Abstract
Purpose
To determine the effect of pH fluctuations of culture media, and the role of co-culture systems on embryo development.
Methods
Mouse embryos were incubated in phosphate buffered solutions (PBSs) with different pH for various lengths of time. After 3 h incubation of embryos at various pH, the embryos were transferred into four media with human (HEF) and mouse (MEF) embryonic fibroblast cells, and without feeder cells; HTF and MEM-α. Developmental rate at day three (morula), four (expanded blastocyst) and five (hatching or hatched blastocyst) was evaluated.
Results
Developmental rate at day three, four and five decreased when the incubation time at pH 6.2 and 8 increased to 3 h and more. In addition, significantly less embryos incubated at pH 6.2 and 8 developed to hatching and hatched blastocysts compared with pH 7.35. Embryos incubated at pH 6.2, co-cultured with MEF or HEF showed a significant improvement (P < 0.05) at day three in HEF compared to HTF, and at day five in MEF compared to HTF. At pH 8, a significant improvement (P < 0.05) was observed at day five in HEF and MEF compared to MEM-α.
Conclusions
Mouse 2-cell embryos could tolerate minor pH fluctuations, but that major pH changes affect subsequent development. Besides, feeder cells could improve embryo development, especially when embryos are prone to rise or fall in pH.
Keywords: Preimplantation embryo, pH, Co-culture, Mouse, Development
Introduction
Fertilization and embryo development in vivo occur in appropriate conditions in mammalian reproductive tract. Whereas in practical methods used to achieve pregnancy in assisted reproductive technology (ART), embryos and oocytes are exposed to various amounts of physical environmental factors such as room temperature, visible light, O2 pressure, and pH fluctuations that may affect embryo development in vitro. During oocyte retrieval, examination and manipulation under the microscope and during embryo transfer, mammalian oocytes and embryos are subject to broad pH changes of the bicarbonate-buffered systems as a response to changes in the environmental CO2 [1, 2].
Most cell types are relatively permeable to H+ and are able to regulate their intracellular pH by mean of plasma membrane proteins, which transport H+ or bicarbonate across the membrane in response to perturbation of intracellular pH. Mouse preimplantation embryos at 2-cell stage do not appear to possess specific pH regulatory mechanisms for relieving acidosis [3]. Internal pH (pHi) is relatively maintained constant and is regulated without any effects by external pH in physiological ranges (7.20–7.80), but in attendance of weak acids such as lactate, an essential component of embryo culture media, pH of early embryo will be perturbed. In contrast, compacted embryos are capable of regulating pHi when challenged with an acid load [4]. In contrast to mouse embryos, hamster 1-cell embryos could not recover from an intracellular rise in pH, resulting in a reduced ability of embryos to develop to the morula/blastocyst stage in vitro [5]. However, alleviating from alkalosis or acidosis in pre-embryos has been shown to be related to the strain of mice as well [6]. Although some studies have addressed the effects of external pH on pHi of mouse [6], hamster [5], and human [7] embryos, the developmental competence of mouse embryos; as a model to imitate human embryo behavior, following exposure to various pH ranges, and the effect of incubation period has yet to be documented.
In spite of recent improvements in the composition of culture media and laboratory conditions [8], and even at highly controlled conditions, in vitro grown embryos are less potent than in vivo grown embryos at the same developmental stage [9]. Some strategies have been introduced to diminish the probable deleterious effects of environmental factors, including pH, on embryo development. The most ordinary methods are the improvement in culture components and conditions [10, 11], using sequential culture media [12], and conditioned media [13, 14], and often feeder cells [15, 16]. Many investigators suggest co-culture systems could improve embryo quality and in vitro cleavage rate efficiently [15, 17–19]. While, few studies have reported no improvement in the embryo quality and implantation rates following co-culture of embryos with somatic feeder cells [20, 21]. In addition, some studies have revealed the beneficial effects of feeder cells on embryo development is more apparent when preimplantation embryos encounter suboptimal conditions [22–24].
During in vitro preimplantation embryo culture and manipulations, embryos are prone to environmental factors such as pH fluctuations. Therefore, the present study was undertaken to investigate the effects of a wide range of pH changes on the developmental competence of 2-cell mouse embryos and to examine whether two embryonic fibroblast feeder layers of different species may improve in vitro culture conditions further. In the first experiment, we incubated 2-cell mouse embryos in various pHs for different length of times and the developmental rates were determined. In the second experiment, 2-cell embryos were incubated in various pHs, and then were cultured to the blastocyst stage in presence or absence of human and mouse embryonic fibroblasts. The efficacy of co-culture systems was determined by assessing developmental rate of embryos to the anticipated stages for 120 h.
