Abstract
Cleidocranial dysplasia (CCD) is a rare autosomal dominant skeletal dysplasia due to mutations causing haploinsufficiency of RUNX2, an osteoblast transcription factor specific for bone and cartilage. The classic form of CCD is characterized by delayed closure of the fontanels, hypoplastic or aplastic clavicles and dental anomalies. Clinical reports suggest that a subset of patients with CCD have skeletal changes which mimic hypophosphatasia. Mutations in RUNX2 are detected in approximately 65% of cases of CCD, and microdeletions occur in 13%. We present clinical and radiological features in a 6-year-old child with severe CCD manifested by absence of the clavicles marked calvarial hypomineralization, osteoporosis and progressive kyphoscoliosis. Hypophosphatasia features included Bowdler spurs, severe osteopenia and low alkaline phosphatase. Following negative mutation analysis of RUNX2, comparative genomic hybridization (CGH) microarray was performed. The result revealed a microdeletion in RUNX2, disrupting the C-terminal part of the gene.
Keywords: cleidocranial dysplasia, hypophosphatasia, RUNX2, osteoporosis, kyphoscoliosis, aCGH, microdeletion, C-terminus
INTRODUCTION
Cleidocranial dysplasia (CCD; OMIM 119600) and hypophosphatasia (HPP; OMIM 241500) are two distinct skeletal disorders. CCD is an autosomal dominant disorder caused by haploinsufficiency of RUNX2 “Runt related transcription factor 2”. [Mundlos et al, 1995; Mundlos 1997; Lee et al., 1997]. RUNX2 is a transcription factor essential for osteoblast cell commitment and chondrocyte maturation [Ducy et al, 1997].
Delayed closure of the fontanels, hypoplastic or aplastic clavicles and dental anomalies characterize CCD. Inter and intra-familial variable expressivity [Chitayat et al., 1992] range from a mild isolated dental phenotype to severe CCD complicated by recurrent fractures and generalized osteoporosis [Quack et al.,1999]. Dental anomalies and clavicular hypoplasia are consistent features of this disorder [Otto et al., 2002; Quack et al., 1999]. Craniofacial features include brachycephaly, delayed closure of the fontanelles and sutures, wormian bones, frontal and biparietal bossing, relative macrocephaly, depressed nasal bridge, midface hypoplasia, unerupted teeth with supernumerary permanent teeth and delayed union of the mandibular symphysis. Other skeletal features include short stature (mild to moderate), genu valgum, delayed ossification of the pubic symphysis and pubic bones, tall/ hypoplastic iliac wings, pes planus, brachydactyly, joint laxity and scoliosis, [Cooper et al., 2001;Mendoza-Londono and Lee, 2006]. Debilitating kyphoscoliosis is not common in CCD. Scoliosis was reported in 16/90 (17%) of patients with CCD, usually manifesting at a later age. Three of the affected patients in that study used a back brace, and none required surgery [Cooper et al, 2001].
Hypophosphatasia is an inborn error of bone metabolism caused by mutations in the tissue non-specific alkaline phosphatase (TNSALP) gene [Whyte, 1994]. Both autosomal recessive and dominant forms have been described. Clinical manifestations of hypophosphatasia vary by age at clinical presentation and are divided into: perinatal, childhood, adult forms, as well as odontohypophosphatasia where only dental manifestations are noted. The level of serum alkaline phosphatase (ALP) activity seems to be correlated with the severity of the disorder [Whyte, 1994].
Biochemical markers used for the diagnosis of HPP include low serum alkaline phosphatase, increased phosphoethanolamine (PEA) in urine and plasma, increased inorganic plasma pyrophosphate (PPi) and increased pyridoxal 5’-phosphate (PLP) in absence of vitamin B6 intake [Whyte 1994; Whyte 2001]. The term “severe CCD” has been used to describe patients who have CCD, osteoporosis and early recurrent fractures [Quack et al., 1999], and severe parietal bone hypoplasia [Cunningham et al., 2006]. In 2002, two separate groups described patients who had combined features of CCD and HPP. One group found no mutations in RUNX2 or TNSALP[Unger et al., 2002], while the other reported a heterozygous mutation (Arg169Pro caused by nucleotide change 506G>C) in RUNX2 in a mother and daughter [Morava et al., 2002]. A CCD-Like phenotype has also been reported with 8q22 deletions [Breuten et. al, 1992]. We describe herein the clinical- radiologic and genetic-metabolic findings of a child with severe CCD featuring short stature, osteoporosis and progressive kyphoscoliosis. He also had Bowdler spurs, and subnormal serum alkaline phosphatase levels suggestive of HPP. The underlying genetic defect was found to be a microdeletion in the C-terminal region of RUNX2.
