Abstract
Purpose
To gain insight into the morphology of the first polar body (1 PB) in ICSI patients and to explore whether it could predict mature oocyte viability and performance in this setting.
Methods
Seventy two consecutive women planned to perform ICSI treatment were prospectively recruited for this study. All oocytes retrieved underwent evaluation for nuclear maturity and accurate assessment of 1 PB morphology. MII oocytes were cultured in separate groups in each woman in accordance with two different categories of 1 PB morphology. Category A included normal intact round or ovoid 1 PB and category B included abnormal fragmented 1 PB. Each oocyte was followed throughout fertilization, embryo cleavage and embryo transfer. Cycles that reached embryo transfer, were divided into three groups in accordance with 1 PB morphology. Group I included only category A 1 PB embryos, group II included categories A and B 1 PB embryos, whereas group III included only category B 1 PB embryos.
Results
A total of 687 oocytes were aspirated and 553 MII oocytes underwent ICSI leading to 410 zygotes showing normal fertilization on day one. Three hundred ninety seven embryos cleaved on day two and a total of 176 embryos were replaced into the uterus. Clinical implantation and pregnancy rates were significantly correlated with the morphology of the 1 PB corresponding to 31%, 9% and 2% and 61%, 24% and 5%, in groups I, II and III respectively.
Conclusions
Our findings demonstrate that 1 PB morphology is related to mature oocyte viability and it has the potential to predict oocyte performance and pregnancy achievement in infertile women undergoing ICSI treatment.
Keywords: Apoptosis, First polar body, ICSI, Ovarian reserve
Introduction
It is generally accepted that breakdown of the germinal vesicle (GV) signifies resumption of meiosis, whereas the extrusion of the first polar body (1 PB) indicates completion of the first meiotic division in human oocytes. These developments are major checkpoints throughout the process of oogenesis that render the oocyte its competence in preparation for fertilization. This complex occurrence normally follows a highly coordinated and synchronized series of events that involves nuclear and cytoplasmic maturation within the oocyte coupled with adequate maturation of somatic cells contained by the follicle [1].
It has been assumed that 1 PB extrusion substantiates the correct progression of oocyte meiotic apparatus to metaphase II and therefore it may mark an important transition into the acquisition of meiotic competence. However, this hypothesis was not tested in the human in the early days of IVF-ET since oocyte morphology was not widely applied in embryo selection. Moreover, it has been shown that in some cases 1 PB extrusion is no definite marker of nuclear maturation since the oocyte could also be in the telo- or pro-metaphase I [2]. Following the introduction of ICSI and the requisite to remove the cumulus cells before fertilization, the studying of the oocyte morphology became easily accessible. As a result, several investigators have explored whether 1 PB morphology, may be an indicator, at least in part, of oocyte quality and viability.
Indeed, in the last several years the prognostic value of 1 PB morphology as an indicator for oocyte performance in an assisted reproduction setting was assessed alone or in combination with other criteria such as the periveltelline space and cytoplasmic appearance [3]. Several investigators have questioned whether normal development of the 1 PB morphology is correlated to fertilization rate [4–11], cleavage rate [4, 7, 10], embryo quality [4–13], blastocyst formation [8, 12] as well as implantation [5, 8, 10–13] and pregnancy rates [5, 6, 10, 13].
The studies available are distributed between pros and cons and as a result the assumption is still in debate. Part of these studies has shown a correlation between the 1 PB morphology and oocyte performance during ICSI treatment [4–6, 12] while others did not [8, 9, 11, 13]. Moreover, the grounds for this discrepancy between the studies are still not clear.
In a previous prospective study presented by our group we have shown that ICSI cycles that had at least one transferred embryo with normal morphology of 1 PB and two pronuclei (2PN) combined, including nucleolar precursor bodies, had better implantation and pregnancy rates than controls [3]. The question of which of these two early structures, the 1 PB or the 2PN contributes more to the embryo implantation potential, is of significant importance.
Therefore, we have performed this targeted prospective study to carefully investigate the morphology of the 1 PB in ICSI patients and to explore whether it could predict mature oocyte viability, performance and pregnancy achievement in this setting.