Materials and methods
The present study was carried out in accordance with Kerman University of medical sciences ethics committee for working on animals. Cell culture dishes and centrifuge tubes were purchased from Falcon® and all the chemicals were purchased from Sigma-Aldrich Chemical Company (Saint Louis, MO, USA), unless otherwise stated.
Superovulation and embryos collection
National medical research institute (NMRI) mice; an outbred strain, were purchased from Razi institute (Karaj, Iran). Animals maintained in an animal room with 12/12 h light/dark cycle, 20 to 24°C temperature and free access to water and rodent chewing food. Six to 10-week-old female mice were superovulated with intraperitoneal injection of 10 IU pregnant mare’s serum gonadotropin (PMSG; Folligon, Intervet, Belgium) followed 48 h later by 10 IU human chorionic gonadotropin (hCG; Serono, Italy). The females were caged individually overnight with fertile male from the same strain. Females with a vaginal plug were considered pregnant and were sacrificed by cervical dislocation, 44–46 h post hCG administration. Uterine tube and the distal portion of uterine horn were excised and placed in drops of human tubal fluid [25] with 20 mM HEPES (hHTF) and 3 mg/ml BSA (fraction V; Roche, Germany). Two-cell stage embryos were flushed out with a 30-gauge needle attached to a syringe filled with pre-warmed hHTF. The embryos were pooled in a 100 μl drop of culture medium under light paraffin oil and morphologically normal embryos were used for the experiments after three washes in hHTF supplemented with 3 mg/ml BSA.
Preparation of feeder cells
Fibroblast cells were prepared in the laboratory as shown elsewhere [22]. Mouse embryonic fibroblast (MEF) was prepared from 13 to 14 day old mouse embryos. Briefly, embryos were removed from the uterine horn. The amniotic membrane, the placenta, the head, and the abdominal viscera were removed carefully in sterile conditions. Embryos were cut into fragments in PBS and digested with 0.5 g/l trypsin/0.2 g/l EDTA for 30 min. Cells were harvested at a density of 3 × 105 cells/ml in MEM-α supplemented with 10% FBS (Gibco® , USA), 100 IU/ml Penicilline G and 60 µg/ml streptomycin. Attached cells were sub-cultured after the cells being reached confluence of >90%. Cells at passages 2 were used either as feeder layer or cryopreserved for further use. The procedure for collecting human fetus was approved by the local ethics committee at Kerman University of Medical Sciences, Kerman, Iran .Human embryonic fibroblast (HEF) was prepared by the method described for MEF preparation [26], with some modifications, after a written consent was obtained from the parents for work on the aborted fetus. Briefly, the upper limb of a 13 week old aborted male fetus with normal karyotype was carefully dissected free of the skin. Underlying connective tissues were detached and digested with 0.1% hyaluronidase followed by 0.25% trypsin in Hank’s balanced salt solution (HBSS). The HEF cells were cultured at the same conditions as those of MEF cells and the cells at passage 2–7 were used for experiments. MEF and HEF cells were cultured in MEM-α supplemented with 10% FBS at a density of 2 × 105 cells/ml in 50 μl drops of medium overlaid with light paraffin oil, for 24 h. The medium was refreshed 24 h before co-culture was carried out.
Phosphate buffered solutions
Phosphate buffered solutions (PBS) with a pH of 6.2 to 8 were prepared by adding different concentrations of Na2HPO4 and KH2PO4 to one litter double distilled deionized water. NaCl (5.93 g/l), KCl (0.4 g/l) and D-glucose (0.5 g/l) were added to these solutions. The pH of PBSs was carefully measured by a pH meter (Hanna, Italy) with 0.01-degree accuracy and was adjusted to desired pH with the aim of 0.1 M HCl and 0.1 M NaOH if necessary. The Osmolarity was also adjusted between 280 and 290 mOsmol. PBSs were sterilized by a 0.22 µm Millipore filter units into sterile glass container and preserved refrigerated until use (maximum 3 months). To approximate the conditions to the mammalian embryo culture systems, PBSs were kept in a 37°C humidified incubator with 5.5% CO2 in air for 2 h and the pH was measured again and adjusted to desired pH: 6.2, 6.6, 7, 7.35, 7.6 and 8 if applicable.
Effect of incubation period on the cleavage rate of embryos
Four hundred and forty-eight morphologically normal 2-cell mouse embryos were randomly allocated in two treatment (pH 6.2 and 8), and one control group; hHTF. Embryos in different groups were incubated at pH 6.2, 8 and hHTF for 1 to 5 h with 1 h interval, and were then transferred into drops of HTF with 3 mg BSA under light mineral oil and incubated at 37°C, 5.5% CO2 in humidified air. developmental rates were assessed every 24 h for 120 h.