CLINICAL REPORT
A 6-year-old male, with normal intellect, was referred to the skeletal dysplasia clinic because of CCD complicated by severe progressive kyphoscoliosis. His prenatal history and ultrasound were normal. He is the only child, born to a young, nonconsanguineous Caucasian couple. The father is 196 cm and the mother 163 cm. Delivery was by cesarean at 41 weeks gestation due to cephalo-pelvic disproportion. At birth, his weight was 3.65 kg, length 50.8 cm, and head circumference 35 cm.
He was noted to have a soft cranium and narrow, sloping shoulders. The chest wall was not small or deformed. He had peripheral cyanosis and mild tachypnea that resolved by hood O2 as well as hypoglycemia that resolved by feeding. The family history revealed that the proband’s parents were healthy and a distant maternal male cousin had short stature and thyroid disease.
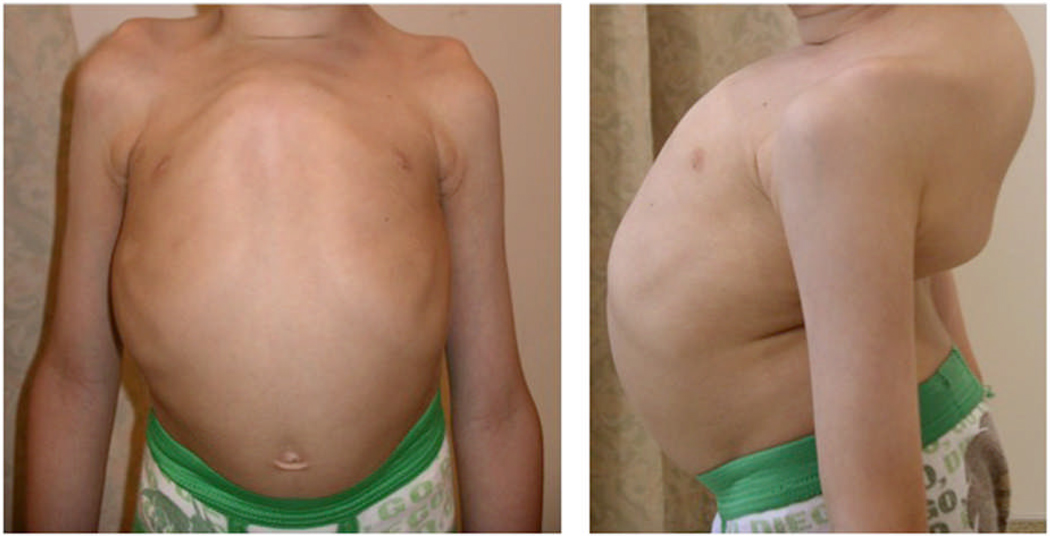
The patient had widened fontanels and relative macrocephaly. Progressive growth failure began at 3 months of age. A full endocrine and gastrointestinal work up revealed no explanation for his growth failure. His dental development was characteristically abnormal showing supernumerary teeth that were shed spontaneously and delayed eruption of permanent teeth. He had recurrent otitis media, requiring grommet tube insertions, and developed profound hearing loss-requiring hearing aids. He also had chronic sinusitis and wheezing episodes. Kyphoscoliosis was apparent before 1 year of age, requiring a back brace at the age of 3 years, with no improvement. Figure 1, demonstrates the severity of his bone deformity at the age of 7 years.
Figure 1.
Shows the appearance of the patient’s chest wall and back/spine
There is marked pectus and kyphoscoliosis at the age of 7 years .
