbilical cord immediately after birth (<10 seconds, usual practice), or the cord was clamped at 30 to 45 seconds and the infant was held in a sterile blanket approximately 10–15 inches below the placenta. The subsequent clinical management of the infant was at the discretion of the attending neonatologist.
Neurodevelopmental Assessments at Seven Months
At the median age of seven months corrected age, the survivors were seen at our Follow-Up Clinic. Bayley Scales of Infant Development-II (BSID-II) mental and motor scales17 were administered to the study infants by trained certified psychologists whose inter-rater reliability was .90. This was followed by a medical history and physical exam completed by a physician or nurse practitioner. The staff was masked to the assigned study groups. BSID-II scores of 100 (± 15) represent the mean and ± one standard deviation. A score below 70 is 2 standard deviations below the mean.
Statistical Methods
Two-sample t-tests and Chi-square analyses were used to test for differences between the groups on demographics, historical and physical findings, and motor function at seven months corrected age. Correlations were conducted to determine which variables were most strongly associated with an infant’s motor scores and to identify potentially confounding variables. A multiple linear regression model for main effects was used to account for the effect of late cord clamping while controlling for potentially confounding variables. An Interaction Term Model was used because of the strength of gender in the regression model. Statistical analyses were performed using SAS software (SAS Institute, Inc, Cary, NC). All reported p values are two-tailed.
RESULTS
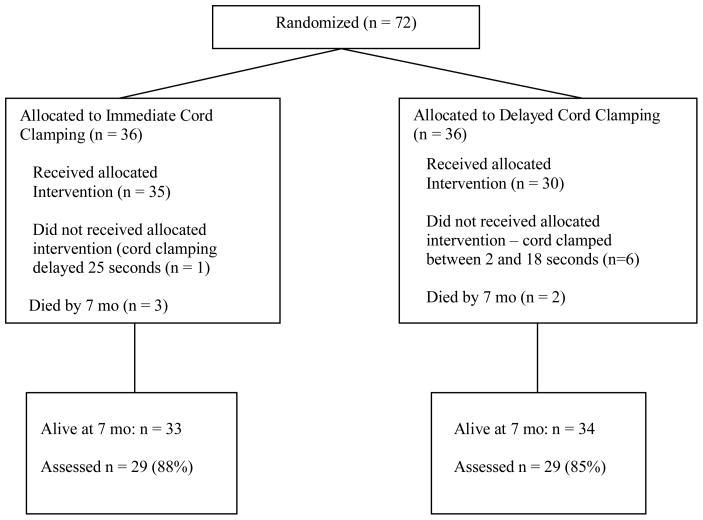
Five children died before the seven month visit and fifty-eight (87%) of the 67 survivors at seven months corrected age were seen (Figure 1). This included 29 (88%) of the infants in the ICC group and 29 (85%) in the DCC group. Nine children were either lost to follow up or did not have a complete BSID II administered for health, transportation, or behavioral reasons. The infants lost to follow up were older at birth (30 vs. 28 weeks, p = 0.008), larger (1352 vs. 1158 grams, p = 0.14), had fewer days of oxygen use (15 vs. 32 days, p = 0.28), and shorter lengths of stay (55 vs. 67 days, p = 0.33) than infants evaluated.
Figure 1.
Flow chart of children included in the Cord Clamping Study and 7 Month Follow-Up
Table 1 and 2 show the maternal and child characteristics. There were no significant differences in demographic and perinatal characteristics between the ICC group and the DCC group. Note that the median corrected age of assessment is 7.3 months in both groups.
Table 1.
