Abstract
Purpose
Cryopreservation of blastocysts, especially those subjected to the trauma due to blastomere biopsy for the purposes of pre-implantation genetic screening (PGS), requires significant optimization. Laboratory and clinical outcomes were compared to determine the effect of two different cryopreservation techniques on the development of human pre-implantation embryos that underwent blastomere biopsy and blastocoel drainage prior to cryopreservation.
Design
Retrospective clinical study.
Patient(s)
Women who requested cryotransfer of supernumerary blastocysts were analyzed by FISH.
Results
The main outcome measures were post-thaw survival (SR), pregnancy (PR), and implantation (IR). The SR of slowly frozen blastocysts was 83% compared to 97% for vitrified blastocysts. In 160 cases where biopsied embryos were cryotransferred, the results for slowly frozen versus vitrified blastocysts were: SR (71% vs. 95%), PR (23% vs. 37%), and IR (26% vs. 36%, P < 0.05), respectively.
Conclusion
The results revealed that vitrified blastocysts provided higher SR, PR and IR as compared to slowly frozen counterparts.
Keywords: Conventional freezing, Vitrification, Human embryo biopsy, Implantation rate (IR), Pregnancy rate (PR)
Introduction
The high rate of multiple gestations resulting from IVF has complications with severe financial and obstetrical ramifications [1]. It has been well established that both implantation and pregnancy rates can be improved by selectively transferring only those embryos that have developed into blastocysts stage 5–6 days post-fertilization in vitro [2–7]. We recently reported that >90% of embryos that fail to develop into blastocysts are in fact aneuploid [8]. Furthermore, even in young women, at least 60% of embryos on day 3 are aneuploid; therefore, blastocyst transfer could improve IVF outcome by eliminating some karyotypically abnormal embryos [8, 9].
Numerical chromosomal aberrations, particularly aneuploidies (i.e. the most common form of karyotypic abnormalities affecting human embryos) are detrimental to early embryonic, fetal, and prenatal development. Exclusion of aneuploid embryos from being transferred has been demonstrated to improve implantation and pregnancy rates, and thus to reduce the incidence of aneuploidy-related miscarriages and birth defects, especially in women who had repeated IVF failure [10, 11]. Mastenbroek et al. [12] postulated in their multicenter randomized control trial that PGS did not increase but significantly reduced the rates of ongoing pregnancies and live births after IVF in women of advanced maternal age. Verpoest et al [13] demonstrated in 1498 couples enrolled prospective cohort study that age has a significantly negative effect on outcome of PGD, due to poor reproductive performance of female partners 40 years of age and older. It has been demonstrated that the effect of PGS was highly dependent on appropriate methods and patient selection [14, 15]. In addition, PGS using FISH has inherited limitations to screen up to 12 chromosomes out of 23. PGS became one of the routine procedures during last decade, but it should be considered a diagnostic procedure. Consequently, patients could benefit from detailed assessment of aneuploidy risk and avoid transfer of aneuploid embryos [16]. With the advance of PGS techniques such as comparative genomic hybridization (CGH), new culture media and protocols, more blastocysts are available for embryo transfer or cryopreservation after PGS. Therefore, there is more than ever a need for safe and effective method by which to cryobank human embryos.
Embryos derived from various species have been successfully cryopreserved by one of the following two procedures: 1) equilibrium freezing, often referred to as “slow freezing” [17, 18]; 2) non-equilibrium freezing, often referred to as “ultra-rapid cooling” or vitrification [19]. Slow freezing was first used successfully for the cryopreservation of mouse embryos and has been subsequently applied to embryos from other species including human [20]. A major change occurred in the field of cryobiology in 1985, a novel cryopreservation method for oocytes and embryos was introduced for mammalian species including human. This procedure simply exposes the embryos to high concentrations of cryoprotective agents (CPA) in combination with direct plunging into liquid nitrogen (LN2) in order to vitrify embryos, and substantially avoiding lethal intracellular ice crystal formation [21]. A growing number of studies have supported the hypothesis that vitrification has significant potential to become the method of choice for embryo cryopreservation due to its simplicity and its association with better cryosurvival and pregnancy rates [22–24]. Although cryopreservation procedure introduces multiple potential insults on embryonic cells such as CPA exposure, anisosmotic stress, hypothermic stress and freezing, long term effects of the vitrification procedure on human embryos are yet to be determined.
