Abstract
Purpose
The objective of this study was to investigate the effects of vitrification on the preimplantation developmental competence of mouse 2-cell, 4-cell and 8-cell stage embryos.
Methods
Mouse 2-cell, 4-cell and 8-cell stage embryos were cryopreserved using the cryotop vitrification method and subsequently warmed on a later date. The embryos were then assessed by their morphology, blastocyst formation and hatching rates. Additionally, trophectoderm (TE) and inner cell mass (ICM) cell numbers were compared in hatched blastocysts from the control and experimental groups.
Results
Vitrified embryos at the 2-cell, 4-cell and 8-cell stages appeared morphologically normal after warming. The overall survival rate of vitrified embryos at various stages after warming was 96.7% and there were no significant differences among 2-cell stage (96.0%), 4-cell stage (96.8%) and 8-cell stage (97.1%) embryos (P > 0.05). The blastocyst formation rate (69.4%) and hatching rate (52.6%) of vitrified 2-cell embryos were significantly lower than that from the control group and vitrified 8-cell embryos (P < 0.05). In the vitrified 4-cell embryo group, the blastocyst formation rate (90.3%) was similar to the 8-cell group (91.2%), but the hatching rate (60.0%) was significantly lower than that of the non-vitrified control ( 84.1%) and vitrified 8-cell embryo (78.4%) groups (P < 0.05). When further development to the fully hatched blastocyst stage was compared, hatched blastocysts derived from vitrified 2-cell, 4-cell and 8-cell embryos had significantly lower cell counts both in the ICM and TE, as compared to fresh blastocysts (P < 0.05). Among the vitrified 2-cell, 4-cell and 8-cell embryo groups, there were no significant differences in the cell counts of ICM and TE (P > 0.05).
Conclusions
Although cryotop vitrification was suitable for the cryopreservation of mouse embryos from the 2-cell stage, 4-cell stage and 8-cell stage without significant loss of survival, vitrification had an adverse effect on the development of 2-cell embryos. Mouse embryos at the 8-cell stage had the best tolerance for vitrification and would yield the highest level of post-vitrification developmental competence among early cleavage stage embryos. Nevertheless, it is unclear how these findings can be extrapolated to human embryos.
Keywords: Vitrification, Cleavage, Embryos, Development, Cryopreservation, Mouse
Introduction
Ever since the first studies demonstrating the feasibility of vitrification technique for the cryopreservation of mammalian embryos [1], this method has become increasingly popular among ART laborotories worldwide. This is due to the fact that vitrification has a number of significant advantages pertaining to cost and time requirement, as compared to the conventional slow-freezing method. The vitrification technique involves rapid cooling and warming rates, and the use of high concentrations of cryoprotectant solutions (at high viscosity and small volumes) to bring about a transition into a glass-like physical state without ice crystallization [2]. Vitrification is rapid, inexpensive, and has been used to cryopreserve embryos (from several mammalian species) at various stages of development [2].
In 1990, vitrification was successfully used for the first time to cryopreserve human cleavage-stage embryos, resulting in a live birth [3]. Since then, vitrification has been widely used for the cryopreservation of human oocytes [4, 5], in addition to pronuclear stage [6, 7], cleavage stage [4, 8–11], and blastocyst-stage [4, 12–14] embryos. Human embryo vitrification has been attempted with a variety of vessels such as electron microscope grids [15, 16], open pulled and hemi-straws [17], the Flexipet [18], the Cryotop [4] and the CryoLoop [19, 20]. However, for vitrification to eventually become the method of choice for the cryopreservation of human embryos, its efficiency at different developmental stages needs to be systematically evaluated.
Successful vitrification of rat embryos was first reported using blastocysts [21]. Later, 1-cell embryos [22], 2-cell embryos [23], and morulae-blastocysts [24] were also successfully vitrified. Subsequently, it was demonstrated that the efficiency of embryo cryopreservation depended not only on the cryopreservation method but also on the developmental stage of the embryos [25]. Several previous studies have attempted to systematically evaluate the most suitable developmental stage for mouse embryo cryopreservation (26 ∼ 28). However, there were conflicting results with different studies concluding that either the morula stage [26], early blastocyst stage [27] or the 2-cell stage [28] as being the most optimal for embryo cryopreservation and cryotolerance.
