Abstract
Objective
To evaluate the sperm’s chromatin quality in couples with spontaneous recurrent abortion.
Methods
Thirty couples with spontaneous recurrent abortion (case group) and 30 fertile couples (control group) referring to Zeinabieh Gynecology clinic of Shiraz were included. Semen samples were collected for each participant and were used for standard semen analysis and sperm nuclear maturity tests including Chromomycin A3 (CMA3), Aniline Blue (AB) staining and Acridine Orange (AO) test (by light microscopy).
Result
Patients in case group had significantly higher percentage of CMA3 (p < 0.001) and AB (p < 0.001) positive spermatozoa compared to controls. However AO results did not differ significantly between groups (p = 0.656). Sperm morphology and progressive motility were negatively correlated with CMA3 (p = 0.001 and p = 0.043) and AB (p = 0.015 and p = 0.031) respectively.
Conclusion
Evaluation of the sperm’s quality via CMA3 and AB staining could be considered as one of the complementary tests of semen analysis for assessment of male factor in couples with spontaneous recurrent abortion.
Keywords: Spontaneous recurrent abortion, Chromomycin A3 (CMA3), Aniline blue (AB), Acridine orange (AO)
Introduction
Semen analysis is traditionally used as the first step to evaluate the male factor infertility and it is believed that semen analysis is insufficient to determine the fertility in-vivo or in-vitro. Semen quality is determined according to motility, morphology and concentration of the spermatozoa. However, a failure of the conventional semen parameters to predict fertilization indicates that hidden anomalies lie at the sperm membrane level or at the chromatin level. In this respect, indirect methods for assessment of the amount of protamines or measuring chromatin structure based on different staining methods or fluorochromes have also been used [1, 2]. These staining methods include Aniline blue (AB), Acridine Orange (AO) and Chromomycin A3 (CMA3) staining. CMA3 is a flurochrome which has been shown to compete with the protamines for binding to the minor groove of DNA and detects protamine deficiency in loosely packed chromatin and is correlated to extent of nicked DNA [2]. During spermiogenesis, DNA histones are mainly replaced by protamines in order to acquire a highly packed sperm chromatin structure. Another indirect approach is the use of AB staining to detect the presence of extra histones and, therefore, indirectly infer the presence of lower amounts of protamines in the sperm nucleus. In the other words AB staining detects presence of remaining histones by binding to lysine-rich histones [3]. AO test is a fluorescence staining called sperm chromatin structural assay (SCSA) which reflects sperm chromatin denaturation (single strand DNA vs. double strand DNA) [4].
Spontaneous pregnancy loss is one of the most common complications of pregnancy occurring in about 1% of couples [5]. Even after a thorough evaluation, the potential cause remains unexplained in about one third to one half of the cases [5]. However, unable to find the cause of these losses, it is reasonable to suppose that factors within sperm may have an influence on these pregnancy losses [6, 7]. Attempts to show a relationship between RPL and sperm quality, evaluated through conventional internationally established guidelines for semen analyses, have been controversial. Attempts to find differences between couples with RPL and the general population or fertile men in terms of morphology, concentration, motility, hypoosmotic swelling test, acrosomal status, viability, or seminal leukocytes have not been successful [7–9]. Some previous studies have shown that nuclear maturation defects affects the embryo development and causes poor reproduction outcomes [1, 11]. Nasr-Esfahani et al. [12] have shown that CMA3 and AB stainings can predict fertilization rate in patients undergoing intracytoplasmic sperm injection (ICSI). Lolis et al. [13] have reported similar results in patients undergoing in vitro fertilization (IVF). Therefore we aimed to evaluate the sperm’s chromatin quality using CMA3, AB and AO staining in couples with spontaneous recurrent abortion.
Materials and methods
In this prospective study, 30 couples with recurrent abortion and 30 fertile couples were enrolled at Infertility clinic of Shiraz University of Medical Science (Shiraz, Iran) between December 2006 and October 2008. All the participants were divided into two groups: Case group (n = 30) consisted of the men whose partners had ≥3 unexplained recurrent spontaneous abortion at less than 20 weeks of gestation. Unexplained recurrent abortion was diagnosed after exclusion of the causes of recurrent abortion such as hormonal disorders, infections, genetic anomalies, immunologic problems and abnormal anatomic structures. Control group (n = 30) consisted of healthy men (with no known medical conditions) whose partners had at least two pregnancies with no history of pregnancy loss and whose last child was conceived a maximum of 1 year before the present study or whose female partner was pregnant with a gestation age >3 months.