His past history was significant for 2 separate fractures following minor trauma: a posterior cortical fracture of the distal tibia which required casting for 2 weeks and a hairline fracture of the foot. He also required frenuloplasty for ankyloglossia, adenoidectomy and inguinal hernia repair. He had nasalized speech which was otherwise normal. He had short trunked dwarfism and was approximately 4 standard deviations (SD) below the mean for height, and 2 SD below the mean for weight. He had brachycephaly and frontal bossing. The anterior and posterior fontanels, as well as the metopic suture were wide open. He had hypertelorism and slight depression of his nasal bridge. His palate was narrow and high arched. He had supernumerary teeth, while other teeth were missing. His shoulders were narrow, sloping, and could be opposed easily. The back and spine showed severe kyphoscoliosis. The chest wall was asymmetrically deformed, protruding anteriorly at the upper part of the sternum. The cardiopulmonary exam was normal. The abdominal exam showed no organomegaly, the genitourinary exam was unremarkable. He had brachydactyly, with broad, proximally-placed thumbs.
There was swelling at the middle inter-phalangeal joints and laxity of the joints of the fingers, wrists, and elbows. There were bilateral skin dimples in the region of the mid shaft of the tibiae, and pes planus. The neurological exam was essentially normal with no evidence of nerve compression. The radiological findings are demonstrated in Figure 2 and Figure 3 respectively, taken during the first week of life. A prominent finding was generalized osteopenia. The skull was brachycephalic with markedly diminished ossification of membranous bone, wide separation of the sutures, wormian bones and hypoplastic teeth. The clavicles were absent bilaterally and the posterior ribs were widely separated from the spinal elements. There were coronal and sagittal cleft- like ossification defects in the lumbar vertebrae, and kyphoscoliosis with lumbar lordosis. There was absent ossification of the pubic and ischial bones. The hands revealed hypoplastic metacarpals and distal phalanges of the thumbs. Both fibulae showed osteochondral projections (Bowdler spurs) at the midshaft of the fibulae, but the long bones revealed no evidence of metaphyseal abnormalities. The patient’s most recent pulmonary function testing was restricted by his severe kyphoscoliosis. His maximum inspiratory and expiratory pressures were low at 16% of predicted for maximum inspiratory pressure and 36% of predicted for maximum expiratory pressure. Bone mineral density done at the age of 7 years revealed severe demineralization of the lumbar spine corresponding to a calculated z-score of −3.4 and z- score average of both femoral necks was −4.87.
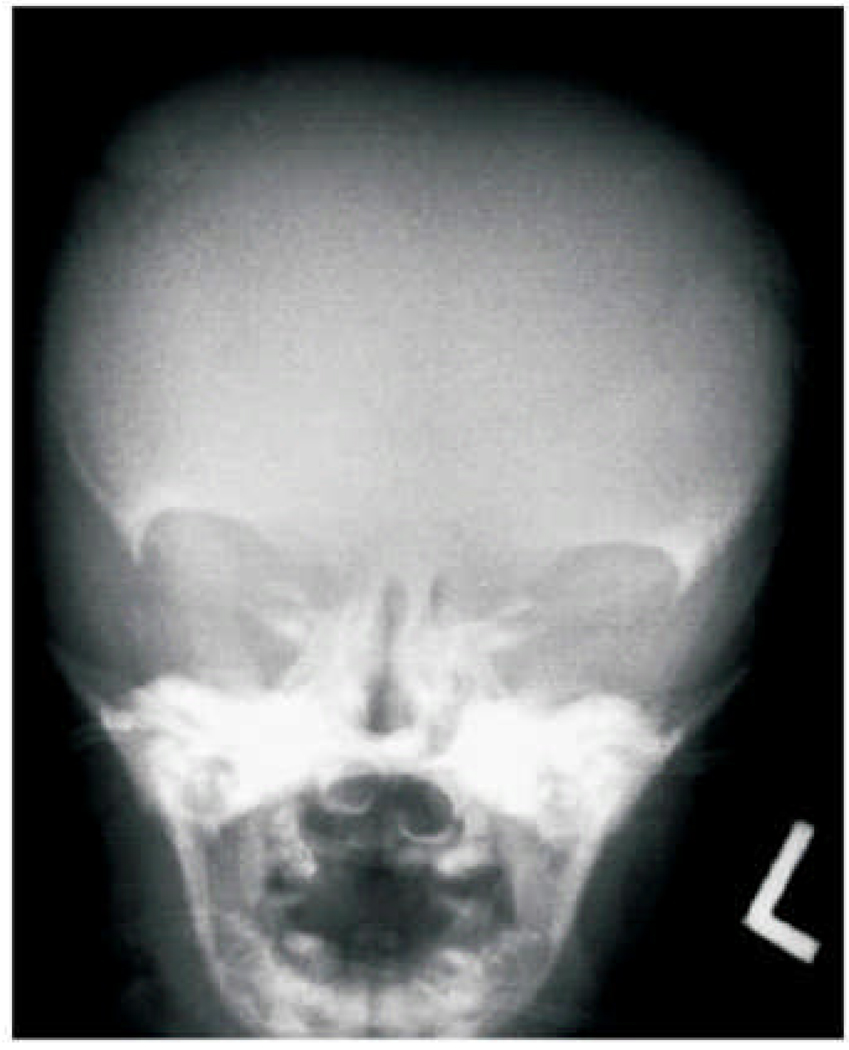
Figure 2.