Effects of 3 h incubation in PBSs with various pH on embryo developmental rate
Incubation of 2-cell embryos for more than 3 h in PBSs with pH 6.2 and 8 resulted in more than 50% degenerated embryos at the end of culture period (Table 1). To assess the effects of incubation of embryos in various pH for 3 h, fifty µl drops of PBSs with various pH and hHTF (control) were put in a 60 × 10 mm culture dish and overlaid with 7 ml mineral oil. As CO2 is essential for embryo development [27], the culture dishes were incubated at 37○ C humidified environment with 5.5% CO2 in the air for 2 h. Incubation of PBSs in a CO2 incubator did not alter pH significantly; in a pilot study when PBSs were incubated at 5.5% CO2 in the air the pH raised about 0.02 degree after 5 h incubation. Two hundred and seventy-nine, 2-cell mouse embryos with normal morphology were rinsed three times in PBS; pH 7.2–7.3 containing 4 mg/ml BSA. The embryos were randomly divided between drops of PBSs with various pH and hHTF as control, and incubated for 3 h in the same conditions. Embryos in treatment and control groups were then transferred into drops of HTF supplemented with 4 mg/ml BSA; 5 to 7 embryos per drop, and incubated at 37°C, 5.5% CO2 in humidified air for 120 h.
Table 1.
Effect of incubation of 2-cell embryos in various conditions for different lengths of times on embryo developmental rates
| Incubation time (h) | Groups | No. of 2-cell embryos | Development (%) | ||
|---|---|---|---|---|---|
| M | E-Bl | H-Bl and Hd-Bl | |||
| 1 | hHTF | 32 | 28 (87) | 24 (75) | 21 (65) |
| pH 6.2 | 32 | 25 (78) | 20 (62) | 17 (53) | |
| pH 8 | 34 | 28 (82) | 21 (62) | 20 (59) | |
| 2 | hHTF | 30 | 28 (92) | 24 (80) | 20 (66) |
| pH 6.2 | 35 | 22 (63) | 17 (49) | 15 (42) | |
| pH 8 | 33 | 25 (76) | 18 (54) | 17 (51) | |
| 3 | hHTF | 30 | 25 (83) | 22 (73) | 19 (63) |
| pH 6.2 | 32 | 12 (37) | 10 (31) | 7 (22)a | |
| pH 8 | 32 | 15 (47) | 12 (37) | 9 (28) | |
| 4 | hHTF | 32 | 26 (81) | 23 (72) | 20 (62) |
| pH 6.2 | 30 | 10 (33)a | 8 (27)a | 5 (17)a | |
| pH 8 | 32 | 12 (37) | 11 (34) | 7 (22)a | |
| 5 | hHTF | 30 | 24 (80) | 19 (63) | 17 (57) |
| pH 6.2 | 33 | 11 (33)a | 4 (12)b | 1 (3.0)c | |
| pH 8 | 34 | 13 (39) | 11 (32) | 5 (17)a | |
Values are pooled from 3 independent experiments. M, morulae at day 3, E-Bl, expanded blastocyst at day 4, H-Bl, hatching blastocyst; Hd-Bl, hatched blastocyst at day 5. a, b, c: P < 0.05, P < 0.01, P < 0.001 (χ2 test) respectively, compared to hHTF (control) at the same developmental stage
Effect of various feeder cells on developmental rate of embryos following 3 h incubation in PBSs with various pH
To determine the possible effects of different fibroblast feeder cells on the development of embryos pre-treated for 3 h in PBSs with various pH (6.2, 6.6, 7, 7.35, 7.6 and 8), 1167 morphologically normal 2-cell mouse embryos were first incubated for 3 h in PBSs with various pH or hHTF as control, and then were randomly transferred into drops of MEF, HEF, MEM-α or HTF as control. All media were supplemented with 10% FBS.
Data collection and statistical analysis
Embryos in treatment and control groups were carefully assessed for any progress in developmental rate every 24 h at the same time for 120 h after embryo collection under an inverted microscope (Nikon TS100, Japan). Day 1 was the day at which 2-cell embryos were flushed from the uterine horns. Development to morula at day 3; expanded blastocyst at day 4 and hatching or hatched blastocyst at day 5 was recorded. Proportions of embryos developed to each stage in different experimental groups was statistically analyzed by χ2 test. A difference with P ≤ 0.05 was considered statistically significant.