Here we report 5 years retrospective data regarding post-thaw viability and corresponding pregnancy and implantation rates of human blastocysts after slow freezing or vitrification. Technical and clinical results from cryotransfers encouraged us to analyze the clinical contribution of vitrification to our PGD program and the data was compared to slow frozen counterparts. Since blastomere biopsy procedure could distress viability of embryos, we included non-biopsied control treatments for both groups.
Materials and methods
Institutional review board (IRB) approval was obtained for this retrospective research (RMIRB #102709), and all clinical research conducted was in full compliance with the guidelines of the American Society of Reproductive Medicine and met ethical principles involving human subjects as defined by the Declaration of Helsinki in 1964.
All women who were included in this retrospective study between 2003–2008 had similar baseline clinical parameters (patient age, day 3 FSH level, and cycle number); IVF cycle parameters (type of protocol, daily dose of gonadotropin, number of oocytes retrieved, number of mature oocytes, fertilization rate, embryo quality, and number of embryos transferred. All recipients had a medical indication for ART, a normal uterine cavity, an endometrial thickness ≥9.0 mm on the day of hCG. Four hundred and nine (409) women requested transfer of their supernumerary cryopreserved embryos. The blastocysts of 160 of these women had undergone PGS performed as blastomere biopsy for FISH. In 249 cases no biopsy was performed. The mean ages of the PGS group and those that did not have PGS were 37.2 ± 2.2 years and 36.2 ± 3.8 years, respectively.
Indications for PGS were as follows: history of recurrent pregnancy loss (n = 76), repeated implantation failure with IVF (n = 53), advanced maternal age (n = 74), or polycystic ovarian syndrome (n = 17; PCO is not an industry standard for PGS screening). Patient characteristics are summarized in Table 1.
Table 1.
Demographics and a comparison of outcomes of slowly frozen and vitrified blastocysts that had undergone blastomere biopsy at day 3
| Slow freezing | Vitrification | |||
|---|---|---|---|---|
| Non- biopsied | pBiopsied | Non- biopsied | Biopsied | |
| Age (± SD) | 36.5 ± 3.4 | 37.8 ± 2.4 | 35.6 ± 3.2 | 37.2 ± 2.2 |
| Prior IVF/ICSI attempts | 3.5 | 3.4 | 2.8 | 3.6 |
| Number of initiated FET cycles | 120 | 85 | 129 | 75 |
| Number of FET cycles cancelled (%) | 10 (8) | 4 (5) | 4 (3) | 3 (4) |
| Blastocysts thawed/warmed | 335 | 165 | 340 | 127 |
| Blastocysts survived (%) | 278 (83) a | 115 (71) b | 330 (97) c | 120 (95) c |
| Blastocysts transferred | 278 | 146 | 330 | 99 |
| Blastocysts transferred/ET (± SD) | 2.2 ± 0.8 | 1.9 ± 0.4 | 2.1 ± 0.3 | 1.4 ± 0.4 |
| oPregnancy rate (%) | 41 (34) h | 20 (23) i | 49 (38) h | 28 (37) h |
All blastocysts were undergone blastocoel puncture
a–mDifferent superscripts in the column denotes statistical difference at p < 0.05
oPregnancy rate is defined as cardiac activity following ultrasound pelvic evaluation at 12 weeks
pPGD for Day 3 embryos were done for 9 chromosomes (13, 15, 16, 17, 18, 21, 22, X, Y)
Blastocyst production
Women were treated with GnRH agonist (GnRHa) and gonadotropins using a long treatment protocol. The injection of hCG was given when at least 2 dominant follicles reached a diameter of 18 mm. Oocytes were collected 36 h after hCG administration using vaginal ultrasound-guided needle aspiration. All MII oocytes underwent ICSI at 4 h post-retrieval and were individually cultured in Global One medium (IVF Online, Guelph, ON) containing 10% (vol/vol) synthetic serum supplement (SSS-Irvine Scientific, Irvine, CA) at 37°C in an atmosphere of 6% CO2, 5% O2, and 89% N2. All normally fertilized oocytes were cultured to day 5–6 post-ICSI in Global One medium. Blastocysts were graded using the same developmental grading classification previously reported by Keskintepe et al [25]. Briefly, blastocysts were classified as cavitated, expanded, and hatching or hatched. Only those blastocysts with well-defined inner cell masses and trophectoderms (grade I or II) were deemed eligible for fresh ET or for cryopreservation.