Materials and methods
We prospectively evaluated 72 consecutive and unselected infertile women throughout their ICSI cycle. Women were referred to ICSI treatment for male factor infertility. All women were regularly menstruating with both intact ovaries. They had normal uterine cavities determined by hysterosalpingography and/or hysteroscopy. The study was performed in a prospective manner in a university affiliated reproductive medicine unit. Embryologists in IVF laboratory, technicians in the endocrinology laboratory as well as ovarian sonographers were all blinded to the clinical data.
The study was exempt from institutional Review Board Approval since no additional interventions were performed to the standard and routine IVF treatment in our unit. Informed consent for IVF treatment was obtained by writing from each woman participating in the study.
We performed basal ovarian reserve studies to all women enrolled (as routinely performed in our unit), in a natural cycle 1 month before starting treatment and at least 3 months of no hormonal therapy. Basal endocrine studies included day 3 serum FSH and E2 levels in addition to FSH/LH ratio. Sonographic studies included day 3 ovarian volume employing a two-dimensional endo-vaginal probe with a frequency of 5–9 MHz (Voluson 730 expert, General Electric Medical System, Kretz Ultrasound, Austria). Ovarian volume was calculated as the volume of an ellipsoid, i.e. length × width × depth × π/6 in both ovaries.
We employed the long gonadotropin releasing hormone agonist (GnRH-a) protocol in all cycles. Down regulation was accomplished following IM administration of Decapeptyl CR 3.75 mg (Ferring, Malmo, Sweden) and was confirmed by serum E2 levels of ≤30 pg/mL and P ≤ 0.5 ng/mL. Super-ovulation was carried out with IM human menopausal gonadotropin (hMG) (Menogon, Ferring, Malmo, Sweden) 3 ampoules per day for the first 5 days. Menogon dosage was thereafter tailored in each patient, in accordance with transvaginal scanning (TVS) of follicular development and serum E2 levels. Human chorionic gonadotropin (hCG) (Pregnyl, NV Organon, Oss, The Netherlands) 10,000 IU, was administered when TVS showed ≥2 follicles with diameter of 18–20 mm and serum E2 level ≥400 pg/mL. Oocyte pick-up was performed 34–35 h following hCG administration. All women received luteal support employing vaginal micronized progesterone (Utrogestan, Besins International Laboratories, Paris, France) 800 mg/day.
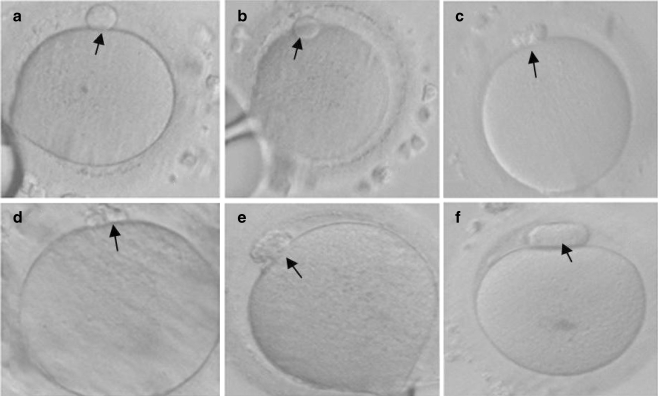
Following pick-up, the cumulus oocyte complexes were incubated for 3–4 h in P1 medium (Scientific Irvine, Santa Ana, CA) with 5% human serum albumin (Scientific Irvine, Santa Ana, CA) at 37°C in a 5% CO2 atmosphere. Nuclear maturity and accurate assessment of 1 PB development was evaluated following a 20 s exposure to hyaluronidase (20 IU/mL; Scientific Irvine, Santa Ana, CA) to facilitate mechanical removal of the cumulus cells. This was performed 38–39 h following hCG administration, i.e. 3–4 h following retrieval in all cycles. Oocytes were rotated so that both the side view and the top view of 1 PB were optimally observed. Normal and abnormal appearing 1 PB oocytes were defined in accordance with Ebner et al. [6]. Mature MII oocytes were cultured separately in each woman in accordance with the two different categories of 1 PB morphology. Category A included intact round or ovoid 1 PB and category B included fragmented or large (huge) 1 PB (Fig. 1).
Fig. 1.