Results
Embryo developmental rates following incubation of embryos in alkaline and acidic buffers for various length of time
Incubation of 2-cell embryos in alkaline (pH 8) and acidic pH (pH 6.2) resulted in a gradual decrease in developmental rate as incubation period increased from 1 to 5 h. A significant decrease was detected after 3 h incubation in acidic pH. While, only after 4 to 5 h incubation in alkaline pH, a significant (P < 0.05) decrease was detected in the rate of hatching and hatched blastocyst transformation. In addition, incubation of embryos in acidic pH decreased developmental rate significantly regarding the incubation period and the developmental stage (Table 1).
Embryo developmental rates following incubation of embryos in PBSs with various pH
Morphologically normal 2-cell mouse embryos were allocated randomly into experimental and control groups. Embryos were kept for 3 h in different PBSs; pH 6.2 to 8 with ∼0.4 interval and hHTF as control. The embryos were then cultured in HTF with 4 mg/ml BSA and evaluated for 120 h. Although developmental rates decreased from pH 7.35 towards pH 6.2 or 8, there was no significant difference among the groups on day 3, 4 and 5 except for pH 6.2 compared to PBS with pH 7.35 on day 3 and 5 (P < 0.05), (Table 2).
Table 2.
Developmental rate of embryos following 3 h incubation in PBSs with various pH, and HTF followed by culture for 120 h in appropriate conditions
| pH | No. of embryos | Development (%) | ||
|---|---|---|---|---|
| Morulae at day 3 | Expanded blastocyst at day 4 | Hatching and hatched blastocyst at day 5 | ||
| HTF (7.3) | 42 | 33 (78.6) | 30 (71.4) | 25 (59.5) |
| 6.2 | 36 | 15 (41.7)a | 18 (50) | 12 (33.3)a |
| 6.6 | 40 | 22 (55) | 26 (65) | 20 (50) |
| 7 | 40 | 31 (77.5) | 24 (60) | 20 (50) |
| 7.35 | 42 | 37 (88.1)b | 30 (71.4) | 31 (73.8)b |
| 7.6 | 39 | 27 (69.2) | 25 (64.1) | 20 (51.3) |
| 8 | 43 | 23 (53) | 21 (49) | 17 (39) |
Experiments were replicated seven times with at least five morphologically normal embryos in each group. In each column figures with different superscripts are statistically different; P < 0.05
Developmental rates of embryos after 3 h incubation in PBSs with various pH followed by co-culture with human and mouse embryonic fibroblasts
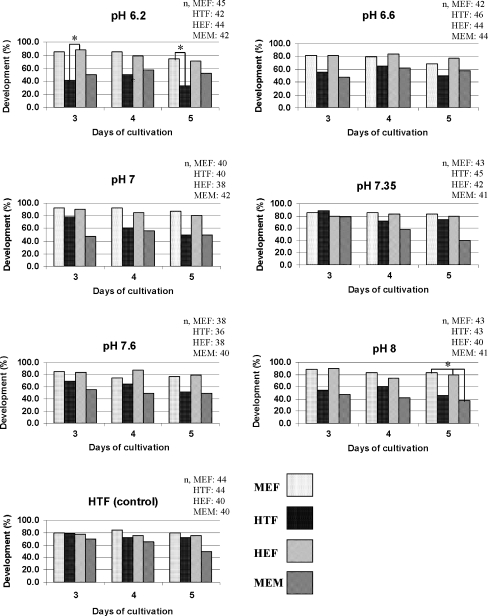
Morphologically normal 2-cell mouse embryos were incubated in PBSs with various pH or hHTF as control for 3 h and then were transferred randomly into four media comprise of MEF, HEF, MEM-α and HTF. Developmental rate of the co-cultured embryos was higher than non-co-cultured embryos in either pH studied, but there was only a significant increase in co-cultured embryos incubated at pH 6.2 and 8 as shown in Fig. 1. At pH 6.2, 37/44 (84%) embryos in HEF group developed to the morulae stage on day 3, that was significantly higher (P < 0.05) than HTF (control) group (17/42, 41%). In addition, in MEF group more embryos (34/45, 75%) developed to the hatching or hatched blastocyst stage on day 5 that was also significantly higher (P < 0.05) than HTF (14/42, 34%) group. At pH 8, development to the hatching or hatched blastocyst stage on day 5 was significantly higher (P < 0.05) in MEF (36/43, 83%) and HEF (32/40, 82%) groups compared to MEM-α group (16/41, 39%). The developmental rates were comparable between embryos having had cultured with mouse and human embryonic fibroblast feeder layers during all stages of development (Fig. 1).
Fig. 1.