Blastomere biopsy
Blastomere (BL) biopsies for PGS/FISH on day-3 were carried out as previously described [9]. After removal of a single BL, embryos were individually cultured in Global One (IVF Online, Guelph, ON) medium until they reach to the blastocyst stage.
Pre-implantation genetic screening by FISH
All structural abnormalities (translocation) were excluded from this study and FISH-PGS for 9 chromosomes were performed by a reference laboratory (Reprogenetics, Livingston NJ and ReproCure LLC, Las Vegas, NV).
Blastocyst vitrification, slow freezing and thawing procedures
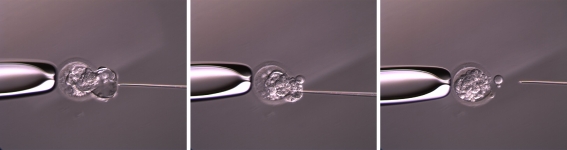
All blastocysts had their blastocoels artificially collapsed through assertive needle aspiration prior to vitrification using a modification of the method previously described by Mukaida et al. [23]. The modified technique which involved the use of an ICSI needle was as follows: About 10 min prior to vitrification, expanded blastocysts were placed in 50-µl drops of mHTF (IVF Online, Guelph, ON) with 10% SSS, vol/vol (Irvine Scientific, Irvine, CA). The blastocyst was transfixed with the ICM in the 6 or 12 o’clock position using a holding pipette connected to a micromanipulator. A 30 degree ICSI needle was introduced via the cellular junction of the trophectoderm into the blastocoel cavity and the fluid was aspirated until the blastocyst had completely collapsed (Fig. 1), then they were cryopreserved with cryoloop vitrification method previously described [22, 23]. Briefly, blastocysts were placed in 0.5 mL of modified HTF [mHTF (Base solution.)] supplemented with 10% (vol/vol) SSS for 2 minutes at 37°C. Then, the blastocysts were placed in base solution + 0.96 mol/L DMSO (D 5879, Sigma, St. Louis, MO) + 1.2 mol/L Ethylene Glycol (EG; P 3265, Sigma) for 90 s. Blastocysts were placed in base solution. + 1.9 mol/L DMSO + 2.4 mol/L EG + 1 mol/L Sucrose (S 7903, Sigma) + 0.1 mol/L Ficoll (F 8636, Sigma) for 30 s. Finally, each blastocyst was individually placed on a cryoloop (Hampton Research, Aliso Viejo, CA) with 1 µL of cryosolution and plunged into liquid nitrogen. For thawing of vitrified blastocyst, the cryoloop was removed from its vial and immersed into base solution + 0.34 mol/L sucrose for 2 min at 37°C. The embryo was then moved to base solution + 0.17 mol/L sucrose for 3 min, and placed in base sol for 5 min. Intact embryos were kept in 50 µL of Global One medium at 37°C in a 6% CO2 for minimum 2 h in culture to evaluate blastocoel re-expansion.
Fig. 1.
Depiction of the aspiration of blastocoel fluid using an ICSI needle to collapse the blastocyst immediately prior to cryopreservation
For conventional slow freezing and thawing, commercially available kits were employed (Irvine Scientific, Irvine, CA). Briefly, for freezing, blastocysts after 5–6 days of culture were exposed to %5 glycerol, and 10% glycerol + 0.2 M sucrose solutions for 10 min consecutively at 20–22°C. They (2 embryos per straw) were then loaded into 0.25 mL of freezing straws (IMV, France) and placed in controlled freezing machine (Cryologic CL-5000, Australia). The straws containing blastocysts were cooled (2°C/min) to −7°C, hold at −7°C for 5 min, seeded by precooled forceps, and hold at this temperature for another 5 min. The temperature was lowered 0.2°C/min to −32°C, and then the straws were quickly plunged into the LN2. For thawing, the straws were hold in air for 30 s, and subsequently emerged into water bath at 33°C for 50 s. Blastocysts were then first expelled into a 30 mm Petri dish containing 5% glycerol + 0.4 M sucrose solution for 1 min, and then 0.4 M sucrose for 5 min, and 0.2 M sucrose solutions for 5 min at 20–22°C. Blastocysts were washed 3 times with mHTF and cultured in 6% CO2, 5% O2, and 89% N at 37°C for 4 h before embryo transfer.
Different cryopreservation protocols, slow freezing (PGD cycles between 2003–2005) vs. vitrification (PGD cycles between 2006–2008) were employed for two different cohorts of patients in this retrospective study.