AP skull radiograph in the newborn period showed absent lateral orbital and poor membraneous skull ossification. The teeth are hypoplastic
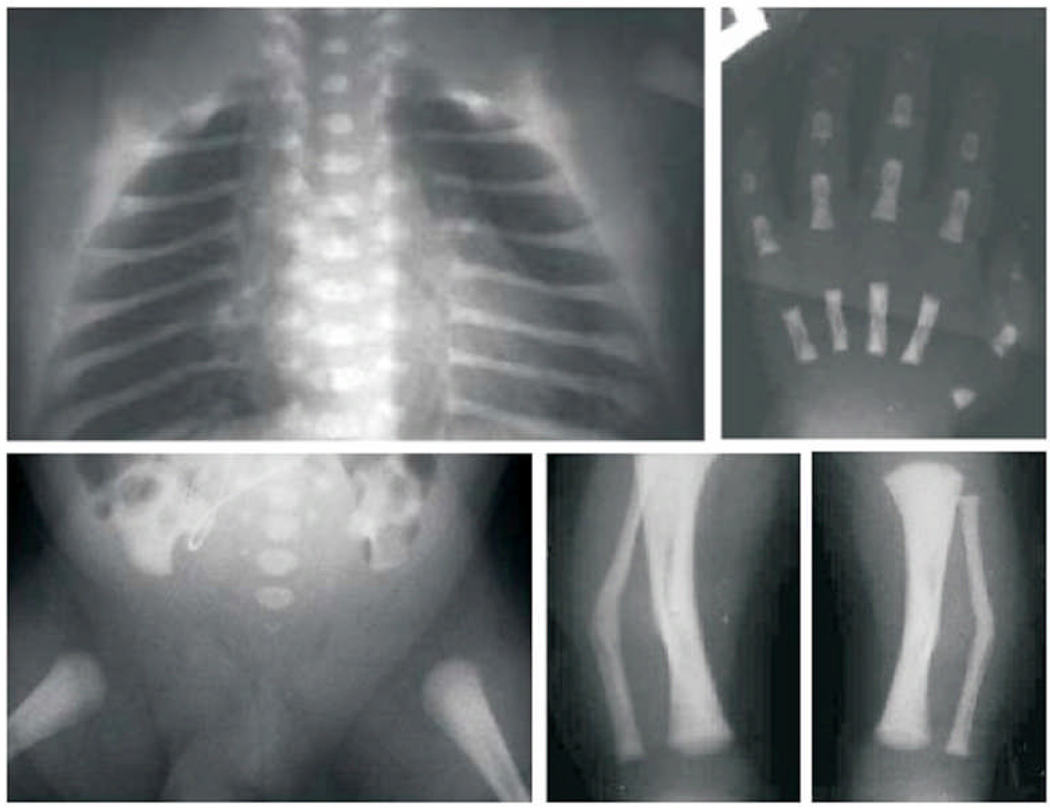
Figure 3.