Embryos were incubated for 3 h in PBSs with various pH and cultured in MEF, HEF, HTF and MEM-α for 120 h. Experiments were replicated 7 times. Day 3, 4 and 5 are days at which embryos cleaved to morulae, expanded blastocyst and hatching or hatched blastocyst respectively. Figures are showing developmental rates in each group. *P < 0.05
Discussion
In the present study, we have investigated the developmental rates of preimlantation mouse embryos when they were incubated in phosphate buffered solutions with alkaline (8) and acidic (6.2) pH for various lengths of time. Incubation of embryos both in acidic pH and in alkaline pH for 3 h and longer reduced developmental rates. Also when the embryos were incubated for 3 h in different phosphate buffered solutions with a pH range of 6.2 to 8. Incubation in acidic pH resulted in lower developmental rates compared to alkaline pH. It seems that minor pH fluctuations in the embryo culture media will not inhibit early mouse embryo development, especially when these changes are towards alkaline pH. pHi regulation is an important homeostatic function of living cells. Mammalian embryo development is disturbed when pHi drifts beyond a relatively small fluctuation [28]. External pH of culture media formulated for preimplantation embryos is commonly between 7.3 and 7.4, but as an exception, hamster embryos could develop from 2-cell to the blastocyst stage in a wide range of pHi; 7.38 to 7.09. However, a sharp reduction in developmental rate have been observed beyond these values [29]. Exposure of mouse embryos to acidic and alkaline pH for less than 3 h did not alter developmental rate significantly in any pHs studied. While an incubation time of up to 12 h in alkaline and acidic media of hamster embryos had no detrimental effects on transformation to blastocysts. Sensitivity of mouse 2-cell embryos to pH changes in contrast to hamster embryos has been shown previously [6]. Plasma membrane of 2-cell mouse embryo is highly permeable to H+. Thus, passive efflux of H+ continues until equilibration reestablishes. Major pHi–regulatory mechanisms in early embryos are: the HCO−3/Cl− exchanger ,which alleviates alkalosis, and the Na+/H− antiporter which recovers embryos from acidosis [6]. HCO−3/Cl− exchanger activity is present in all stages of preimplantation embryo in the hamster and mouse and is required for the maintenance of normal pHi and recovery from intracellular alkalosis [30]. While, there are controversies in the presence of a Na+/H− antiporter mechanism in 2-cell mouse embryos. Baltz et al. (1993) demonstrated that the 2-cell embryo did not employ conventional mechanisms common to somatic cells to recover from acid load but relied on the slow passive efflux of protons [3]. However, Gibb et al. (1995) reported that 2-cell mouse embryo did possess mechanisms that allowed the embryo to actively recover from an acid load [31]. In our study, the embryos were moderately affected by exposure to alkaline pH (Ph = 8), and highly affected by exposure to acidic pH (pH = 6.2). The ability to remove pHi perturbation appears to be related to how far the external pH is from standard pHi and small pH changes would be alleviated via pHi -regulatory mechanisms in mouse early embryos.
Baltz et al. (1993) have shown only compacted mouse embryos are capable of regulating pHi when challenged with an acid load [3]. Nevertheless, in our experiments moderate acid as well as alkaline pH fluctuations were well tolerated at 2-cell stage. Strain differences [6] may explain inconsistent results obtained in our study which requires further researches.
Some reports have suggested that co-culture of embryos with different somatic cells may rescue poor quality embryos even comparable to those present in vivo [15, 32]. We have transferred the embryos incubated in PBSs with various pH, into four media with feeder cells; MEF or HEF, and without feeder cells; MEM-α or HTF to investigate the impact of co-culture on embryo development after exposure to pH fluctuations. Both feeder cells improved nonsignificantly and significantly embryos developmental rates irrespective of pH range. By using feeder cells in in vitro embryo procedures, in vitro environments may near the in vivo conditions resulting in higher rates of development and implantation [33].
The ability of co-culture systems to improve developmental competence of preimplantation embryos is known to be through two putative mechanisms, so-called positive and negative conditioning. In the negative conditioning, embryos are protected against oxidative stress and deleterious components which are regularly generated during in vitro embryo cultivation [20, 34]. In the positive conditioning, embryo development is enhanced by the secretion of embryotrophic factors [35] such as leukemia inhibitory factor (LIF) [36, 37], fibroblast growth factor (FGF) [38], epidermal growth factor (EGF) [39], and insulin-like growth factor (IGF) into the culture medium [35]. Our study could not provide enough evidences which mechanism have been most probably involved in the improvement of the embryo development following co-culture of embryos with either mouse or human embryonic fibroblasts. Since we have exposed embryos to pH fluctuations, that is an example of suboptimal conditions [29], we may suggest human and mouse embryonic fibroblasts used in the present study have most likely neutralized the pernicious reactive oxygen species to which embryos are vulnerable during in vitro culture; an example of negative conditioning. In contrast to our suggestion, some authors have proposed; addition of embryotrophic substances which are usually secreted by the somatic cells into the simple culture media, may improve embryo quality without the need of feeder cells [33, 40]. However, recent studies have shown a direct contact between the embryo and feeder cells enhance embryo development better than the embryos cultured in presence of feeder cells without a direct contact [18]. Besides, this assumption may be true if the negative conditioning hypothesis was neglected.