Recipient hormonal treatment and embryo transfer
Following the onset of birth control pill-induced menstruation, oestradiol valerate IM (4–8 mg) I.M. was administered every 3 days for a period of 8–12 days, until endometrial thickness had reached ≥9 mm in sagital diameter and the plasma E2 had stabilized at 1300–3000 Pmol/L. At that point, daily IM injections of 100 mg progesterone in oil (PIO) were initiated. On the 6th day of PIO, subject to patient choice and availability, <4 thawed/warmed blastocysts were cryotransferred. An ultrasound pelvic evaluation was carried out at 6–7 weeks in positive cases and cardiac activity at this stage was defined as positive pregnancy. In the event of an ultrasound-confirmed pregnancy, an estrogen/progesterone supplementation regime was continued until the 10th week of gestation. In all cases where negative beta hCG testing on 8 and 10 days after ET were obtained, hormonal treatment was immediately stopped. The patient’s age, number of cryopreserved and thawed/warmed blastocysts, blastocyst grading at vitrification, recovery rate, post thaw/warming survival rate, cancellation rate, and the number of transferred blastocysts were registered. The main clinical outcome measures were: SR, PR, and IR.
Statistical analysis
Differences between groups were evaluated by using Student’s t tests. Differences in rates and proportions were evaluated by using Fisher's exact test of independence. Significance was set at P = .05. Odds ratios and 95% confidence intervals were calculated for significant differences between groups.
Results
Table 1 summarizes patient characteristics and tabulates SR, PR, IR, and miscarriage rate in cases where blastocysts were slowly frozen vs. those vitrified. Each group was further subcategorized as non-biopsied or biopsied.
After warming, blastocysts from the slow freezing group had lower (P < 0.05) survival rates (83% for non-biopsied and 71% biopsied) than those vitrified blastocysts (97% for non-biopsied and 95% biopsied). Average number of blastocysts transferred after thawing and was not statistically different. After warming, similar transfer cancellation rates were observed in slow freezing and vitrification groups (8% and 5% vs. 3% and 4%, respectively; overall, 5%). At transfer, a comparable mean number of blastocysts per patient were eventually replaced (2.2 ± 0.8 vs. 2.1 ± 0.3, respectively; overall, 1.9 ± 0.5; P > .05).
The pregnancy, rates for non-biopsied and biopsied slow freezing groups were 34%, and 23% respectively, whereas 38%, and 37% for non-biopsied and biopsied vitrification groups.
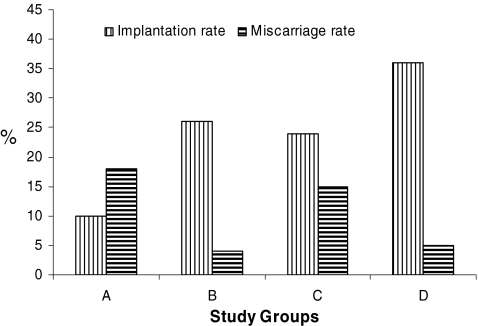
Figure 2 depicts IR and miscarriage rates following cryotransfer of previtrified blastocysts with those where cryopreservation was performed by way of slow freezing non-biopsied (Group A) or biopsied (Group B) and vitrification non-biopsied (Group C) or biopsied (Group D). In Group A, the implantation and miscarriage rates were 26% and 4%, 26% and 4% for Group B, 24% and 15% for Group C, 36% and 5% Group for Group D, respectively. The overall efficacy of the embryo vitrification process after PGS is supported by the fact that vitrified blastocysts had a implantation rate of 36% (Group D) and 26% (Group B), which was statistically significant (P < 0.05). The IR per warmed non-biopsied vitrified blastocyst was 24% (Group C) whereas 10% for those non-biopsied slow freezing group (Group A), further pointing to the utility of vitrification (P < 0.05).
Fig. 2.
Comparison of implantation and miscarriage rate following transfer of slowly frozen vs vitrified blastocysts after FISH-PGS. Group A and B comprises those blastocysts were cryopreserved with the slow freezing method, Group B included blastocyst which were biopsied on day 3; Group C and D comprises blastocysts cryopreserved with vitrification, Group D included blastocysts vitrified after FISH-PGS on day 3
Discussion
Effective cryopreservation of gametes and embryos not only solves logistical problems associated with achieving embryo-endometrium synchrony during IVF treatment, but it also provides adequate time required for accurate screening for genetic disorders and transmitted diseases. In this study, we aimed at comparing cryosurvival, pregnancy, and implantation rates of human embryos that have undergone blastomere biopsy for PGS and subsequently cryopreserved by either slow freezing or vitrification methods at blastocyst stage. It is also important to note that all vitrified blastocysts were subjected to blastocoel drainage prior to vitrification in order to minimize lethal ice formation in the blastocoel cavity which contains considerable amount of water molecules.