MII oocytes following cumulus cell removal and before ICSI performance showing normal morphology of 1 PB (a, b), fragmented 1 PB (c, d, e) and large 1 PB (f)
Subsequently to 1 PB assessment, MII oocytes underwent the routine ICSI treatment. The ICSI procedure was carried out in accordance with Van Steirteghem et al. [14]. An inverted microscope with ×200 magnification (Olympus IX70, Tokyo, Japan) and Hoffman modulation contrast (Modulation Optics inc., Greenvale, NY, USA) were employed.
Each oocyte was followed throughout fertilization, embryo cleavage and transfer or embryo cryopreservation. All women that reached embryo transfer were divided into three groups in accordance with 1 PB morphology. Group I included only category A 1 PB embryos, group II included category A and B 1 PB embryos, whereas group III included only category B 1 PB embryos. Fertilzation and cleavages rates, embryo quality as well as clinical pregnancy and implantation rates were evaluated in correlation with the morphology of the 1 PB.
Embryo transfer during the study period was performed 48 h following oocyte retrieval. Embryo grading before transfer was performed in accordance with the classical criteria i.e. number of blastomeres, their homogeneity and the degree of anucleated fragments [15]. To properly evaluate embryo fragmentation a score of 1, 2 and 3 were given to embryos in accordance with their fragmentation degree of 0–20%, 21–50% and >50%, respectively.
Serum FSH and LH levels were analyzed by microparticle enzyme immunoassay (AxSYM®, Abbott, Abbott Park, IL, USA). The intra-assay and inter-assay coefficients of variation were <5% and <11%, respectively for FSH and <7% and <8%, respectively for LH. Serum E2 was assayed by solid-phase, competitive chemiluminescent enzyme immunoassay (Immulite 2000, DPC, Los Angeles, CA, USA). The intra-assay and inter-assay coefficients of variation were, <10% and <16%, respectively.
We analyzed all data using the Software Package for Social Sciences (SPSS) for windows version 15.0 (SPSS Inc. 2006, Chicago, IL, USA). Two-independent samples t-tests were used wherever appropriate. Moreover, linear logistic regressions were employed for each independent variable in order to evaluate significant factors that could predict clinical pregnancy rate and to adjust for possible confounders. All variables were tested for normal distribution prior to statistical analyses using Shapiro-Wilk test for normality. Significance was interpreted as P < 0.05. All data are presented as the mean ± SD.
Results
Six out of the 72 consecutive ICSI recruited cycles were excluded from the final evaluation since they did not reach ET. Three cases did not achieve normal fertilization or cleavage and the other three cycles were considered high risk to develop OHSS and therefore all of their embryos were cryopreserved. Noticeably, all cycles that did not achieve normal fertilization or cleavage had abnormal 1 PB morphology.
All other 66 cycles were eligible for the final evaluation. A total of 687 oocytes were retrieved and carefully studied in these cycles. No oocytes were frozen and therefore excluded from evaluation. Five hundred fifty-three mature MII oocytes underwent an ICSI procedure achieving 397 2PN zygotes on day one and 397 cleaved embryos on day two. These figures corresponded to a fertilization rate of 74% and a cleavage rate of 96%, respectively. One hundred seventy-six embryos were eventually transferred to the 66 women in the study achieving 13% and 28% clinical implantation and pregnancy rates, respectively.
The 66 women included in the final evaluation were subdivided into three groups in accordance with 1 PB morphology. Eighteen women were included in group I (only normal 1 PB embryos transferred), 30 in group II (normal and abnormal 1 PB embryos transferred) and 18 in group III (only abnormal 1 PB embryos transferred). Patients’ characteristics including age, BMI, infertility duration, etiology and order as well as previous IVF cycles were comparable between the three groups (Table 1).
Table 1.