We found a relatively identical developmental rate when embryos were co-cultured with fibroblasts obtained from mouse embryos compared to fibroblasts obtained from human embryo. Although fibroblast cells from fetal bovine uterine tube have been successfully used in human IVF procedures after initial screening for viral contaminations [41], the risk of contamination and transfection of embryos by genetic materials secreted by feeder cells remains a challenge in the utilization of feeder cells when the feeder cells are obtained from a foreign species [21, 42]. Human embryonic fibroblast cells propagate readily in the laboratory and their morphology remains undisturbed during passages (our unpublished observations). These cells could be an appropriate candidate for feeder cells obtained from a foreign species in human co-culture systems as well as animal studies. The use of serum in culture media have been reported to have some adverse effects on in vitro produced embryos. Serum was found to affect postimplantation viability of embryos and also resulted in lighter mouse fetuses compared to control [43]. While culture of ovine embryos in presence of serum resulted in heavier lambs [44], the consequence of co-culture with granulosa cells and supplementation of culture media with serum also led to large offspring syndrome (LOS) in ovine fetuses [45]. Although studies have addressed some adverse effects of serum and co-culture with granulose cells on preimplantation embryos, more studies are required to examine the probable adverse effects of serum supplementation and co-culture in in vitro blastocyst transformation and fetal development and health in other species including human. In addition, the appropriate cell density in co-culture systems, the culture media capable of supporting both feeder cells and embryos, and establishment of a serum-free media that could sufficiently support both feeder cell growth and embryo development remains to be investigated.
In conclusion, the data presented in this study demonstrate that mouse early embryos would tolerate minor pH fluctuations during in vitro development. However, adverse effects of major pH fluctuations in culture media could be alleviated by co-culture with either mouse or human embryonic fibroblasts.
Acknowledgments
Authors thank M. Kianinejad for his technical assistance. This study was supported by grant No.81–9 from Kerman University of medical sciences, Kerman, Iran.
Footnotes
Capsule
Mouse embryos were incubated in various pH solutions and co-cultured with embryonic fibroblasts. The developmental rate in co-cultured embryos was higher especially at acidic pH.
References
- 1.John DP, Kiessling AA. Improved pronuclear mouse embryo development over an extended pH range in Ham’s F-10 medium without protein. Fertil Steril. 1988;49:150–5. [DOI] [PubMed]
- 2.Loutradis D, Drakakis P, Kallianidis K, Sofikitis N, Kallipolitis G, Milingos S, et al. Biological factors in culture media affecting in vitro fertilization, preimplantation embryo development, and implantation. Ann N Y Acad Sci. 2000;900:325–35. [DOI] [PubMed]
- 3.Baltz JM, Biggers JD, Lechene C. A novel H+ permeability dominating intracellular pH in the early mouse embryo. Development 1993;118:1353–61. [DOI] [PubMed]
- 4.Edwards LJ, Williams DA, Gardner DK. Intracellular pH of the preimplantation mouse embryo: effects of extracellular pH and weak acids. Mol Reprod Dev. 1998;50:434–42. [DOI] [PubMed]
- 5.Lane M, Baltz JM, Bavister BD. Bicarbonate/chloride exchange regulates intracellular pH of embryos but not oocytes of the hamster. Biol Reprod. 1999;61:452–7. [DOI] [PubMed]
- 6.Steeves CL, Lane M, Bavister BD, Phillips KP, Baltz JM. Differences in intracellular pH regulation by Na(+)/H(+) antiporter among two-cell mouse embryos derived from females of different strains. Biol Reprod. 2001;65:14–22. [DOI] [PubMed]
- 7.Phillips KP, Leveille MC, Claman P, Baltz JM. Intracellular pH regulation in human preimplantation embryos. Hum Reprod. 2000;15:896–904. [DOI] [PubMed]
- 8.Fujiwara M, Takahashi K, Izuno M, Duan YR, Kazono M, Kimura F, et al. Effect of micro-environment maintenance on embryo culture after in-vitro fertilization: comparison of top-load mini incubator and conventional front-load incubator. J Assist Reprod Genet. 2007;24:5–9. [DOI] [PMC free article] [PubMed]