None of the women in control group had a history of preeclampsia or intrauterine growth retardation. None of the participants were smoker and none of them had history of alcohol consumption. Participants who had previous exposure to anesthetic gases, perchorethylene (a dry-cleaning solvent), heavy metals (mercury, lead) and isotretinoin (Accutane) were excluded from the study. None of the men were painters or factory workers. The study protocol was approved by the institutional review board of Shiraz University of Medical Sciences. Ethical approval was obtained from the Ethics Committee. Informed consent explaining the objectives and implications of the tests performed on each ejaculate was obtained. It included a form with identification data, reproductive history, and a survey asking for certain potential risk factors for each individual.
The semen samples of the two groups were collected by masturbation after 3–4 days of abstinence. After complete liquefaction of the sample, semen analysis was performed according to World Health Organization guidelines [14] and sperm morphology was analyzed following the Kruger strict criteria [15]. Sperm count was performed in a Neuberger counting chamber. After immobilizing the cells with distilled water, morphology was evaluated by the hematoxylin staining technique. Motility was expressed as a percentage of rapid and/or progressive spermatozoa. Thereafter the protocol preparation, several smears were prepared from each specimen to record chromatin status, using aniline blue, CMA3 and acridine orange staining. A total of 200 sperms were evaluated per smear in all tests.
Chromomycin A3 staining
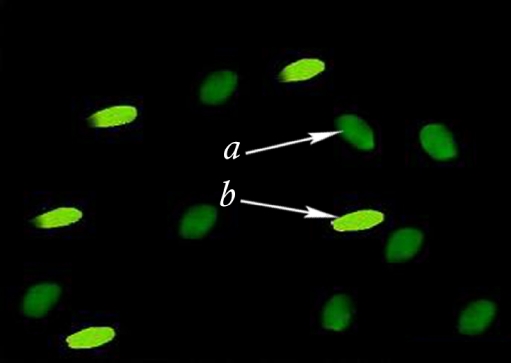
Spermatozoa retrieved from double-wash swim up sample as well as semen smears were fixed in methanol/glacial acetic (3:1) at 40°C for 5 min. Each slide was treated for 20 min with 100 µl CMA3 solution (0.25 mg/ml in McIlvane’s buffer, PH 7.0, containing 10 mM MgCl2). The slides were rinsed in buffer and mounted with buffered glycerol. Fluorescence was performed using an Olympus microscope (Zeiss Company, Germany). The spermatozoa were evaluated on each slide. Two types of staining patterns were identified, namely bright green fluorescence of the sperm head (abnormal chromatin packaging), and dull green staining (normal chromatin packaging) (Fig. 1) [13].
Fig. 1.
Chromomycin A3 (CMA3) staining of sperm chromatin of a man with spontaneous recurrent abortion (case group) showing normal spermatozoa (a) versus spermatozoa with protamin deficiency (b)
Aniline blue staining
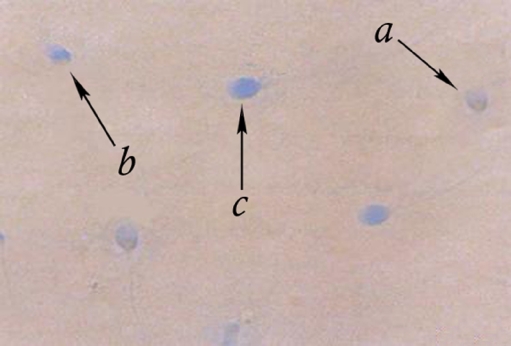
A drop of semen (swim-up sample) was spread on the glass slides and allowed to air- dry. All the smears were fixed in 3% buffered glutaraldehyde for 30 min. The slides were then stained with 5% aqueous Aniline Blue and mixed with 4% acetic acid (PH 3.5) for 7 min. Three classes of head staining intensities were noted, namely unstained (gray/white), partially stained, and entire sperm head stained dark blue (Fig. 2) [16].