Our results with 409 patients showed that in those patients who underwent blastocysts cryotransfers (Tables 1 and 2), slowly frozen blastocysts resulted in a lower post-thaw morphologic survival rate when compared to those vitrified blastocysts (83% versus 97%, P < 0.05). This suggests that the vitrification method used in this study yields a higher percentage of embryos that are eligible for transfer. These results were in concordance with the previous reports on non-biopsied blastocyst vitrification studies [19, 27]. More importantly, cryotransfer of vitrified blastocysts resulted in a higher IR than those slowly frozen counterparts, i.e. 24% versus 10%, respectively (P < 0.05). Similarly, Balaban et al. [28] demonstrated that vitrified cleavage stage embryos had better SR, PR and IR than their conventionally frozen counterparts, and vitrified cleavage stage embryos had higher metabolic rate assessed by higher pyruvate uptake.
Table 2.
Clinical outcome following the transfer of FISH/PGS blastocysts: A comparison of conventional freezing versus vitrification
| aFISH/PGS | |||
|---|---|---|---|
| Group A | Group B | p Value | |
| Number of cycles with cryopreserved blastocyst transfer | 85 | 75 | |
| Mean age of patients (± SD) | 37.8 ± 2.4 | 38.2 ± 3.4 | |
| Mean no. of blastocysts transferred per woman (± SD) | 1.9 ± 0.4 | 1.4 ± 0.6 | |
| Births and ongoing pregnancies (%) | 20 (23) a | 28 (37) b | <0.05 |
| Implantation rate (%) | 38/146 (26) c | 36/99 (36) d | <0.05 |
| Singletons | 15 | 22 | |
| Multiples | 5 | 6 | |
| Miscarriages | 4% | 5% | |
aEmbryos were tested for 9 chromosomes (13, 15, 16, 17, 18, 21, 22, X, Y)
Group A: Conventional freezing
Group B: Vitrification
It has been suggested that cryopreservation by slow freezing is often associated with poor intracellular solute dispersion and lethal ice formation [29]. Furthermore, slow freezing of gametes and embryos is a protracted process where several hours are required before storage in LN2. This might compromise embryo cryosurvival due to in part solution effects [30] and subsequently lower downstream PR and IR following ET. Lane et al. [19] introduced cryoloop vitrification method for human embryos in an effort to increase cooling rates to enhance vitrification. Subsequently, several modifications were presented for human embryo vitrification [23, 27, 31] . The benefit of human blastocysts being cryopreserved using vitrification has been demonstrated by other groups [23, 27, 32]. Takahashi et al. [26] reported no increase in adverse prenatal outcomes and/or birth defects in children resulting from the transfer of vitrified blastocysts. The current data thus suggest that vitrification should be considered as the preferred method to cryopreserve blastocysts after biopsy for PGS.
Concerns have been raised if embryo biopsy may damage the embryo, leading to a diminution in the viability and implantation potential of both fresh and pre-cryopreserved embryos [33, 34, 35]. Our findings as well as those of others [36, 37] suggest that, with the hand of a skilled operator, no such reduction in embryo viability would be observed. The PR and IR were highest in cases where vitrified blastocysts that had previously undergone blastomere biopsy (37% and 36%) versus those where cryopreserved by conventional freezing (23% and 26%). The fact that our pregnancy rate with intact vitrified human embryos was about 40% for patients under 38 years strongly suggests that human embryos subjected to invasive blastomere biopsy were not adversely affected even after vitrification. In fact, we noted that vitrification improved blastocyst post-thaw PR and IR regardless of their biopsy for PGS comparing to their slowly frozen counterparts. Zheng et al. [38] demonstrated that after blastomere biopsy of human of embryos, those blastomeres nearest to the created aperture were more prone to undergo post-thaw lyses following slow freezing. In contrast, Zech et al. [39] showed that making a trans-zonal aperture in the ZP of human blastocysts prior to vitrification yielded an 80% post-thaw survival and 26% implantation rates, respectively. Our results are in agreement with a similar study reported by Agca et al. [40] who determined post-thaw survival and pregnancy rates of IVF bovine blastocyst that had previously undergone blastomere biopsy for gender determination. They compared post-thaw survival of blastocyst following slow freezing and vitrification. In their study, while only 36% (13/36) of the embryos survived after slow freezing whereas 100% (16/16) of the vitrified embryos did. Their results were further validated by ET in that 44% (7/16) and 23% (3/13) pregnancy rate were achieved for vitrified and slowly frozen embryos, respectively.