In our previous study, we had successfully developed a cryotop method of ultra-rapid vitrification that is currently employed in our laboratory [29]. In the present study, we vitrified mouse embryos at early developmental stages utilizing this optimised protocol, so as to determine the optimal embryonic developmental stage for cryopreservation. Although it is unclear how the results obtained with mouse embryos can be extrapolated to human embryos, it is hoped that the data could provide some insight regarding the choice of developmental stage for vitrification in human application.
Materials and methods
Superovulation, mating of mice and harvesting of embryos
Six-to-eight week-old female ICR mice were induced to superovulate with an injection of 10 IU PMSG (Sansheng Pharmaceuticals, Ningbo, China) followed with 10 IU hCG (Pregnyl TM, Livzon Syntpharm, Zhuhai, China) administered 48 h later. Females were mated with males from the same strain and inspected for the presence of vaginal plugs on the following morning. Female mice with the presence of vaginal plugs were considered to be pregnant and were sacrificed by cervical dislocation 38–40 h post-hCG for 2-cell embryos, 48–50 h for 4-cell embryos and 60–62 h for 8-cell embryos. Embryos were flushed from the dissected oviducts using Morpholinepropanesulfonic acid (MOPS)-buffered medium (G-MOPSTM) (Vitrolife, Gothenburg, Sweden). Only embryos at the two-to-eight cell stage, having an intact zona pellucida and being graded as excellent or good with regard to morphological appearance, were selected for experiments. After an intial wash in flushing medium, the embryos were transferred to G-1™ (Vitrolife, Gothenburg, Sweden) media containing 10% SSS for continuous culture at 37°C within a humidified atmosphere of 6% CO2 in air. Embryos were cultured in groups of 20 in 50 µl droplets of culture medium under paraffin oil (OVOIL™, Vitrolife, Gothenburg, Sweden) for 1 h prior to the vitrification study.
Vitrification
Mouse embryos at the 2-cell to 8-cell stage were vitrified by a two-step procedure with the KITAZATO Vitrification KIT (Kitazato BioPharmaceuticals, Shizuoka, Japan) using the cryotop as carrier, as described by Kuwayama et al. [4]. Embryos were initially equilibrated in ES (equilibration solution) consisting of 7.5% (v/v) ethylene glycol (EG) and 7.5% (v/v) dimethylsulfoxide (DMSO) at room temperature for 10 min, and subsequently placed in VS (vitrification solution) consisting of 15% (v/v) EG , 15% (v/v) DMSO and 0.5 mol/L sucrose and washed 3∼4 times. Within less than 60 s, two to three embryos in minimal VS (<1.0 µl) were placed onto the inner surface of the cryotop carrier. The cryotop was plunged vertically into liquid nitrogen, and inserted into a protective straw-cap prior to cryo-storage within liquid nitrogen.
Warming
After cryo-storage for 10–14 days, the embryos were warmed using a four-step dilution procedure with sucrose (KITAZATO Vitrification KIT, Japan). Briefly, the cryotop containing the embryos were removed from the protective straw-cap and dipped into warming solution (WS) containing 1.0 mol/L sucrose at 37°C. After 1 min equilibration in WS, the embryos were moved into diluent solution (DS) containing 0.5 M sucrose for 3 min. Subsequently, the embryos were transferred to 0 M sucrose washing solution 1 (WS1) for 5 min, followed by a final transfer to 0 M sucrose washing solution 2 (WS2) for 5 min at 37°C. The survival rate of embryos was assessed by observing the intactness of blastomeres and zona pellucida. The recovered embryos were cultured in G-1™ (Vitrolife, Gothenburg, Sweden) medium with 10% SSS (Synthetic Serum Substitute) under mineral oil at 37°C within a humidified atmosphere of 6% CO2 for 24 h for 2-cell embryos, 48 h for 4-cell embryos and 60 h for 8-cell embryos.