Fig. 2.
Aniline blue (AB) staining of sperm chromatin of a man with spontaneous recurrent abortion (case group) showing normal spermatozoa (a) versus spermatozoa with moderate level of remaining histones (b) and spermatozoa with remained histones (c)
Acridine orange test
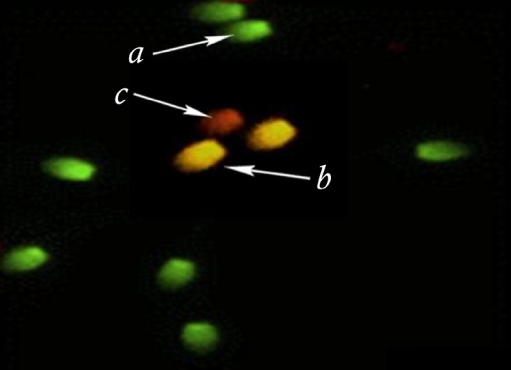
A drop of semen (swim-up samples) was spread on the glass slides and allowed to air-dry. All the smears were fixed in methanol/acetic acid (3:1). The slides were then stained with 2–3 cc Acridine Orange solution %19 in phosphate citrate for 10 min in each slide. The sperms were evaluated with fluorescence microscope (Zeiss Company, Germany). Three types of staining patterns were identified; green sperms (double-stranded DNA), yellow and red sperms (single-stranded DNA) (Fig. 3) [17].
Fig. 3.
Acridine orange (AO) staining of sperm chromatin of a man with spontaneous recurrent abortion (case group) showing normal (a) moderately denaturated (b) and completely denaturated (c) spermatozoa
Statistical analysis
All statistical analyses were performed with the Statistical Package for Social Sciences version 15.0 (SPSS Inc., Chicago, IL, USA). Utilizing student’s t-test was used to compare between parametric data sets. Pearson’s correlation coefficients were used to calculate the correlation between paired data Sets. A p-value less than 0.05 was considered statistically significant.
Result
All the patients completed the study. Mean age of the men in case and control groups didn’t differ significantly (34.6 ± 5.6 vs. 33.8 ± 6.3; p = 0.153). This finding was consistent for women (29.4 ± 6.6 vs. 29.7 ± 5.9; p = 0.281). The semen analysis was found to be normal in all the patients according to WHO criteria (Table 1). However, the percentage of sperm progressive motility was significantly lower in patients with spontaneous recurrent pregnancy loss (p = 0.002). In the same way men in case group had significantly higher percentage of morphological defects (p = 0.001) especially head defects (p = 0.024) and multiple defects (p = 0.043).
Table 1.
Seminal parameters of 30 couples with spontaneous recurrent abortion (Case group) compared to 30 fertile couples (Control group)
| Semen parameters | Case group (n = 30) | Control group (n = 30) | p-value |
|---|---|---|---|
| Ejaculation Volume (mL) | 3.5 ± 1.3 | 3.8 ± 0.9 | 0.092 |
| Concentration (×106/mL) | 65.4 ± 28.9 | 66.8 ± 30.2 | 0.157 |
| Progressive motility (%) | 53.6 ± 25.4 | 64.6 ± 28.3 | 0.002 |
| Morphologic alterations | |||
| Head defect (%) | 9.6 ± 6.3 | 7.9 ± 5.8 | 0.024 |
| Acrosome defects (%) | 2.8 ± 1.2 | 2.5 ± 0.9 | 0.253 |
| Neck defects (%) | 2.9 ± 0.8 | 3.1 ± 1.3 | 0.151 |
| Midpiece defects (%) | 4.7 ± 2.7 | 4.5 ± 2.2 | 0.361 |