Radiographic images obtained during the Newborn period: Upper row: left to right depicts AP Thorax showing absent clavicles, large separation of the posterior ribs from the spinal elements, sagittal clefting is present. AP radiograph of the Hand revealed a hypoplastic metacarpal and distal phalanx of the thumb. Lower row, left to right: depicts AP film of the Pelvis and Hips showing tall narrow iliac wings, absent pubic and ischial ossification, absent lower sacral pedicle ossification. AP radiographs of both Tibias and Fibulas showing fibula angulation with tiny osteochondral projections [Bowdler spurs] at the site of the skin dimples
METHODS AND RESULTS
Biochemical studies revealed a serum calcium level of 9.9 mg/dl (8.7–10.5), with normal albumin and phosphorus levels. The serum alkaline phosphatase levels were low on two occasions 102 U/L in 2007 (134–346) and 118 (145–320 U/L) in 2005.
His urine spot phosphoethanolamine was 108mmol/mg creatinine, (within the normal range) while the osteocalcin level was 8 ng/ml( normal <7.2). Cytogenetic analysis of peripheral blood showed a normal 46,XY karyotype. RUNX2 mutation analysis was negative. Peripheral blood was sent to Signature Genomics Laboratories, LLC (Spokane, Washington) for array Comparative Genomic Hybridization (aCGH). Array CGH analysis of 1543 loci using almost 5000 Bacterial Artificial Chromosome (BAC) clones was used to explore DNA gains or losses in a peripheral blood specimen from this patient. Loss of a single BAC signal was reported at chromosome 6p12.3 (CTD-2528C1; 45,544,848-45,692bp). This deletion was confirmed and narrowed to ~70kb (45,593,804-45,643,200bp, +/− 9kb for each nearest proximal and distal flanking probes) using an oligonucleotide aCGH approach where seven contiguous oligonucleotide probes were lost.
The magnitude of fluorescence lost was equivalent to a single copy deletion using either aCGH approach. The deletion disrupts the 3' end of the RUNX2 gene (OMIM #600211), including an additional 25Kb of non-coding sequence past the stop codon (Fig. 4). This deletion is predicted to truncate the transcript and prevent translation of acids 327 to 521. No deletion, or alteration, was observed at 8q22, a region when deleted causes a CCD-like phenotype [Brueton et al, 1992].
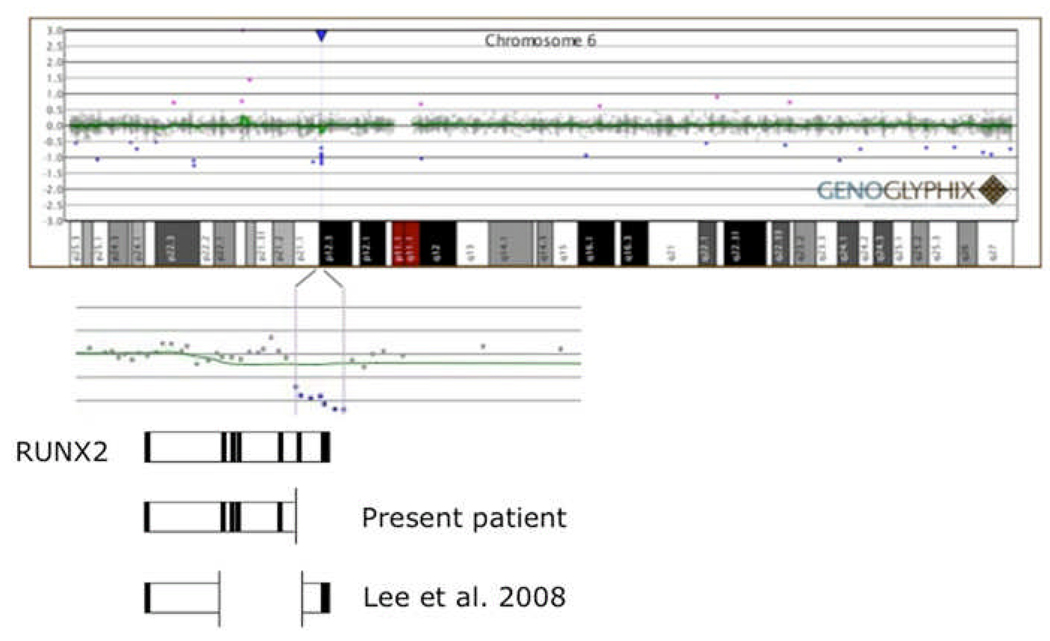
Figure 4.