- 9.Baltz JM, Biggers JD, Lechene C. Two-cell stage mouse embryos appear to lack mechanisms for alleviating intracellular acid loads. J Biol Chem. 1991;266:6052–7. [PubMed]
- 10.Gao G, Yi J, Zhang M, Xiong J, Geng L, Mu C, et al. Effects of iron and copper in culture medium on bovine oocyte maturation, preimplantation embryo development, and apoptosis of blastocysts in vitro. J Reprod Dev. 2007;53:777–84. [DOI] [PubMed]
- 11.Babaei H, Nematallahi-Mahani SN, Kheradmand A. The effects of Vitamin A administration on the development of vitrified-warmed mouse blastocyst. Anim Reprod Sci. 2006;95:125–33. [DOI] [PubMed]
- 12.Menezo YJ, Hamamah S, Hazout A, Dale B. Time to switch from co-culture to sequential defined media for transfer at the blastocyst stage. Hum Reprod. 1998;13:2043–4. [DOI] [PubMed]
- 13.Eyestone WH, First NL. Co-culture of early cattle embryos to the blastocyst stage with oviducal tissue or in conditioned medium. J Reprod Fertil. 1989;85:715–20. [DOI] [PubMed]
- 14.Fujita T, Umeki H, Shimura H, Kugumiya K, Shiga K. Effect of group culture and embryo-culture conditioned medium on development of bovine embryos. J Reprod Dev. 2006;52:137–42. [DOI] [PubMed]
- 15.Orsi NM, Reischl JB. Mammalian embryo co-culture: trials and tribulations of a misunderstood method. Theriogenology 2007;67:441–58. [DOI] [PubMed]
- 16.Qian Y, Shi WQ, Ding JT, Liu JY, Sha JH, Fan BQ. Effects of type and state of co-culture cells on in-vitro development of porcine oocytes matured and fertilized in vitro. J Assist Reprod Genet. 2005;22:233–8. [DOI] [PMC free article] [PubMed]
- 17.Lane M, Gardner DK. Embryo culture medium: which is the best? Best Pract Res Clin Obstet Gynaecol. 2007;21:83–100. [DOI] [PubMed]
- 18.Omar Farouk FN, Vlad M. In vitro development of mouse pronuclear embryos to blastocysts in sequential media with and without co-culture of autologous cumulus cells. J Reprod Dev. 2008;54:385–90. [DOI] [PubMed]
- 19.Parikh FR, Nadkarni SG, Naik NJ, Naik DJ, Uttamchandani SA. Cumulus coculture and cumulus-aided embryo transfer increases pregnancy rates in patients undergoing in vitro fertilization. Fertil Steril. 2006;86:839–47. [DOI] [PubMed]
- 20.Bavister BD. Co-culture for embryo development: is it really necessary? Hum Reprod. 1992;7:1339–41. [DOI] [PubMed]
- 21.Kattal N, Cohen J, Barmat LI. Role of coculture in human in vitro fertilization: a meta-analysis. Fertil Steril. 2008;90:1069–76. [DOI] [PubMed]
- 22.Nematollahi-mahani SN, Pahang H, Moshkdanian G, Nematollahi-mahani A. Effect of embryonic fibroblast cell co-culture on development of mouse embryos following exposure to visible light. J Assist Reprod Genet. 2009;26:129–35. [DOI] [PMC free article] [PubMed]
- 23.Johnson JE, Higdon HL 3rd, Boone WR. Effect of human granulosa cell co-culture using standard culture media on the maturation and fertilization potential of immature human oocytes. Fertil Steril. 2008;90:1674–9. [DOI] [PubMed]
- 24.Saikhun J, Sriussadaporn S, Thongtip N, Pinyopummin A, Kitiyanant Y. Nuclear maturation and development of IVM/IVF canine embryos in synthetic oviductal fluid or in co-culture with buffalo rat liver cells. Theriogenology 2008;69:1104–10. [DOI] [PubMed]
- 25.Quinn P, Warnes GM, Kerin JF, Kirby C. Culture factors affecting the success rate of in vitro fertilization and embryo transfer. Ann N Y Acad Sci. 1985;442:195–204. [DOI] [PubMed]
- 26.Wobus AM, Holzhausen H, Jakel P, Schoneich J. Characterization of a pluripotent stem cell line derived from a mouse embryo. Exp Cell Res. 1984;152:212–9. [DOI] [PubMed]
- 27.Quinn P, Wales RG. Growth and metabolism of preimplantation mouse embryos cultured in phosphate-buffered medium. J Reprod Fertil. 1973;35:289–300. [DOI] [PubMed]
- 28.Lane M, Baltz JM, Bavister BD. Regulation of intracellular pH in hamster preimplantation embryos by the sodium hydrogen (Na+/H+) antiporter. Biol Reprod. 1998;59:1483–90. [DOI] [PubMed]
- 29.Squirrell JM, Lane M, Bavister BD. Altering intracellular pH disrupts development and cellular organization in preimplantation hamster embryos. Biol Reprod. 2001;64:1845–54. [DOI] [PMC free article] [PubMed]
- 30.Phillips KP, Baltz JM. Intracellular pH regulation by HCO3-/Cl- exchange is activated during early mouse zygote development. Dev Biol. 1999;208:392–405. [DOI] [PubMed]
- 31.Gibb CA, Poronnik P, Day ML, Cook DI. pH regulatory mechanisms in the 2-cell mouse embryo. Proc Aust Physiol Pharmacol Soc. 1994;25:123–9.