| Tail defects (%) | 1.8 ± 1.1 | 1.4 ± 0.7 | 0.164 |
| Multiple defects (%) | 5.9 ± 4.2 | 4.8 ± 3.9 | 0.043 |
| Total (%) | 27.7 ± 15.6 | 24.2 ± 13.6 | 0.001 |
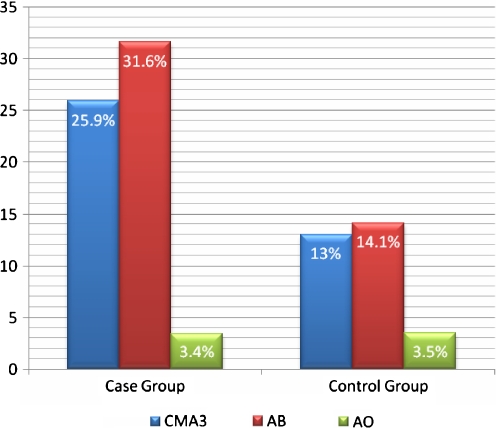
A substantial difference was observed in the percentage of positive CMA3 stained spermatozoa in case group compared to control group (25.9% ± 13.1% vs. 13% ± 5.5%; p < 0.001). There was higher number of AB positive spermatozoa from men with spontaneous recurrent abortion compared to fertile ones which was statistically significant (31.6% ± 16.6% vs. 14.1% ± 7.0%; p < 0.001). The percentage of denaturated spermatozoa (positive AO) didn’t differ significantly between groups (3.4% ± 1.8% vs. 3.5% ± 1.07%; p = 0.656) (Fig. 4).
Fig. 4.
Results of Chromomycin A3 (CMA3), Aniline blue (AB) and Acridine orange (AO) staining in case and control groups. Comparison between result of CMA3, AB and AO test in case and control groups
Table 2 demonstrates correlation between sperm parameters and sperm chromatin quality tests. Sperm morphology was negatively correlated with CMA3 (r = −0.651, p = 0.001) and aniline blue (r = −0.572, p = 0.015) staining, respectively. In the same way sperm progressive motility was negatively correlated with CMA3 (r = −0.316, p = 0.043) and aniline blue (r = −0.439, p = 0.031) staining.
Table 2.
Correlation between semen parameters and sperm chromatin quality tests
| Sperm parameters | Correlation coefficient | p-value |
|---|---|---|
| Chromomycin A3 | ||
| Age (yr) | 0.236 | 0.216 |
| Volume (mL) | 0.198 | 0.311 |
| Concentration (×106/mL) | 0.019 | 0.816 |
| Progressive motility (%) | −0.316 | 0.043 |
| Normal sperm morphology (%) | −0.651 | 0.001 |
| Aniline blue | ||
| Age (yr) | 0.382 | 0.145 |
| Volume (mL) | 0.261 | 0.086 |
| Concentration (×106/mL) | 0.085 | 0.765 |
| Progressive motility (%) | −0.439 | 0.031 |
| Normal sperm morphology (%) | −0.572 | 0.015 |
| Acridine orange | ||
| Age (yr) | 0.087 | 0.712 |
| Volume (mL) | 0.025 | 0.915 |
| Concentration (×106/mL) | 0.188 | 0.545 |
| Progressive motility (%) | 0.076 | 0.891 |
| Normal sperm morphology (%) | −0.166 | 0.461 |
Discussion
We are witnesses of a dramatic increase in the extent of knowledge of human gamete biology and reproductive medicine. Andrological investigations still rely on a thorough history and physical examination of the male partner. Standard semen analysis is routinely used to evaluate the male infertility; however, some researchers believe that sperm functional test should also be used with standard semen analysis for evaluation of male fertility. The success rate of fertilization is greatly affected by the quality of sperm used for insemination [18, 19].