Disruption of RUNX2 by genomic deletion. An array comparative genomic hybridization (aCGH) plot (Genoglyphix; courtesy Signature Genomic Laboratories) is presented at the top of the figure, above a chromosome 6 ideogram. The deletion encompasses 7 oligonucleotide probes at 6p12.3 which are expanded below the ideogram. The expanded view of the deleted region is overlayed on a cartoon representation of the exon/intron structure of the RUNX2 gene, drawn to approximate horizontal scale. The deletion is predicted to disrupt the C-terminal end of the protein (starting with exon 6), which is involved in transactivation/repression, protein-protein interaction, and includes the nuclear matrix-targeting signal (see text for details). This region is essential for integrating TGFB and BMP2 signaling pathways which are important for osteogenesis.
DISCUSSION
The diagnosis of CCD is usually based on clinical and radiological findings [Lee and Zhou, 2004]. A subset of patients with CCD have radiographic and biochemical features that overlap with HPP [Wyckoff et al., 2005; Morava et al., 2002; and Unger et al., 2002]. In infancy, it may be difficult to clinically differentiate between both disorders because both present with a soft cranium. RUNX2, the gene mutated in CCD is a transcription factor that regulates intra-membranous and endochondral ossification during embryonic and postnatal skeletogenesis [Ducy et al., 1997]. Because the clavicles ossify embryologically by both routes, the presence of significantly hypoplastic or aplastic clavicle(s) suggest CCD [Zeng et al., 2005; Huang et al., 1997]. Complete absence of the clavicle has not been reported in HPP [Shohat et al., 1991]. Currently, there is no effective treatment for either disorder, but for purposes of genetic counseling and assessment of the prognosis, it is prudent to identify the underlying genetic/metabolic defect in patients with overlapping features.
In this report, the proband had clinical features suggestive of CCD since birth. Unexplained severe growth failure, dentition problems, and progressive kyphoscoliosis started in infancy proceeding into childhood. At the age of six years, he became a candidate for corrective spinal surgery to prevent pulmonary restriction. The pulmonologist and multiple orthopedic surgeons suggested intervention to allow pulmonary expansion by either using a growing rod or vertical expandable titanium rib (VEPTR). The decision to perform this surgery electively has been delayed due to concerns regarding the skeletal architecture and defective bone mineralization. Absence of the clavicles, suggested a diagnosis of CCD. Quack et al [1999] described a patient who had an unusual form of CCD, associated with an insertion/frameshift mutation (1205–1206insC). This patient had recurrent fractures that started in utero and severe osteoporosis diagnosed in adulthood. He developed severe scoliosis and lordosis of the spine at a later age necessitating the implantation of a Harrington rod for spinal support [Quack et al., 1999].
It has been shown that Runx2 null mice (−/−) do not form bone, and lose markers of early bone differentiation including alkaline phosphatase while heterozygote Runx2 deficient mice (+/−) have features of CCD with reduced alkaline phosphatase activity [Komori et al., 1997; Otto et al.,1997]. Forced expression of RUNX2 in cell lines of mesenchymal origin lead to upregulation of osteoblast-expressed genes such as osteocalcin and alkaline phosphatase [Ducy, 1999]. Mutant mice expressing a truncated Runx 2 C-protein lack a mineralized skeleton and fail to transactivate the osteocalcin promoter when co expressed with either BMP2 “ bone morphogenic protein 2” or TGFB “ transforming growth factor beta” responsive [Afzal et al., 2005; Choi et al., 2001].