Infant and maternal demographic characteristics
| Variables | ICC (n = 29) | DCC (n = 29) | p value |
|---|---|---|---|
| Birth weight* | 1138 ± 373 | 1178 ± 364 | 0.68 |
| Gestational age at birth | 28.1 ± 2.28 | 28.1 ± 2.14 | 0.91 |
| Gender, % male | 15 (52%) | 18 (62%) | 0.43 |
| Race | |||
| White | 17 (59%) | 12 (41%) | 0.53 |
| Hispanic | 9 (31%) | 10 (35%) | |
| Black | 2 (7%) | 5 (17%) | |
| Other | 1 (3.4%) | 2 (7%) | |
| Maternal education < high school | 6 | 8 | 0.54 |
| Maternal education, years | 13 | 13.3 | 0.71 |
| English spoken in the home | 29 (100%) | 25 (86%) | 0.12 |
| Corrected Age at Assessment (mo) | 8.4 ± 3.1 | 8.9 ± 3.1 | 0.57 |
| Range | (6 to 18.6) | (6.3 to 18.3) | |
| Median | 7.3 | 7.3 | |
mean + SD; n (%) unless otherwise noted
Table 2.
Perinatal characteristics of study subjects.
| Variables | ICC (n = 29) | DCC (n = 29) | p value |
|---|---|---|---|
| Apgar @ 5 min, median | 8 | 8 | 0.88 |
| IVH, all grades* | 10 (34%) | 5* (17%) | 0.13 |
| Late onset sepsis** | 6 (21%) | 1* (3%) | 0.10 |
| Suspected NEC | 16 (55%) | 11(38%) | 0.19 |
| NEC | 1 (3%) | 1 (3%) | 1.0 |
| BPD | 6 (21%) | 6 (21%) | 1.0 |
| Percent of BW < 10th percentile | 1 | 1 | 0.98 |
n(%);
One child had ICC (protocol violation)
The BSID-II MDI and PDI scores at approximately seven months corrected age were similar for the infants in the ICC group and the DCC group (Table 3). There were no group differences in weight, length, or head circumference equal to or less than the 10th percentile, post discharge emergency room visits, hospitalizations or use of medications or early intervention participation (data not shown). Repeating the analyses by actual treatment group rather than by intention to treat group did not change the significance of any of the outcomes.
Table 3.
Bayley Scales of Infant Development motor and mental outcomes at 7 months corrected age of preterm infants born 24 to 31.6 wks
| Variables | ICC (n = 29) | DCC (n= 29) | p value |
|---|---|---|---|
| PDI (Mean + SD)* | 84 ±16 | 84 ± 19 | 0.98 |
| PDI** | |||
| ≥ 85 | 15 (52%) | 19 (66%) | 0.15 |
| 70–84 | 9 (31%) | 3 (10%) | |
| < 70 | 5 (17%) | 7 (24%) | |
| MDI (Mean + SD)* | 88 ± 11 | 84 ± 16 | 0.19 |
| MDI** | (n = 28) | ||
| ≥ 85 | 22 (79%) | 20 (69%) | 0.51 |
| 70–84 | 4 (14%) | 4 (14%) | |
| < 70 | 2 (7%) | 5 (17%) | |
| PDI + SD (n)** | |||
| Males | 81 ± 13 (n = 15) | 86 ± 13 (n = 18) | 0.11 |
| Females | 87 ± 18 (n = 14) | 78 ± 18 (n = 10) | |
| MDI + SD (n)** | |||
| Males | 85 ± 12 (n = 14) | 86 ± 13 (n = 18) | 0.11 |
| Females | 92 ± 8 (n = 14) | 78 ± 19 (n = 10) | |
t-test;
Chi-Square; ICC = immediate cord clamping; DCC = delayed cord clamping; one infant had PDI assessment but was unable to complete MDI assessment due to irritability. One female infant in the DCC group with MDI < 70 was a protocol violation and another developed a devastating syndrome unrelated to birth.
Additional t-tests were used to explore relationships between the PDI and the categorical variables. The infants who had an oxygen requirement at 36 weeks (BPD) had lower PDI scores (72 vs. 88, p < 0.01) compared to infants without oxygen use. Infants with confirmed sepsis also scored lower on the PDI (66 vs. 87, p <0 .01) than those with no sepsis. There was no significant difference in PDI scores between the infants with or without IVH (80 vs. 86, ns). There were no differences in the PDI scores of infants with ICC versus DCC (84 vs. 85, ns) or for males and females (85.6 vs. 82.7, ns).