One of the central events of spermiogenesis is substituation of the chromatin proteins (especially histones) by protamines, allowing a different structural organization to take place in the sperm nucleus. This type of organization provides sperm with a highly condensed nucleus and also protects sperm DNA against the enzymatic attack of nucleases and polymerases. Due to the tight packaging afforded by the protamines, any modification or absence of these proteins lead to an anomaly in the packaging process of sperm nucleus and influence sperm quality (morphology) and fertilizing capacity. Abnormalities such as loosely packaged chromatin and damaged DNA have already been observed in poor quality semen samples [10]. Pervious studies have shown that protamine deficiency measured by CMA3 staining independently effect fertilization [1, 20]. However abnormality in sperm chromatin can take place at several levels including 1) histone/protamine replacement, 2) absence of protamine, 3) epididymal maturation, and 4) chromatin stability during ejaculation. Therefore during this study we observed that number of CMA3 and AB positive spermatozoa are significantly higher in patients with spontaneous recurrent abortion compared to fertile men. As these two tests reflects the quality of sperm chromatin, this can be concluded that semen form men whose partners suffer from spontaneous recurrent abortion has low chromatin quality especially protamine deficiency. We also found that progressive sperm motility (%) and abnormal sperm morphology are strongly correlated to percentage of positive CMA3 and AB positive spermatozoa in a negative fashion. This shows that abnormal sperm chromatin packaging will result in destructed semen parameters. This result is in concordance with many others authors, who have concentrated their attention on the predictive value of morphology, using WHO standards or strict criteria [21–23], and it is felt that normal morphology is indicative of normal sperm function while high level of abnormal spermatozoa in ejaculation is associated with reduce fertilization potential. Even though the role of sperm morphology in prediction of pregnancy and in vitro fertilization outcome is well established in literatures [13], some authors question the sole predictive role of morphology in prediction of male fertility potential [24]. Also, when studying the role of sperm morphology in relation to fertilization rate in ICSI, literature shows that there is no clear relation between percentage of spermatozoa with normal form and fertilization, cleavage, and percentage outcome of ICSI [25, 26].
Positive CMA3 staining has been shown to be increased in the sperm cells of the infertile patients [9]. In some studies, CMA3 fluorochrome has been used to evaluate the sperm quality and fertilization capacity. Sakkas et al. [27] found that men who had a high percentage of anomalies in their chromatin, i.e. >30% CMA3 fluorescence and >10% nicks, had more than double the number of unfertilized oocytes containing spermatozoa that had remained condensed. Bianchi et al. [2] and Lolis et al. [13] showed that a high percentage of CMA3 positivity is present in certain forms of male factor infertility and that such a test may be used to distinguish separate populations in morphologically normal spermatozoa. On the other hands, Oliva [3] found that the percentage of protamines in the fertile men was the same as that in infertile patients with normal seminal parameters, but it differed in the patients with abnormal seminal parameter. It seems likely that poor chromatin packaging may contribute to failure in the decongestion process in specific cases [15]. In our study, although all the semen parameters were normal according to WHO criteria in both groups, patients with spontaneous recurrent abortion had significantly higher percentage of abnormal morphology and lower percentage of progressive motility. Higher percentage of CMA3 and AB positive spermatozoa was observed in case group while all semen parameters were normal.
AB staining detects the presence of histones and, therefore, indirectly infers the presence of lower amounts of protamines in the sperm nucleus [28]. Some studies have reported a correlation between staining of spermatozoa with AB and decreased percentage of normal spermatozoa [28]. Franken et al. [16] showed that there was significantly higher percentage of AB positive spermatozoa among men with teratozoospermia when compared with normozoospermic (51% vs. 26%). Despite normal semen analysis in this study, the mean percentage of positive AB staining was 31.6% in the case group and 14.1% in the control group (p < 0.001).
Among the assays that have been developed to assess DNA damage, the most frequently used in published clinical studies is the Acridine orange staining, which measures the stability of sperm chromatin in acid media with acridine orange. The dye gives rise to green fluorescence when bound to intact DNA and red when bound to fragmented DNA. The proportion of sperm with fragmented DNA is determined by flow cytometric analysis. Using light microscopy for this test is one of the limitations of this study. In some studies, AO test has been used for evaluation of sperm quality. Angelopoulos et al. [29] believed that AO staining does not predict fertilization efficiency or pregnancy outcome in IVF cycles. Oppositely, Virant-Klun et al. [30] found that sperm single-stranded DNA, detected by AO staining, reduces fertilization and quality of ICSI-derived embryos. In our study the percentage of AO positive spermatozoa did not differ between patients and controls that indicates that sperm denaturation may not play a role in spontaneous recurrent abortion.