In humans with CCD, RUNX2 mutations are detected in 65% of cases, while microdeletions occur in 13% of those with normal mutation analysis [Mendoza-Londono and Lee, 2006]. Most deletions of RUNX2 involve only a few bases [Mundlos et al. 1995,1997; Otto et al., 2002; Quack et al., 1999]. A larger deletion spanning both RUNX2 and the upstream gene VEGF “vascular endothelial growth factor” was detected in a patient with CCD and vascular anomalies [Izumi et al., 2006]. Another report described a large intragenic deletion involving exons 2–6 in a three-generation family with typical CCD features, hypoplastic distal phalanges and cone shaped epiphysis of the middle phalanges [Lee et al., 2008]. In our patient, no mutations (base changes or small insertions/deletions) were detected upon sequencing RUNX2, so the possibility of a large-scale genomic change involving RUNX2 was investigated using aCGH. These studies revealed a 50–70Kb deletion, which predicted a disruption of the C-terminal region of RUNX2 encompassing the coding sequence for amino acids (aa) 327 to 521. SMAD 1,2,3,5 binding sites and the nuclear matrix targeting signal (NMTS) regions are within the deleted region (aa 369–417 and aa 390–427, respectively; while the RUNT domain is not. A similar mutation was described by Quack et al. [1999] in a patient with severe CCD. They reported an insertion/frameshift 3' of the RUNT domain, which left the DNA binding domain intact, but without activation potential, and they postulated that this alteration may result in a dominant negative effect [Quack et al. 1999].
Within the constraints of the current clinical study, we cannot discriminate a dominant negative effect from haploinsufficiency. The hypophosphatasia features in our patient may be secondary to reduced osteoblast differentiation and bone formation or abnormal RUNX2-SMAD interaction. The findings in this patient emphasize the importance of defining the clinical and metabolic phenotype in patients with severe disease and the need to search for deletions when sequencing of the target gene is normal. It also sheds light on the integral role of the C-terminal region of RUNX2 in human osteogenesis and osteoblast differentiation.
ACKNOWLEDGMENTS
This work is supported in part by an NIH Program Project Grant HD22657
The authors acknowledge the patient and his family for their kind participation in this case study.
REFERENCES
- Afzal F, Pratap J, Ito K, Ito Y, Stein JL, van Wijnen AJ, Stein GS, Lian JB, Javed A. Smad function and intranuclear targeting share a RUNX2 motif required for osteogenic lineage induction and BMP2 responsive transcription. J. Cell. Physiol. 2005;204:63–72. doi: 10.1002/jcp.20258. [DOI] [PubMed] [Google Scholar]
- Brueton LA, Reeve A, Ellis R, Husband P, Thompson EM, Kingston HM. Apparent cleidocranial dysplasia associated with abnormalities of 8q22 in three individuals. Am J Med Genet. 1992;1192(43):612–618. doi: 10.1002/ajmg.1320430322. [DOI] [PubMed] [Google Scholar]
- Chitayat D, Hodgkinson KA, Azouz EM. Intrafamilial variability in cleidocranial dysplasia: a three generation family. Am J Med Genet. 1992;42:298–303. doi: 10.1002/ajmg.1320420307. [DOI] [PubMed] [Google Scholar]
- Choi J-Y, Pratap J, Javed A, Zaidi SK, Xing L, Balient E, Dalamangas S, Boyce B, van Wijnen AJ, Lian JB, Stein JL, Jones SN, Stein GS. Sub nuclear targeting of RUNX2/Cbfa/AML factors is essential for tissue-specific differentiation during embryonic development. Proc Natl Acad Sci USA. 2001;98:8650–8655. doi: 10.1073/pnas.151236498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SC, Flaitz M, Johnston DA, Brendan L, Hecht JT. A natural history of Cleidocranial dysplasia. Am J Med Genet. 2001;104:1–6. doi: 10.1002/ajmg.10024. [DOI] [PubMed] [Google Scholar]
- Cunningham ML, Seto ML, Hing AV, Bull MJ, Hopkin RJ, Leppig KA. Cleidocranial dysplasia with severe parietal bone dysplasia: C-terminal RUNX2 Mutations. Birth Defects Research(A) 2006;76:78–85. doi: 10.1002/bdra.20231. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G. ACbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev. 1999;13:1025–1036. doi: 10.1101/gad.13.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LF, Fukai N, Selby PB, Olsen BR, Mundlos S. Mouse clavicular development: analysis of wild type and cleidocranial dysplasia mutant mice. Dev Dyn. 1997;210:33–40. doi: 10.1002/(SICI)1097-0177(199709)210:1<33::AID-AJA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Izumi K, Yahagi N, Fujii Y, Higuchi M, Kosaki R, Naito Y, Nishimura G, Hosokai N, Takahashi K, Kosaki K. Cleidocranial dysplasia plus vascular anomalies with 6p21.2 microdeletion spanning RUNX2 and VEGF. Am J Med Genet Part A. 2006;140A:398–401. doi: 10.1002/ajmg.a.31061. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura A, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Lee B, Thirunavukkarasu K, Zhou L, Pastore L, Baldini A, Hecht J, Geoffrov V, Ducy P, Karsenty G. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF/CBFA1 in cleidocranial dysplasia. Nat Genet. 1997;16:307–310. doi: 10.1038/ng0797-307. [DOI] [PubMed] [Google Scholar]
- Lee B, Zhou G. RUNX2 and cleidocranial dysplasia. In: Epstein CJ, Erickson RP, Wynshaw-Boris A, editors. Inborn errors of development. New York: Oxford; 2004. pp. 331–339. [Google Scholar]
- Lee MT, Tsai AC, Chou CH, Sun FM, Huang LC, Yen P, Lin CC, Liu CY, Wu JY, Chen YT, Tsai FJ. Intragenic microdeletion of RUNX2 is a novel mechanism for cleidocranial dysplasia. Genomic Med. 2008;2:45–49. doi: 10.1007/s11568-008-9024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Londono R, Lee B. Cleidocranial dysplasia. Gene Rev. 2006:1–18. http:/www.geneclinics.org.