A Main Effects Regression Model (See Table 4) was used to predict the PDI as a continuous variable for VLBW infants with ICC or DCC, controlling for gestational age, male gender, IVH, oxygen use at 36 weeks, and sepsis. (None of the variables used for socioeconomic status - maternal age, education, marital status, type of insurance - were correlated with either DCC or PDI or any of the predictor variables and were not included.) These predictor variables explained 29% of the variance in the PDI scores (p = 0.007). The model indicates that LOS and oxygen use at 36 weeks significantly lowered motor scores by 19 and 13 points respectively when the other variables are held constant. Male gender appeared to influence the model as it trended toward significance (p = 0.06).
Table 4.
Regression Analysis of Predictors of Motor Outcomes (PDI Scores) at 7 Months Corrected Age
In the Main Effects model, the second column, “b” (regression coefficient) represents the effect of the predictor variable on the dependent variable, PDI, taking into account each level of the the other predictor variables. Thus, “b” represents the number of units that the PDI scores would be expected to change, given the condition listed in the first column, while holding the values for all the other variables constant. For example, if an infant had sepsis, one could expect that the PDI score for that infant would be 18.9 points less than if he did not have sepsis. Being male raised the score by 8 points suggesting the need for an interaction term.
In the Interaction Term Model in the third column, “b” for the interaction term (male X late clamping) is 18.3 points indicating that if an infant was male and had late clamping, his score on the PDI would be 18 points higher than if he had early clamping. In the presence of an interaction term in the model, the coefficients for the lower-order terms (male, late clamped alone) no longer represent main effects on PDI and are not independent of the other variables in the model. They represent the effects of male when NOT late clamped, and the effects of late clamp when NOT male.
| Main Effects Model | Interaction Term Model | |||
|---|---|---|---|---|
| Predictor Variables | b | p value | b | p value |
| Gestational Age | −0.66 | 0.56 | −0.7 | 0.51 |
| IVH | 0.52 | 0.88 | 3.5 | 0.51 |
| BPD | −12.71* | 0.01 | −17.5** | 0.006 |
| Sepsis | −18.9** | 0.007 | −16.6* | 0.01 |
| Late Clamp | −3.9 | −13.8* | 0.01 | |
| Male | 8.33 | 0.06 | −0.2 | 0.96 |
| Male X Late Clamp | 18.3* | 0.04 | ||
| R2 | 29%** | 0.007 | 35%** | 0.005 |
b: unstandardized regression coefficient;
p < 0.05;
p < 0.01
Because of the strength of the variable Male in our Main Effects Model and our previous finding that DCC appeared to be more protective for males with lower rates of IVH and LOS, an interaction term was created between male gender and DCC (Table 4). In this model, the regression coefficient for the product term, Male × Late Clamped, indicates that male infants who had DCC, had PDI scores 18 points higher compared to females or infants with ICC when controlling for the other variables. The coefficient of multiple determination, R2, is 35% for the Interaction Model (p = < 0.005).
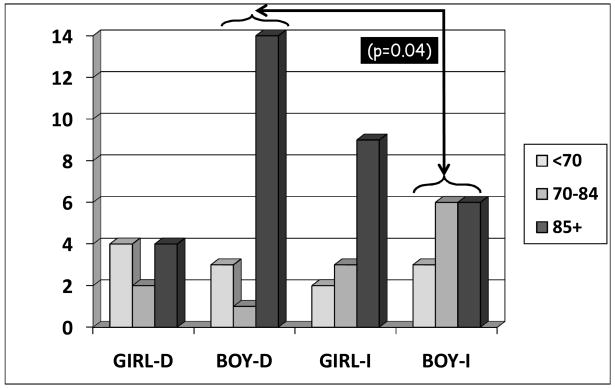
Figure 2 shows distribution of infants by gender who fell into PDI ranges of <70, 70 to 84, and 85 points or greater. Differences between male infants with ICC versus DCC reached significance.
Figure 2.