This study has also revealed that expanded blastocysts have a better survival rate than their less developed counterparts, but it is in disagreement with the findings of Vanderzwallmen et al. [41] and Mukaida et al. [42] who found that the efficiency of blastocyst vitrification was negatively correlated with the expansion of the blastocoel. We postulate that this difference could be due to the fact that by removing a blastomere on day 3 embryos could cause retardation in blastocyst formation. Mammalian blastocoel cavity formation is dependent on development of the trophectoderm epithelium, and is initiated following the establishment of ion gradients and osmotic fluid accumulation across this cell layer mediated by Na+ and K+ adenosine triphosphatase (Na/K-ATPase) [43]. The blastocyst stage embryos consist of inner cell mass and trophectoderm that are known to give rise to embryo and placenta, respectively [44]. It is expected that the formation of large ice crystals during freezing and thawing in the blastocoelic cavity might preferentially rupture the trophectoderm cells that are responsible for hatching, placental formation, and subsequent implantation in-utero [21]. Vanderzwallmen et al [30] elegantly overcame this problem by puncturing through trophectoderm cells, allowing the reduction of blastocoelic fluid prior to vitrification, a technique we adopted. This study is in agreement with the Vanderzwallmen study, suggesting a beneficial effect of pre-vitrification aspiration of blastocoel fluid to collapse the blastocyst prior to cryopreservation.
In conclusion, the data presented here suggest that blastocyst vitrification yields greatly improved embryo SR, PR, and IR than does slowly freezing. Furthermore, the combination of assisted hatching and pre-vitrification with artificial blastocoel collapse could represent a method of choice for cryostorage of biopsied human blastocysts. The current results also strongly suggest that cryopreservation of human blastocysts using vitrification is superior to slowly freezing methods regardless of their biopsy status.
Footnotes
Capsule
A reliable procedure for cryopreservation of blastocysts after preimplantation genetic screening is much needed. Transfer of ≤2 (1.4 ± 0.4) vitrified blastocysts after PGS yielded an implantation and ongoing pregnancy rate of 36% and 37%, respectively.
References
- 1.Fisch JD, Adamowicz M, Hackworth J, Ginsburg M, Keskintepe L, Sher G. Single embryo transfer (SET) on day 3 versus day 5 based on Graduated Embryo Score (GES) and soluble Human Leukocyte Antigen-G (sHLA-G): Preliminary results of a prospective, randomized controlled trial. Fertil. Steril. 2007;88:S315. [DOI]
- 2.Scholtes MC, Zeilmaker GH. A prospective, randomized study of embryo transfer results 3 or 5 days of embryo culture in vitro fertilization. Fertil Steril. 1996;65:1245–8. [DOI] [PubMed]
- 3.Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69:84–8. [DOI] [PubMed]
- 4.Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod. 1998;13:3434–40. [DOI] [PubMed]
- 5.da Motta ELA, Alegretti JR, Baracat EC, Olive D, Serafini PC. High implantation and pregnancy rates with transfer of human blastocysts developed in preimplantation stage one and blastocyst media. Fertil Steril. 1998;70:659–63. [DOI] [PubMed]
- 6.Behr B, Pool TB, Milki AA, Moore D, Gebhardt J, Dasig D. Preliminary clinical experience with human blastocyst development in vitro without co-culture. Hum Reprod. 1999;14:454–7. [DOI] [PubMed]