- Morava E, Karteszi J, Weisenbach AC, Mundlos S, Mehes K. Cleidocranial dysplasia with decreased bone density and biochemical findings of hypophosphatasia. Eur J Pediatr. 2002;161:619–622. doi: 10.1007/s00431-002-0977-x. [DOI] [PubMed] [Google Scholar]
- Mundlos S, Mulliken JB, Abramson DL, Warman ML, Knoll JH, Olsen BR. Genetic mapping of cleidocranial dysplasia and evidence of a microdeletion in one family. Hum Mol Genet. 1995;56:938–943. doi: 10.1093/hmg/4.1.71. [DOI] [PubMed] [Google Scholar]
- Mundlos S. Cleidocranial dysplasia: clinical and molecular genetics. J Med Genet. 1999;13:177–182. [PMC free article] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmore KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Otto F, Kanegane H, Mundlos S. Mutations in the RUNX2 gene in patients with Cleidocranial dysplasia. Hum Mutat. 2002;19:209–216. doi: 10.1002/humu.10043. [DOI] [PubMed] [Google Scholar]
- Quack I, Vonderstrass B, Stock M, Aylsworth AS, Becker A, Brueton L, Lee PJ, Majewski F, Mulliken JB, Suri M, Zenker M, Mundlos S, Otto F. Mutation analysis of core binding factor A1 in patients with cleidocranial dysplasia. Am J Hum Genet. 1999;65:1268–1278. doi: 10.1086/302622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohat M, Rimoin DL, Gruber HE, Lachman RS. Perinatal lethal hypophosphatasia: clinical, radiologic and morphologic findings. Pediatr Radiol. 1991;21:421–427. doi: 10.1007/BF02026677. [DOI] [PubMed] [Google Scholar]
- Unger S, Mornet E, Mundlos S, Blaser S, Cole DEC. Severe cleidocranial dysplasia can mimic hypophosphatasia. Eur J Pediatr. 2002;161:623–626. doi: 10.1007/s00431-002-0978-9. [DOI] [PubMed] [Google Scholar]
- Whyte MP. Hyposphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev. 1994;15:439–446. doi: 10.1210/edrv-15-4-439. [DOI] [PubMed] [Google Scholar]
- Whyte MP. Hypophosphatasia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill; 2001. pp. 5313–5329. [Google Scholar]
- Wyckoff MH, El-Turk C, Laptook A, Timmons C, Gannon FH, Zhang X, Mumm S, Whyte MP. Neonatal lethal Osteochondrodysplasia with low serum levels of alkaline phosphatase and osteocalcin. J Clin Endocrinol Metab. 2005;90:1233–1240. doi: 10.1210/jc.2004-0251. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Sebald E, Guang Z, Zhou G, Chen Y, Wilcox W, Lee B, Krakow D. Dysregulation of chondrogenesis in human Cleidocranial dysplasia. Am J. Hum. Genet. 2005;77:305–312. doi: 10.1086/432261. [DOI] [PMC free article] [PubMed] [Google Scholar]