Patients’ Characteristics in the three groups of the study
| Group I | Group II | Group III | P | |
|---|---|---|---|---|
| No. of cases | 18 | 30 | 18 | |
| Age (y) | 30.8 ± 4.7 | 31.9 ± 6.3 | 32.8 ± 7.3 | NS |
| BMI | 26.8 ± 6.6 | 24.1 ± 3.3 | 23.3 ± 3.1 | NS |
| Infertility duration (y) | 5.7 ± 3.9 | 5.4 ± 3.7 | 4.3 ± 2.7 | NS |
| Infertility order | ||||
| Primary (%) | 53% | 42% | 60% | NS |
| Secondary (%) | 47% | 58% | 40% | NS |
| Previous IVF cycles | 1.8 ± 0.9 | 1.7 ± 1.1 | 2.2 ± 1.4 | NS |
The fertilization rate, cleavage rate and embryo quality were similar in all three groups (Table 2). The number of transferred embryos to the uterus was comparable between the groups. However, women in group I achieved significantly better clinical implantation rate as opposed to group II and group III, corresponding to 31%, 9% and 2% respectively. Moreover, the clinical pregnancy rate was significantly higher in group I as related to group II and group III, corresponding to 61%, 24% and 5%, respectively. Interestingly, the total number of oocytes retrieved was different among the three groups; whereas there was a significant different between group I and III (P < 0.05) but not between group I and group II , corresponding to 11.9 ± 7.5, 9.6 ± 4.2 and 6.1 ± 3.9, for groups I, II and III, respectively (Table 2).
Table 2.
Fertilization rate, cleavage rate, embryo quality and pregnancy rate in the three groups of the study
| Group I | Group II | Group III | P | |
|---|---|---|---|---|
| No of cases | 18 | 30 | 18 | |
| No of oocytes | 11.9 ± 7.5a | 9.6 ± 4.2ab | 6.1 ± 3.9b | <0.04 |
| No of MII oocytes | 9.5 ± 5.5a | 8.0 ± 3.6a | 5.2 ± 3.7b | <0.01 |
| Fertilization rate | 0.82 ± 0.16 | 0.70 ± 0.19 | 0.85 ± 0.19 | NS |
| Cleavage rate | 0.98 ± 0.06 | 0.96 ± 0.11 | 0.97 ± 0.12 | NS |
| No. of embryos | 7.2 ± 4.2a | 5.6 ± 3.3a | 4.0 ± 2.9b | <0.04 |
| No. of transferred embryos | 2.5 ± 0.8 | 2.9 ± 0.7 | 2.5 ± 1.1 | NS |
| Embryo quality: | ||||
| No of blastomeres | 2.9 ± 1.0 | 3.4 ± 1.6 | 3.2 ± 1.4 | NS |
| Fragmentation | 1.4 ± 0.7 | 1.1 ± 0.3 | 1.2 ± 0.4 | NS |
| Clinical implantation rate | 31% (14/45)a | 9% (8/87)b | 2% (1/44)b | <0.001 |
| Clinical pregnancy rate | 61% (11/18)a | 24% (8/33)b | 5% (1/18)b | <0.001 |
Different superscripts a and b indicates significant statistical difference (P < 0.05, using Bonferroni multiple comparison test and Z-test for two proportions) whereas similar superscript indicates non-significance
Table 3 summarizes basal ovarian reserve parameters as well as maximal E2 level achieved and gonadotropin requirement in the three different groups of the study. Whereas some parameters showed better ovarian reserve in group I when compared to group III, others did not. Group I showed a lower basal FSH level as related to group II and group III. As well, maximal E2 level was higher in group I and in group II as related to group III. However, other basal ovarian reserve parameters, including day 3 E2 level, FSH/LH ratio and ovarian volume did not differ significantly between the groups. Moreover, gonadotropin requirement was similar in all three groups.
Table 3.
Basal ovarian reserve studies, maximal E2 serum level and gonadotropin requirement in the three groups of the study
| Group I | Group II | Group III | P | |
|---|---|---|---|---|
| FSH | 5.8 ± 2.4a | 7.7 ± 3.6b | 8.1 ± 4.1b | <0.03 |
| LH | 7.6 ± 6.4 | 6.1 ± 3.6 | 5.4 ± 1.8 | NS |
| E2 pg/mL | 24 ± 22 | 30 ± 31 | 36 ± 37 | NS |
| FSH/LH ratio | 0.9 ± 0.5 | 1.3 ± 0.6 | 1.6 ± 0.4 | NS |
| Ovarian volume (cm3) | 15.8 ± 10.4 | 15.3 ± 11.4 | 10.2 ± 3.8 | NS |
| Maximal E2 (pg/mL) | 1868 ± 788a | 2263 ± 1206a | 1335 ± 609b | <0.004 |
| Number of ampoules | 42.8 ± 17.5 | 50.4 ± 19.0 | 48.9 ± 13.0 | NS |
Different superscripts a and b indicates significant statistical difference (P < 0.05, using Bonferroni multiple comparison test and Z-test for two proportions) whereas similar superscript indicates non-significance
In-order to examine whether the association between 1 PB morphology and clinical pregnancy rate was confounded by the ovarian reserve of the women in the study, a logistic regression analysis was performed. The independent variables employed into the logistic model included age, infertility duration, day 3 FSH, maximal E2 leve1, gonadotropin amount employed for stimulation, number of oocytes retrieved, number of MII oocytes, embryos achieved and 1 PB morphology. Indeed, the logistic regression analysis controlling for ovarian reserve has shown that only 1 PB morphology significantly explained the clinical pregnancy rate (P < 0.05). All other independent factors examined did not affect clinical pregnancy rate (Table 4).