To the best of our knowledge, it is the first study using CMA3, AB and AO test to evaluate the male factor in couples suffering from spontaneous recurrent abortion. This study sheds light on the pathogenesis of spontaneous recurrent abortion. However, the results of this study indicate that low sperm chromatin quality is one of the common etiologies of spontaneous recurrent abortion. So assisted reproduction techniques including ICSI and IVF using CMA3 and AB negative spermatozoa could be a novel treatment option in these patients.
In conclusion, CMA3 and AB staining are useful tools for evaluating of the sperm’s chromatin quality in couples with spontaneous recurrent abortion, because we found that there is a higher percentage of CMA3 and AB positive spermatozoa between the patients and controls. This study also showed that abnormal sperm parameters including progressive motility and abnormal morphology are correlated with protamines deficiency detected by both CMA3 and AB staining.
Acknowledgments
The authors wish to thank all the couples who participated in this study.
Footnotes
The work was performed in
Infertility Research Center, Department of Obstetrics and Gynecology, Zeinabieh Hospital, Shiraz University of Medical Science, Shiraz, Iran.
Capsule
Evaluation of the sperm’s quality via CMA3 and AB staining is useful for assessment of male factor in couples with spontaneous recurrent abortion.
References
- 1.Iranpour FG, Nasr-Esfahani MH, Valojerdi MR, al-Taraihi TM. Chromomycin A3 staining as a useful tool for evaluation of male fertility. J Assist Reprod Genet. 2000;17(1):60–6. [DOI] [PMC free article] [PubMed]
- 2.Bianchi PG, Manicardi GC, Urner F, Campana A, Sakkas D. Chromatin packaging and morphology in ejaculated human spermatozoa: evidence of hidden anomalies in normal spermatozoa. Mol Hum Repord. 1996;2(3):139–44. [DOI] [PubMed]
- 3.Oliva R. Protamines and male infertility. Hum Reprod Update. 2006;12(4):417–35. [DOI] [PubMed]
- 4.Tejada RI, Mitchell JC, Norman A, Maric JJ, Fridman S. A test for the practical evaluation of male infertility by Acridine orange (AO) fluorescence. Fertil Steril. 1984;42:87–91. [DOI] [PubMed]
- 5.Scientific Advisory Committee of the Royal College of Obstetricians and Gynaecologists. The management of recurrent miscarriage. RCOG ‘Green-top’ Guideline No. 17, 2001.
- 6.Nanassy L, Carrell DT. Paternal effects on early embryogenesis. J Exp Clin Assist Reprod. 2008;5:2. [DOI] [PMC free article] [PubMed]
- 7.Puscheck EE, Jeyendran RS. The impact of male factor on recurrent pregnancy loss. Curr Opin Obstet Gynecol. 2007;19:222–8. [DOI] [PubMed]
- 8.Hill JA, Abbott AF, Politch JA. Sperm morphology and recurrent abortion. Fertil Steril. 1994;61:776–8. [PubMed]
- 9.Sbracia S, Cozza G, Grasso JA, Mastrone M, Scarpellini F. Semen parameters and sperm morphology in men in unexplained recurrent spontaneous abortion, before and during a 3 year follow-up period. Hum Reprod. 1996;11:117–20. [DOI] [PubMed]
- 10.Saxena P, Misro MM, Chaki SP, Chopra K, Roy S, Nandan D. Is abnormal sperm function an indicator among couples with recurrent pregnancy loss? Fertil Steril. 2008;90:1854–8. [DOI] [PubMed]
- 11.Liu DY, Baker HW. Sperm nuclear chromatin normality: relationship with sperm morphology, sperm-zona pellucida binding and fertilization rates in vitro. Fertil Steril. 1992;58(6):1178–84. [DOI] [PubMed]
- 12.Nasr-Esfahani MH, Salehi M, Razavi S, Anjomshoa M, Rozbahani S, Moulavi F, et al. Effect of sperm DNA damage and sperm protamine deficiency on fertilization and embryo development post-ICSI. Repord Biomed Online. 2005;11(2):198–205. [DOI] [PubMed]
- 13.Lolis D, Georgiou I, Syrrou M, Zikopoulos K, Konstantelli M, Messinis I. Chromomycin A3 staining as an indicator of protamine deficiency and fertilization. Int J Androl. 1996;19(1):23–7. [DOI] [PubMed]
- 14.World Health Organization. Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambrige: Cambridge University Press; 1999.