Number of girls and boys in each PDI Score Range with delayed (D) or immediate (I) cord clamping at 7 Months corrected age. The difference between the boys with ICC and DCC is significant (p = 0.04). No difference was noted between the girls (p = 0.33).
DISCUSSION
This is the first report of developmental outcomes of VLBW infants after a cord clamping intervention. No significant differences in Bayley scores were identified using bivariate statistical methods between the infants in the ICC and DCC groups at seven months corrected age. However, using regression analyses to control for potential confounding factors, a brief delay (30–45 seconds) in cord clamping with the infant held below the level of the placenta, was found to be associated with higher Bayley PDI scores for VLBW male infants at seven months corrected age. Male infants with delayed cord clamping had motor scores more than one standard deviation higher than males with ICC when controlling for NICU morbidities.
Being born preterm places an infant at greater risk for both cognitive and motor delay. Approximately 30–40% of VLBW infants experience some delay in motor functioning sometime during childhood.18 The exact mechanisms contributing to motor delay are unknown although data suggests that physiologic stressors associated with premature birth can disrupt regionally specific brain maturation.19 Motor compromise is more common among VLBW infants with brain injury.20 Damage occurring in the motor portions of the cortex, corpus callosum, and basal ganglia appears to cause children to have a predisposition for motor disturbances.19 We speculate that hypovolemia, secondary to immediate cord clamping, may be disruptive to the developing brain resulting in subsequent motor delay. One mechanism may be that having less blood volume may contribute to cardiovascular instability resulting in the loss of autoregulation within the brain. Poor perfusion can lead to ischemic damage by reducing oxygen delivery to the motor cortex in the first few days of life. Hypovolemia immediately after birth, however, is extremely difficult to diagnose, measure, or confirm. Alternatively, our findings of lower PDI scores in the ICC group may be related to the higher incidence of IVH and LOS. Both of these conditions have been found to be associated with developmental delay.15
VLBW infants are at greater risk than term infants for hypovolemia when the cord is immediately clamped at birth. Proportionately more of preterm infants’ fetal-placental blood volume is in the placenta.21 Generally, DCC results in more blood and red cell volume at birth.1 At birth, the cardiac output to the lung must increase from the 8% level in fetal life to the 45% needed for neonatal life and adult circulation. Therefore, some of the blood from the fetal “lung”, the placenta, is needed by the neonatal lung for adequate expansion and recruitment of lung tissue. When there is little opportunity for placental transfusion, the infant is left with only the blood that was in the body at the time of cord clamping. Placental transfusion creates an increase in the circulatory bed at the same time that the infant’s organs (lung, liver, kidney, etc) begin to assume the functions sustained by the placenta during fetal life.
Data to support this hypothesis is limited in part due to the lack of a simple straightforward tool for blood volume measurement. Aladangady et al reported that delayed cord clamping resulted in approximately 12 mL/kg more blood volume in preterm infants.1 Nelle reported improved systemic and cerebral hemoglobin transport in preterm infants (<1500gms) after a 30 second delay in cord clamping while lowering the infant below the placenta.5 Higher blood and red cell volume may offer protection against hypovolemia and the resulting loss of autoregulation and ischemic damage.
Children in this study who had BPD had significantly lower Bayley Motor Scores consistent with the findings of others.15 Our research supports prior studies19,22 showing that preterm children with chronic lung disease score lower in motor ability when assessed at 18 to 22 months. (We could find no reported results at 7 months). Two studies followed large samples of preterm infants with BPD who were born between 1989 and 1991 when surfactant and postnatal steroid use was standard practice. Motor function was delayed at all ages and BPD predicted poorer motor outcome at ages 2.5–3 years after controlling for other risks of age, gender, race and socioeconomic status.23–24
Previously we reported that children in our study who had delayed cord clamping had less late onset sepsis (LOS), especially male infants. (Due to smaller sample size, LOS does not reach significance in this analysis.) Our data suggests that ICC may increase the risk of LOS particularly in male VLBW infants. We found that children who had LOS while in the NICU also had significantly lower motor scores at 7 months corrected age. Stoll and colleagues (2004) found more developmental delays in infants with LOS.25 At 18 to 22 month follow-up, infants with confirmed sepsis were more likely to have a lower BSID-II score, more cerebral palsy, and more vision impairment than infants without sepsis.25
Delayed cord clamping was associated with protection of male infants against IVH and sepsis in the NICU and improved motor outcomes at seven months corrected age.14 Preterm male infants are known to have higher neonatal mortality and more long term impairment when compared to preterm female infants.26 Hintz27 reported neurodevelopmental outcomes of over 2500 NICHD network infants born <28 weeks and <1000 grams. Male infants had significant increase in neurodevelopmental impairment at 18–22 months corrected age when compared to females.27 Constable et al, using MRI, found that former preterm male infants had lower white matter volume and poorer neuromotor integrity compared to term controls at 12 years of age.28 Our findings of male advantage in association with delayed cord clamping are at odds with published data on male disadvantage for acute perinatal morbidity.