Table 4.
Logistic regression analysis results examining age, ovarian reserve parameters and 1 PB morphology and their association to clinical pregnancy rate in the study (66 women)a
| Independent variable | B | Wald | Exp (B) |
|---|---|---|---|
| Age | 0.009 | 0.013 | 1.009 |
| Infertility duration | −0.195 | 2.660 | 0.823 |
| Day 3 FSH | 0.052 | 0.111 | 1.054 |
| Maximal E2 level | 0.001 | 1.791 | 1.001 |
| Gonadotropin requirement | 0.009 | 0.110 | 1.009 |
| Number of retrieved oocytes | −0.240 | 1.083 | 0.787 |
| Number of MII oocytes | 0.439 | 1.494 | 1.551 |
| Number of embryos | −0.348 | 1.552 | 0.706 |
| First polar body morphology* | 1.974 | 5.821 | 7.196 |
aB values are the values that we need to replace in the equation to establish the probability that a case (women) falls into a certain category. Wald statistics tells us whether the B coefficient for that predictor is significantly different from zero
Exp (B)—the odds ratio, is the predicted change in odds for a unit increase in the predictor. The “exp” refers to the exponential value of B
*P < 0.05
Discussion
Our results clearly demonstrate that there is correlation between 1 PB morphology oocyte performance and viability in women undergoing ICSI treatment due to male factor infertility. Although 1 PB morphology did not correlate with fertilization rate, cleavage rate and embryo quality in our study, it was closely associated with clinical implantation and pregnancy rates. Women who received only normal 1 PB embryos has significantly better implantation and pregnancy rates in relation to women that received combined normal and abnormal 1 PB embryos and women that received only abnormal 1 PB embryos. Our finding was further supported by the logistic regression model performed controlling for age and the ovarian reserve of women recruited to the study.
We believe that the better implantation and pregnancy rates achieved following normal 1 PB morphology embryos as opposed to abnormal 1 PB embryos transfer could be attributed to two main explanations. The first related to the natural apoptotic process of the first polar body and the second associated with the chromosomal integrity of the oocyte.
It is well recognized today that after the extrusion of the 1 PB, a programmed cell death of this structure follows by apoptosis [16], leading eventually to its disintegration. This process is usually completed by approximately 20 h following 1 PB extrusion [17]. Our results also show that women in group I with normal 1 PB morphology transferred embryos may have a better ovarian reserve as related to the other groups. Women with low ovarian reserve have a higher proportion of post-mature or dysmature oocytes [18]. Therefore, it could be speculated that the apoptotic process in women with low ovarian reserve is already taking place at least in some of the oocytes at the time of retrieval or denudation. Therefore, this could lead to an early degradation or fragmentation of the 1 PB in these oocytes. The significantly better day 3 FSH and maximal E2 levels in group I as compared to group III may support our speculation.
A second explanation for the high implantation and pregnancy rate in women with normal 1 PB embryos may well be suggested. It has been estimated that abnormal morphology of the 1 PB in MII oocytes could be attributed to chromosomal aneuploidy of the oocyte [1]. As 1 PB extrusion is directly related to spindle formation and sister chromatid exchange, it could be the most likely point of non-disjunction or aneuploidy formation. Therefore, it is suggested that abnormal 1 PB size and appearance can indicate its chromosomal integrity. As a result, it could be speculated that women with normal morphology of 1 PB has a better chromosomal status of their embryos and therefore have a better implantation and pregnancy rates.