- 15.Kruger TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, van Zyl JA, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46:1118–23. [DOI] [PubMed]
- 16.Franken DR, Franken CJ, de la Guerre H, de Villiers A. Normal sperm morphology and chromatin packaging: comparison between aniline blue and chromomycin A3 staining. Andrologia 1999;31(6):361–6. [DOI] [PubMed]
- 17.Tejada RI, Mitchell JC, Norman A, Marik JJ, Friedman S. A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil Steril. 1984;42(1):87–91. [DOI] [PubMed]
- 18.De Vos A, Van De Velde H, Joris H, Verheyen G, Devroey P, Van Steirteghem A. Influence of individual sperm morphology on fertilization, embryo morphology and pregnancy outcome of intracytoplasmic sperm injection. Fertil Steril. 2003;79(1):42–8. [DOI] [PubMed]
- 19.Nagvenkar P, Zaveri K, Hinduja I. Comparison of the sperm aneuploidy rate in severe oligozoospermic and oligozoospermic men and its relation to intracytoplasmic sperm injection outcome. Fertil Steril. 2005;84(4):925–31. [DOI] [PubMed]
- 20.Esterhuizen AD, Franken DR, Lourens JGH, Prinsloo E, Van Rooyen LH. Sperm chromatin packaging as an indicator of in vitro fertilization rates. Hum Reprod. 2000;15:657–61. [DOI] [PubMed]
- 21.Coetzee K, Kruger TF, Lombard CJ. Predictive value of normal morphology a structured literature review. Hum Reprod Update. 1998;4(1):73–82. [DOI] [PubMed]
- 22.Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Perdictive value of abnormal sperm morphology in vitro fertilization. Fertil Steril. 1988;49:112–7. [DOI] [PubMed]
- 23.Hammadeh ME, Stieber M, Haidle G, Schmidt W. Assocciation between sperm cell chromatin condensation, morphology based on strict criteria and fertilization, cleavage and pregnancy rates in an IVF program. J Androl. 1998;30:29–35. [DOI] [PubMed]
- 24.Bartoov B, Elters F, Pansky M, Lederman H, Caspi E, Soffer Y. Estimating fertility potential via semen analysis data. Hum Reprod. 1993;8:65–70. [DOI] [PubMed]
- 25.Hammadeh ME, Al-Hassani S, Stieber M, Rosenbaum P, Kupker D, Diedrich K, et al. The effect of chromatin condensation (aniline blue staining and morphology “strict criteria”) of human spermatozoa fertilization, cleavage and pregnancy rates in an intracytoplasmic sperm injection program. Hum Reprod. 1996;11:2468–71. [DOI] [PubMed]
- 26.Hammadeh ME, Al-Hassani S, Doerr S, Stieber M, Rosenbaum P, Schmidt W, et al. Comparison between chromatin condensation and morphology from testis biopsy extracted and ejaculated spermatozoa and their relationship to ICSI outcome. Hum Reprod. 1999;14(2):363–7. [DOI] [PubMed]
- 27.Sakkas D, Urner F, Bizzaro D, Manicardi G, Bianchi PG, Shoukir Y, et al. Sperm nuclear DNA damage and altered chromatin structure: effect on fertilization and embryo development. Hum Repord. 1998;13(suppl 4):11–9. [DOI] [PubMed]
- 28.Hofmann N, Hilscher B. Use of aniline blue to assess chromatin condensation in morphologically normal spermatozoa in normal and infertile men. Hum Repord. 1991;6(7):979–82. [DOI] [PubMed]
- 29.Angelopoulos T, Moshel YA, Lu L, Macanas E, Grifo JA, Krey LC. Simultaneous assessment of sperm chromatin condensation and morphology before and after separation procedures: effect on the clinical outcome after in vitro fertilization. Fertil Steril. 1998;69(4):740–7. [DOI] [PubMed]
- 30.Evenson DP, Darzynkiewicz Z, Melamed MR. Relation of mammalian sperm chromatin heterogeneity to fertility. Science 1980;210(4474):1131–3. [DOI] [PubMed]