There is considerable speculation as to why male infants may be more susceptible to the negative effects of preterm birth. Derzbach proposes that the influence of estrogen may offer a protective effect to female infants even at an early age.29 Frazier and Werthammer report male infants are 2.5 times more likely to need resuscitation at birth when compared to females.30 Our findings suggest that there is a protective effect from placental transfusion through a brief delay (30–45 seconds) in cord clamping at birth for VLBW infants. The extra newborn blood volume appears to offer gender-specific, neuroprotective and immunoprotective benefit. It is possible that the effect is the result of an increased blood, red cell, and stem cell volume which may be more important for male than female infants.14 These findings are of considerable interest as the evidence of gender-specific differences in VLBW infants continues to unfold.
The sample size of this study is a limitation yet currently this is the largest clinical trial published in the literature on VLBW infants and delayed cord clamping with developmental follow up. Assessments of outcomes at seven months corrected age are limited in interpretation. However, we were constrained by our three year funding mechanism. The wide age range of infants at assessment is offset by the fact that the BSID are age adjusted. Thus any difference in performance due to age should be eliminated. Although most studies of preterm infants follow the children to 18–24 months, it is suggested that four years of age when motor outcomes stabilize and cerebral palsy can be confirmed may be optimal.22 Our compliance rate for our primary outcome of BSID II motor score is consistent with the literature.31
In summary, delayed cord clamping at birth appears to be protective of VLBW male infants against motor disability at 7 months corrected age. Delaying the clamping of the cord for just 30–45 seconds while lowering the infant is a simple perinatal intervention which may offer a gender-specific benefit for improved motor outcome among the VLBW infants. Further research to replicate these findings is essential.
Acknowledgments
We wish to thank the parents and the staff who participated in this study. Their trust and cooperation were essential to our success. We would also like to thank Richard Tucker, Department of Pediatrics, Alpert Medical School of Brown University, Providence, RI, for his expert assistance with data analyses and interpretation.
Supported by National Institute of Health, National Institute for Nursing Research, K23 NR00078
This work was supported by the NIH National Institute for Nursing Research K23NR00078.
Abbreviations
- BPD
bronchopulmonary dysplasia
- BSID, PDI, MDI
Bayley Scales of Infant Development, Psychomotor Developmental Index, Mental Developmental Index
- CA
Age corrected for prematurity (as if infant had been born at term)
- CUS
cranial ultrasound
- DCC
delayed cord clamping
- ICC
immediate cord clamping
- IVH
intraventricular hemorrhage
- LOS
late onset sepsis
- NEC
necrotizing enterocolitis
- NICU
neonatal intensive care unit
- VLBW
very low birth weight
Footnotes
Statistical Consultation: Richard Tucker, M.S., Department of Pediatrics, Women and Infants Hospital, Providence, Rhode Island
Conflict of Interest
None of the authors have any conflict of interest to report.
Contributor Information
Judith S. Mercer, University of Rhode Island, Kingston, RI.
Betty R. Vohr, Warren Alpert Medical School of Brown University, Providence, RI.
Debra A. Erickson-Owens, University of Rhode Island, Kingston, RI.
James F. Padbury, Warren Alpert Medical School of Brown University, Providence, RI.