To the best of our knowledge only one study has examined the actual relation between 1 PB morphology and chromosomal aneuploidy [8]. In this study aneupoidy testing was performed in 395 embryos obtained from 49 patients (50 cycles). However, no relationship was found between 1 PB morphology and chromosomal aneuploidy. Nevertheless, it should be noted that in this report chromosomal status of the 1 PB was performed in only a selected part 50/141 of cycles in the study. Only women that requested pre-implantation genetic diagnosis (PGD) were tested for 1 PB aneuploidy. The indications for PGD were not pointed out in the study. As it is well known, infertile women that request PGD have usually specific medical needs. Moreover, this study originated from a referral center that performs PGD for several other ART units. It is possible that the oocytes in the study were collected from different ART units. Each of these ART units may have a different ICSI protocol and a diverse practice concerning the timing of hCG administration as well as timing of oocyte denudation from cumulus cells following retrieval and ICSI performance. These alterations could influence the morphology of the 1 PB and its integrity and therefore could interfere with the results of the study. Indeed, Ciotti et al. has found different fragmentation rates of the 1 PB, depending on the time elapsing between retrieval, denudation and ICSI performance [13]. Further studies are therefore encouraged to explore the association between 1 PB morphology and aneuploidy.
The lack of correlation between 1 PB morphology and fertilization rate, cleavage rate and embryo quality found in our study is in accordance of previously published studies [5, 7–9, 12, 13]. However, the correlation between 1 PB morphology and implantation and pregnancy rate found in our study is a controversial issue in the literature. Only few published reports have examined the association between 1 PB morphology, implantation rate [5, 6, 8, 11, 13] and pregnancy rate [5, 12, 13]. Our results are in accordance with some of the previously published reports [5, 6] however, they contradict other reports [8–11, 13]. Several explanations could be suggested to clarify this discrepancy. It is our belief that patient selection and methodology of the studies performed seems to be the major reason of this inconsistency.
Part of these studies [4, 6, 7, 10, 13] was performed in a retrospective manner. Moreover, there is a large discrepancy in the literature concerning the timing of 1 PB morphology evaluation ranging from 2 to 11 h following retrieval [4–13]. As has already has been explained this might have a crucial effect on 1 PB morphology. As well, some studies [8, 13] did not include cases with large polar body into consideration. A large 1 PB would indicate a problem with check point genes and mitogen activated protein Kinase, cMOS and involving first meiotic spindle establishment [19]. In addition, few of these studies included only “good quality” infertile women [11, 13] while others defined poor responders as those having below 10 oocytes per retrieval [8]. As well, few studies estimated only a selected number of oocytes from each retrieved infertile woman [9, 13]. Other oocytes were discarded or cryopreserved. This was performed in accordance with the Italian law that allowed a certain number of oocytes to be injected or fertilized. Therefore, many MII oocytes were excluded from ICSI and therefore 1 PB evaluation and further follow-up was unfeasible. The inclusion or exclusion criteria of oocyte selection were not documented in these studies.
Taken together, it seems that considerable methodological variations are encountered when reviewing the relevant published studies. These variations could affect the accumulated data and conclusions of each study and may cause a bias when comparing between various studies. Therefore, these methodological variations may contribute largely to the discrepancy found in the literature.
Our study was performed in a prospective manner, consecutive and unselected infertile women were recruited and all their retrieved oocytes were included in the study. All women received the same super-ovulation protocol and the timing of hCG administration, cumulus cell denudation and ICSI performance was similar in all patients. Women with low ovarian reserve were not excluded from the study. Moreover, no oocytes were discarded or cryopreserved.
Conclusions
First polar body morphology is related to mature oocyte viability and it could predict clinical pregnancy rate in infertile women undergoing ICSI treatment. It is our belief that 1 PB abnormal morphology is related to the normal apoptotic process of the 1 PB that may be more evident in low ovarian reserve women. The association between abnormal 1 PB morphology and chromosomal aneuploidy is encouraged to be further investigated.
Footnotes
Capsule
First polar body morphology is related to mature oocyte viability and it has the potential to predict clinical pregnancy rate in infertile women undergoing ICSI treatment.