- 32.Kim YB, Ahn SH, Chang DY, Chung KN, Koh JW. Vero cell co-culture counteracts the detrimental effects of hydrosalpinx fluid on the development of mouse embryos in vitro. J Korean Med Sci. 2002;17:217–9. [DOI] [PMC free article] [PubMed]
- 33.Desai N, Abdelhafez F, Bedaiwy MA, Goldfarb J. Live births in poor prognosis IVF patients using a novel non-contact human endometrial co-culture system. Reprod Biomed Online. 2008;16:869–74. [DOI] [PubMed]
- 34.Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–89. [DOI] [PubMed]
- 35.Richter KS. The importance of growth factors for preimplantation embryo development and in-vitro culture. Curr Opin Obstet Gynecol. 2008;20:292–304. [DOI] [PubMed]
- 36.Kauma SW, Matt DW. Co-culture cells that express leukemia inhibitory factor (LIF) enhance mouse blastocyst development in vitro. J Assist Reprod Genet. 1995;12:153–6. [DOI] [PubMed]
- 37.Mishra S, Lei ZM, Rao CHV. A novel role of luteinizing hormone in the embryo development in co-cultures. Biol Reprod. 2003;68:1455–62. [DOI] [PubMed]
- 38.Lim JM, Hansel W. Role of growth factores in the development of bovine embryos fertilized in vitro and cultured singly in a defined medium. Reprod Fertil Dev. 1996;8:1199–205. [DOI] [PubMed]
- 39.Kurachi H, Morishige K, Imai T, Homma H, Masumoto N, Yoshimoto Y. Expression of epidermal growth factor and transforming growth factor-alpha in fallopian tube epithelium and their role in embryogenesis. Horm Res. 1994;41:48–54. [DOI] [PubMed]
- 40.Nagaoka M, Hagiwara Y, Takemura K, Murakami Y, Li J, Duncan SA, et al. Design of the artificial acellular feeder layer for the efficient propagation of mouse embryonic stem cells. J Biol Chem. 2008;283:26468–76. [DOI] [PMC free article] [PubMed]
- 41.Wiemer KE, Cohen J, Wiker SR, Malter HE, Wright G, Godke RA. Coculture of human zygotes on fetal bovine uterine fibroblasts: embryonic morphology and implantation. Fertil Steril. 1989;52:503–8. [DOI] [PubMed]
- 42.Aghaee-afshar M, Rezazadehkermani M, Asadi A, Malekpour-afshar R, Shahesmaeili A, Nematollahi-mahani SN. Potential of human umbilical cord matrix and rabbit bone marrow-derived mesenchymal stem cells in repairing of surgically incised rabbit external anal sphincter. Dis Colon Rectum. 2009;52:1753–61. [DOI] [PubMed]
- 43.Khosla S, Dean W, Brown D, Reik W, Feil R. Culture of preimplantation mouse embryos affects fetal development and the expression of imprinted genes. Biol Reprod. 2001;64:918–26. [DOI] [PubMed]
- 44.Thompson JG, Gardner DK, Pugh PA, McMillan WH, Tervit HR. Lamb birth weight is affected by culture system utilized during in vitro pre-elongation development of ovine embryos. Biol Reprod. 1995;53:1385–91. [DOI] [PubMed]
- 45.Sinclair KD, McEvoy TG, Maxfield EK, Maltin CA, Young LE, Wilmut I, et al. Aberrant fetal growth and development after in vitro culture of sheep zygotes. J Reprod Fertil. 1999;116:177–86. [DOI] [PubMed]