Blastocyst cell labeling
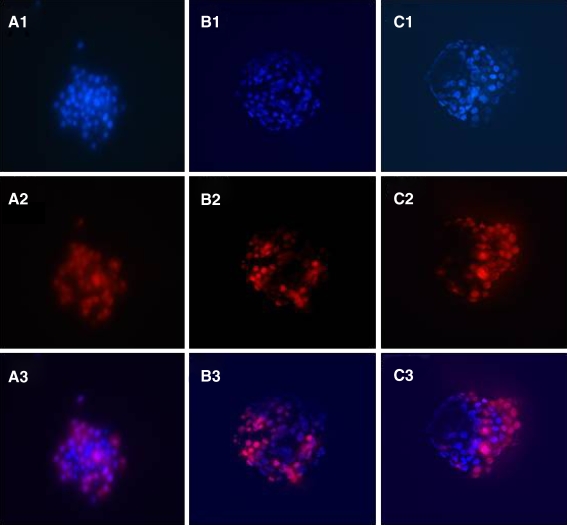
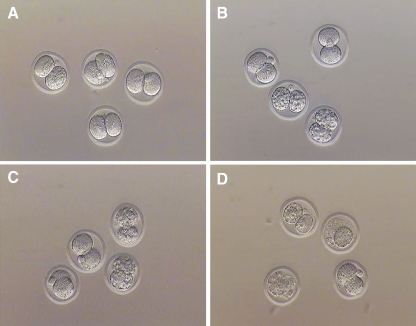
The total number of cells of the trophectoderm (TE) and the inner cell mass (ICM) were determined through labeling with polynucleotide-specific fluorochromes, as descrbed in our previous publication [29]. Briefly, hatched embryos were incubated in 10 mM trinitrobenzene sulfonic acid (TNBS; Sigma-Aldrich Inc., St. Louis, MO, USA) for 15 min on ice, followed by incubation in anti-dinitrophenyl BSA (anti-DNP BSA; Sigma-Aldrich Inc., St. Louis, MO, USA) diluted 1:15 in HTF-Hepes at 37°C for 15 min. After further washing in HTF-Hepes with Polyvinylpyrrolidone (PVP, Sigma-Aldrich Inc., St. Louis, MO, USA), lysis of TE was induced by incubating the embryos in guinea pig complement (Sigma-Aldrich Inc., St. Louis, MO, USA) diluted 1:10 in HTF-Hepes plus PVA (Poly-Vinyl Alcohol) supplemented with propidium iodide (Sigma-Aldrich Inc., St. Louis, MO, USA) at 37°C for 10–15 min. This was followed by incubation in 0.05 mM bisbenzimide (Hoechst 33258, Sigma-Aldrich Inc., St. Louis, MO, USA) in absolute ethanol overnight at 4°C. The propidium iodide will stain only the nucleus of non-viable cells without an intact plasma membrane, whereas bisbenzimide will stain the nucleus of both viable and non-viable cells. Hence, the lysed TE will be stained by both propidium iodide and bisbenzimide, whilst the intact ICM will be stained only by bisbenzimide. Labeled blastocysts were then fixed in absolute ethanol for 24 h before examination. Embryos with labeled nuclei were individually mounted on microscope slides with glycerol, placed underneath a cover-slip, and initially examined with the whole mount. Under fluorescence microscopy (excitation filter at 420 nm, barrier filter at 365 nm), the outer TE cells were identified by the pink fluorescence of propidium iodide, whereas the ICM cells were recognized by the blue fluorescence of the bisbenzimide (Fig. 1). The numbers of ICM and TE nuclei were thus counted under the inverted fluorescence microscope (×200) (TE2000S, Nikon, Japan).
Fig. 1.