- 7.Milki AA, Hinckley MD, Fisch JD. Comparison of blastocyst transfer with day 3 embryo transfer in similar patient populations. Fertil Steril. 2000;73:126–9. [DOI] [PubMed]
- 8.Sher G, Keskintepe L, Keskintepe M, Ginsburg M, Maassarani G, Yakut T, et al. Oocyte karyotyping by comparative genomic hybridization provides a highly reliable method for selecting “competent” embryos, markedly improving in vitro fertilization outcome: a multiphase study. Fertil Steril. 2007;87:1033–40. [DOI] [PubMed]
- 9.Keskintepe L, Sher G, Keskintepe M. Reproductive oocyte/embryo genetic analysis: comparison between fluorescence in-situ hybridization and comparative genomic hybridization. RBM Online. 2007;15:303–9. [DOI] [PubMed]
- 10.Staessen C, Platteau P, Van Assche E, Michiels A, Tournaye H, Camus M, et al. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod. 2004;19:2849–58. [DOI] [PubMed]
- 11.Munne S. Chromosome abnormalities and their relationship to morphology and development of human embryos. RBM Online. 2006;12:234–53. [DOI] [PubMed]
- 12.Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B et al (2007). In vitro fertilization with preimplantation genetic screening. NEJM 2007; 357: 9. [DOI] [PubMed]
- 13.Verpoest W, Haentjens P, De Rycke M, Saessen C, Sermon K, Bonduelle M, Devroey P, Liebaers I. Cumulative reproductive outcome after preimplantation genetic diagnosis: a report on 1498 couples. Hum Reprod 2009; 1–9. [DOI] [PubMed]
- 14.Munne S, Sandalinas M, Escudero T, et al. Improved implantation after preimplantation genetic diagnosis of aneuploidy. Reprod Biomed Online. 2003;7:91–7. [DOI] [PubMed]
- 15.Munne S, Fischer J, Warner A, Cohen S, Zouves C, Cohen J. Preimplantation genetic diagnosis significantly reduces pregnancy loss in infertile couples: a multicenter study. Fertil Steril. 2006;85:326–32. [DOI] [PubMed]
- 16.The Practice Committee of the Society for Assisted reproduction the Practice Committee of the American Society for Reproductive Medicine. Preimplantation genetic testing: a practice committee opinion. Fertil Steril. 2007;88:1497–504. [DOI] [PubMed]
- 17.Mazur P. Equilibrium, quasi-equilibrium, and non-equilibrium freezing of mammalian embryos. Cell Biophys. 1990;17:53–91. [DOI] [PubMed]
- 18.Menezo Y, Nicollet B, Herbaut N, Andre D. Freezing cocultured human blastocysts. Fertil Steril. 1992;58:977–80. [DOI] [PubMed]
- 19.Lane M, Schoolcraft WB, Gardner DK. Vitrification of mouse and human blastocysts using a novel cryoloop container-less technique. Fertil Steril. 1999;72:1073–8. [DOI] [PubMed]
- 20.Whittingham DG, Leibo SP, Mazur P. Mouse embryos frozen to −196°C. Science. 1972;178:411–4. [DOI] [PubMed]
- 21.Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at -196 C degrees C by vitrification. Nature. 1985;313:573–5. [DOI] [PubMed]
- 22.Mukaida T, Nakamura S, Tomiyama T, Wada S, Kasai M, Takahashi K. Successful birth after transfer of vitrified human blastocysts with use of a cryoloop containerless technique. Fertil Steril. 2001;76:618–20. [DOI] [PubMed]
- 23.Mukaida T, Nakamura S, Tomiyama T, Wada S, Oka C, Kasai M, et al. Vitrification of human blastocysts using cryoloops: clinical outcome of 223 cycles. Hum Reprod. 2003;18:384–91. [DOI] [PubMed]
- 24.Kasai M, Mukaida T. Cryopreservation of animal and human embryos by vitrification. RBM Online. 2004;9:164–70. [DOI] [PubMed]
- 25.Keskintepe L, Sher G, Kotze D, Adamowicz M. Blastocyst competency index provides powerful tool for selecting embryos on day 5 for fresh transfer and for the frozen embryo transfer cycle. Presented at the 19th IFFS Conference in Durban, South Africa, 2007.