William Oh, Warren Alpert Medical School of Brown University, Providence, RI.
References
- 1.Aladangady N, McHugh S, Aitchison TC, Wardrop CA, Holland BM. Infants’ blood volume in a controlled trial of placental transfusion at preterm delivery. Pediatrics. 2006;117(1):93–8. doi: 10.1542/peds.2004-1773. [DOI] [PubMed] [Google Scholar]
- 2.Rabe H, Reynolds G, Diaz-Rossello J. Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database Syst Rev. 2004;(4):CD003248. doi: 10.1002/14651858.CD003248.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Rabe H, Reynolds G, Diaz-Rossello J. A systematic review and meta-analysis of a brief delay in clamping the umbilical cord of preterm infants. Neonatology. 2008;93(2):138–44. doi: 10.1159/000108764. [DOI] [PubMed] [Google Scholar]
- 4.Mercer J, McGrath M, Hensman A, Silver H, Oh W. Immediate and delayed cord clamping in infants born between 24 and 32 weeks: a pilot randomized controlled trial. J Perinatol. 2003;23(6):466–72. doi: 10.1038/sj.jp.7210970. [DOI] [PubMed] [Google Scholar]
- 5.Nelle M, Fischer S, Conze S, Beedgen B, Brischke EM, Linderkamp O. Effects of later cord clamping on circulation in prematures (Abstract) Pediatr Res. 1998;44:420. [Google Scholar]
- 6.Rabe H, Wacker A, Hulskamp G, Homig-Franz I, Jorch G. Late cord clamping benefits extrauterine adaptation. Pediatr Res. 1998;44:454. [Google Scholar]
- 7.Kinmond S, Aitchison TC, Holland BM, Jones JG, Turner TL, Wardrop CA. Umbilical cord clamping and preterm infants: a randomized trial. BMJ. 1993;306:172–175. doi: 10.1136/bmj.306.6871.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibrahim H, Krouskop R, Lewis D, Dhanireddy R. Placental transfusion: umbilical cord clamping and preterm infants. J Perinat. 2000;20:351–54. doi: 10.1038/sj.jp.7200408. [DOI] [PubMed] [Google Scholar]
- 9.Oh W, Carlo W, Fanaroff AA, McDonald S, Donovan EF, Poole K, et al. Delayed cord clamping in preterm infants -a pilot randomized controlled trial. Pediatr Res. 2002;51(4 Suppl):365–6. [Google Scholar]
- 10.Strauss RG, Mock DM, Johnson K, Mock NI, Cress G, Knosp L, et al. Circulating RBC volume, measured with biotinylated RBCs, is superior to the Hct to document the hematologic effects of delayed versus immediate umbilical cord clamping in preterm neonates. Transfusion. 2003;43(8):1168–72. doi: 10.1046/j.1537-2995.2003.00454.x. [DOI] [PubMed] [Google Scholar]
- 11.Hofmeyr GJ, Gobetz L, Bex PJ, Van der Griendt M, Nikodem C, Skapinker R, et al. Periventricular/intraventricular hemorrhage following early and delayed umbilical cord clamping. A randomized controlled trial. Online J Curr Clin Trials. 1993 Doc No 110:[2002 words; 26 paragraphs] [PubMed] [Google Scholar]
- 12.Hofmeyr GJ, Bolton KD, Bowen DC, Govan JJ. Periventricular/intraventricular haemorrhage and umbilical cord clamping. Findings and hypothesis. S Afr Med J. 1988;73(2):104–6. [PubMed] [Google Scholar]
- 13.Rabe H, Wacker A, Hulskamp G, Hornig-Franz I, Schulze-Everding A, Harms E, et al. A randomised controlled trial of delayed cord clamping in very low birth weight preterm infants. Eur J Pediatr. 2000;159(10):775–7. doi: 10.1007/pl00008345. [DOI] [PubMed] [Google Scholar]
- 14.Mercer JS, Vohr BR, McGrath MM, Padbury JF, Wallach M, Oh W. Delayed cord clamping in very preterm infants reduces the incidence of intraventricular hemorrhage and late-onset sepsis: a randomized, controlled trial. Pediatrics. 2006;117(4):1235–42. doi: 10.1542/peds.2005-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vohr BR, Wright LL, Poole WK, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks’ gestation between 1993 and 1998. Pediatrics. 2005;116(3):635–43. doi: 10.1542/peds.2004-2247. [DOI] [PubMed] [Google Scholar]