References
- 1.Scott L. The biological basis of non-invasive strategies for selection of human oocytes and embryos. Hum Reprod Update. 2003;9:237–49. [DOI] [PubMed]
- 2.Eichenlaub-Ritter U, Schmiady H, Kenetenich H, Soewarto D. Recurrent failure in polar body formation and premature chromosome condensation in oocytes from a human patient: indicators of asynchrony in nuclear and cytoplasmic maturation. Hum Reprod. 1995;10:2343–9. [DOI] [PubMed]
- 3.Younis JS, Radin O, Mirsky N, Izhaki I, Majara T, Bar-ami S, et al. First polar body and nucleolar precursor bodies morphology is related to the ovarian reserve of infertile women. Reprod Biomed Online. 2008;16:851–8. [DOI] [PubMed]
- 4.Xia P. Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod. 1997;12:1750–5. [DOI] [PubMed]
- 5.Ebner T, Moser M, Yaman C, Feichtinger O, Hartl J, Tews G. Elective transfer of embryos selected on the basis of first polar body morphology is associated with increased rates of implantation and pregnancy. Fertil Steril. 1999;72:599–603. [DOI] [PubMed]
- 6.Ebner T, Yaman C, Moser M, Sommergruber M, Feichtinger O, Tews G. Prognostic value of first polar body morphology on fertilization rate and embryo quality in intracytoplasmic sperm injection. Hum Reprod. 2000;15:427–30. [DOI] [PubMed]
- 7.Mikkelsen AL, Lindenberg S. Morphology of in-vitro matured oocytes: impact on fertility potential and embryo quality. Hum Reprod. 2001;16:1714–8. [DOI] [PubMed]
- 8.Verlinsky Y, Lerner S, Illkevitch N, Kuznetsov V, Kuznetsov I, Cieslak J, et al. Is there any predictive value of first polar body morphology for embryo genotype or developmental potential? Reprod Biomed Online. 2003;7:336–41. [DOI] [PubMed]
- 9.De Santis L, Cino I, Rabellotti E, Calzi F, Persico P, Borini A, et al. Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod Biomed Online. 2005;11:36–42. [DOI] [PubMed]
- 10.Chamayou S, Ragolia C, Alecci C, Storaci G, Maglia E, Russo E, et al. Meiotic spindle presence and oocyte morphology do not predict clinical ICSI outcomes: a study of 967 transferred embryos. Reprod Biomed Online. 2006;13:661–7. [DOI] [PubMed]
- 11.Scott L, Finn A, O’Leary T, McLellan S, Hill J. Morphologic parameters of early cleavage-stage embryos that correlate with fetal development and delivery: prospective and applied data for increased pregnancy rates. Hum Reprod. 2007;22:230–40. [DOI] [PubMed]
- 12.Ebner T, Moser M, Sommergruber M, Yaman C, Pfleger U, Tews G. First polar body morphology and blastocyst formation rate in ICSI patients. Hum Reprod. 2002;17:2415–8. [DOI] [PubMed]
- 13.Ciotti PM, Notarangelo L, Morselli-Labate AM, Felleti V, Porcu E, Venturoli S. First polar body morphology before ICSI is not related to embryo quality or pregnancy rate. Hum Reprod. 2004;19:2334–9. [DOI] [PubMed]
- 14.Van Steirteghem AC, Nagy Z, Joris H, Liu J, Staessen C, Smitz J, et al. High fertilization and implantation rates after intracytoplasmic sperm injection. Hum Reprod. 1993;8:1061–6. [DOI] [PubMed]
- 15.Veeck LL. Atlas of the human oocyte and early conceptus, volume 2. Baltimore: Williams & Wilkins; 1991. p. 121–246.
- 16.Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, et al. The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proc Natl Acad Sci USA. 1996;93:7032–5. [DOI] [PMC free article] [PubMed]
- 17.Ortiz M, Lucero P, Coxatto H. Post ovulatory aging of human ova: spontaneous division of the first polar body. Gamete Res. 1983;7:269–76. [DOI]
- 18.Morita Y, Tilly JL. Oocyte apoptosis: like sand through an hourglass. Dev Biol. 1999;213:1–17. [DOI] [PubMed]
- 19.Verlhac MH, Lefebvre C, Kubiak JZ, Umbhaur M, Rassinier P, Maro CW, et al. Mos activates MAP kinase in mouse oocytes through two opposite pathways. EMBOJ. 2000;19:6065–74. [DOI] [PMC free article] [PubMed]