- 26.Takahashi K, Mukaida T, Goto T, Oka C. Perinatal outcome of blastocyst transfer with vitrification using cryoloop: a 4 year follow-up study. Fertil Steril. 2005;84:88–92. [DOI] [PubMed]
- 27.Liebermann J, Dietl J, Vanderzwalmen P, Tucker MJ. Recent developments in human oocyte, embryo and blastocyst vitrification: where are we now? RBM Online. 2003;7:623–33. [DOI] [PubMed]
- 28.Balaban B, Urman B, Ata B, Isiklar A, Larman MG, Hamilton R, et al. Randomized controlled study of human Day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Human Reproduction. 2008;23(9):1976–82. [DOI] [PubMed]
- 29.Youssry M, Ozmen B, Zohni K, Diedrich K, Al-Hasani K. Current aspects of blastocyst cryopreservation. RBM Online. 2008;16:311–20. [DOI] [PubMed]
- 30.Mazur P, Schneider U. Osmotic responses of preimplantation mouse and bovine embryos and their cryobiological implications. Cell Biophys. 1986;8:259–85. [DOI] [PubMed]
- 31.Reed ML, Lane M, Gardner DK, Jensen NL, Thompson J. Vitrification of human blastocysts using the cryoloop method: successful clinical application and birth of offspring. J. Assist. Reprod. Genet. 2002;19:304–6. [DOI] [PMC free article] [PubMed]
- 32.Vanderzwalmen P, Bertin G, Debauche Ch, Standaert V, Bollen N, van Roosendaal E. Vitrification of human blastocysts with the Hemi-Straw carrier: application of assisted hatching after thawing. Hum Reprod. 2003;18:1504–11. [DOI] [PubMed]
- 33.Joris H, Van den Abbeel E, Vos AD, Van Steirteghem A. Reduced survival after human embryo biopsy and subsequent cryopreservation. Hum Reprod. 1999;14:2833–7. [DOI] [PubMed]
- 34.Magli C, Gianaroli L, Fortini D, Ferraretti AP, Mukaida T, Oka C, et al. Artificial shrinkage of blastocoels using either a micro-needle or a laser pulse prior to the cooling steps of vitrification improves survival rate and pregnancy outcome of vitrified human blastocysts. Hum Reprod. 2006;21:3246–52. [DOI] [PubMed]
- 35.Munné S. Impact of blastomere biopsy and cryopreservation techniques on human embryo viability. Hum. Reprod. 1999;3:770–3. [DOI] [PubMed]
- 36.Valojerdi MR, Eftekhari-Yazdi P, Karimian L, Ashtiani SK. Effect of laser zona pellucida opening on clinical outcome of assisted reproduction technology in patients with advanced female age, recurrent implantation failure, or frozen-thawed embryos. Fertil Steril. 2008;90:84–91. [DOI] [PubMed]
- 37.Hiraoka K, Fuchiwaki M, Hiraoka K, Horiuchi T, Murakami T, Kinutani M, et al. Effect of the size of zona pellucida opening by laser assisted hatching on clinical outcome of frozen cleaved embryos that were cultured to blastocyst after thawing in women with multiple implantation failures of embryo transfer: a retrospective study. J Assist Reprod Genet. 2008;25:129–35. [DOI] [PMC free article] [PubMed]
- 38.Zheng WT, Zhuang GL, Zhou CQ, Fang C, Ou JP, Li T, et al. Comparison of the survival of human biopsied embryos after cryopreservation with four different methods using non-transferable embryos. Hum Reprod. 2005;20:1615–8. [DOI] [PubMed]
- 39.Zech NH, Lejeune B, Zech H, Vanderzwalmen P. Vitrification of hatching and hatched human blastocysts: effect of an opening in the zona pellucida before vitrification. RBM Online. 2005;11:355–61. [DOI] [PubMed]
- 40.Agca Y, Monson RL, Northey DL, Peschel DE, Schaefer DM, Rutledge JJ. Normal calves from transfer of biopsied, sexed and vitrified IVP bovine embryos. Theriogenology. 1998;50:129–45. [DOI] [PubMed]
- 41.Vanderzwalmen P, Bertin G, Debauche Ch, Standaert V, van Roosendaal E, Vandervorst M, et al. Births after vitrification at morula and blastocyst stages: effect of artificial reduction of the blastocoel cavity before vitrification. Hum Reprod. 2002;17:744–51. [DOI] [PubMed]
- 42.Mukaida T, Oka C, Goto T, Takahashi K. Artificial shrinkage of blastocoels using either a micro-needle or a laser pulse prior to the cooling steps of vitrification improves survival rate and pregnancy outcome of vitrified human blastocysts. Hum Reprod. 2006;21:3246–52. [DOI] [PubMed]
- 43.Biggers JD, Bell JE, Benos DJ. Mammalian blastocyst: transport functions in a developing epithelium. Am. J. Physiol. 1988;255:C419–32. [DOI] [PubMed]
- 44.Duc-Goiran P, Mignot TM, Bourgeois C, Ferré F. Embryo-maternal interactions at the implantation site: a delicate equilibrium. Eur J Obstet Gynecol Reprod Biol. 1999;83:85–100. [DOI] [PubMed]