- 16.Nelle M, Zilow EP, Bastert G, Linderkamp O. Effect of Leboyer childbirth on cardiac output, cerebral and gastrointestinal blood flow velocities in full-term neonates. Am J Perinatol. 1995;12(3):212–6. doi: 10.1055/s-2007-994455. [DOI] [PubMed] [Google Scholar]
- 17.Bayley N. Bayley Scales of Infant Development-II. San Antonio, TX: The Psychological Corportation; 1993. [Google Scholar]
- 18.Vohr BR, Msall ME. Neuropsychological and functional outcomes of very low birth weight infants. Semin Perinatol. 1997;21(3):202–20. doi: 10.1016/s0146-0005(97)80064-x. [DOI] [PubMed] [Google Scholar]
- 19.Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. Jama. 2000;284(15):1939–47. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 20.Bracewell M, Marlow N. Patterns of motor disability in very preterm children. Ment Retard Dev Disabil Res Rev. 2002;8(4):241–8. doi: 10.1002/mrdd.10049. [DOI] [PubMed] [Google Scholar]
- 21.Wardrop CAJ, Holland BM. The roles and vital importance of placental blood to the newborn infant. J Perinat Med. 1995;23:139–143. doi: 10.1515/jpme.1995.23.1-2.139. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan MC, Margaret MM. Perinatal morbidity, mild motor delay, and later school outcomes. Dev Med Child Neurol. 2003;45(2):104–12. [PubMed] [Google Scholar]
- 23.Singer L, Yamashita T, Lilien L, Collin M, Baley J. A longitudinal study of developmental outcome of infants with bronchopulmonary dysplasia and very low birth weight. Pediatrics. 1997;100(6):987–93. doi: 10.1542/peds.100.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Northway WH, Jr, Moss RB, Carlisle KB, Parker BR, Popp RL, Pitlick PT, et al. Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med. 1990;323(26):1793–9. doi: 10.1056/NEJM199012273232603. [DOI] [PubMed] [Google Scholar]
- 25.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Jama. 2004;292(19):2357–65. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 26.Elsmen E, Hansen Pupp I, Hellstrom-Westas L. Preterm male infants need more initial respiratory and circulatory support than female infants. Acta Paediatr. 2004;93(4):529–33. doi: 10.1080/08035250410024998. [DOI] [PubMed] [Google Scholar]
- 27.Hintz SR, Kendrick DE, Vohr BR, Kenneth Poole W, Higgins RD For The Nichd Neonatal Research N. Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birthweight infants. Acta Paediatr. 2006;95(10):1239–48. doi: 10.1080/08035250600599727. [DOI] [PubMed] [Google Scholar]
- 28.Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121(2):306–16. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- 29.Derzbach L, Treszl A, Balogh A, Vasarhelyi B, Tulassay T, Rigo J. Gender dependent association between perinatal morbidity and estrogen recepto-alpha Pvull ploymorphism. J Perinat Med. 2005;33(5):461–2. doi: 10.1515/JPM.2005.082. [DOI] [PubMed] [Google Scholar]
- 30.Frazier M, Werthammer J. Post-resuscitation complication in term neonates. J Perinatol. 2007;27(2):82–4. doi: 10.1038/sj.jp.7211644. [DOI] [PubMed] [Google Scholar]
- 31.Castro L, Yolton K, Haberman B, Roberto N, Hansen NI, Ambalavanan N, et al. Bias in reported neurodevelopmental outcomes among extremely low birth weight survivors. Pediatrics. 2004;114(2):404–10. doi: 10.1542/peds.114.2.404. [DOI] [PubMed] [Google Scholar]