Hatched blastocysts on D5 from different groups by dual differential staining. Hoechst 33258 stains all nuclei displaying blue fluorescence, while trophectoderm nuclei are labeled by propidium iodide showing red fluorescence. 2-cell group [A1, A2, A3(merge)], 4-cell group[B1, B2 B3(merge)] and 8-cell [C1, C2, C3(merge)] respectively. Note: Fluorescense imaging of a mouse hatched blastocyst. Blue: inner cell mass, Red: trophoectoderm
Statistical analysis
Post-vitrification survival rates, blastocyst and hatched blastocyst formation rates among experimental groups were compared and analyzed with the Chi squared test. Differences in total cell numbers and TE and ICM cell number counts were analyzed for significance using the Student’s t-test. A normality test was performed before any statistical analysis was carried out. Statistical significance was defined as P < 0.05.
Results
Vitrified embryos at the 2-cell, 4-cell and 8-cell stages appeared morphologically normal after Wing (Figs. 2, 3, 4). Embryonic development after vitrification and subsequent warming is summarized in Table 1. There were no significant differences in the post-vitrification survival rates of vitrified mouse embryos at the 2-cell (96.0%), 4-cell (97.0%) and 8-cell (97.0%) stages (P > 0.05). The developmental rates of vitrified 2-cell embryos to the blastocyst (69.4%) and hatched blastocyst (52.6%) stages were significantly lower than that of the control group (97.7% and 80.3%, respectively), as well as that of vitrified 8-cell embryos (91.1% and 78.4%, respectively) (P < 0.05). 93.3% of vitrified-warmed 4-cell embryos developed to the blastocyst stage and 60.0% of these embryos hatched from the zona pellucida. The hatching rate (60.0%) for vitrified 4-cell embryos was significantly lower than that of the non-vitrified control group ( 84.1%) and vitrified 8-cell embryos (78.4%) (P < 0.05). The blastocyst formation rate for vitrified 8-cell embryos was lower than that of the non-vitrified control group (91.1% versus 97.9%, P < 0.05), whereas the hatching rate was not significantly different from the control group (78.4% versus 86.0%, P > 0.05). Among the experimental groups, the blastocyst formation rate for the vitrified 2-cell embryo group was the lowest (P < 0.05), while the hatching rate was highest for vitrified 8-cell embryos (P < 0.05).
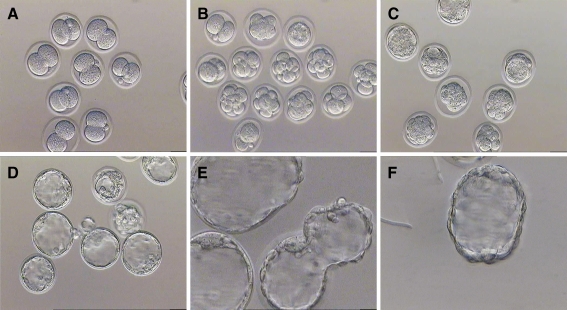
Fig. 2.
Development Photomicrographs of mouse vitrified 2-cell embryos. a 2 h later: 2-cell stage, Magnification ×200. b 24 h later: 4-cell or 8-cell stage, Magnification ×200. c 48 h later: morula stage and early blastocyst stag, Magnification ×200. d–f 96 h later or hatched blastocyst stage. Magnification ×400
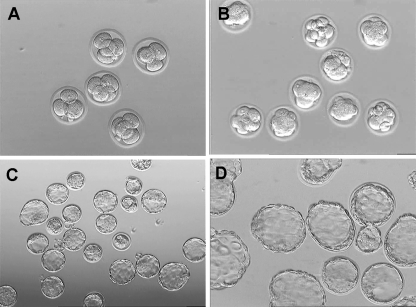
Fig. 3.
Development of mouse vitrified 4-cell embryos. a 2 h later: 4-cell stage, Magnification ×200. b 24 h later: 8-cell or morula stage, Magnification ×200. c 48 h later: blastocyst stage, Magnification ×200. d 72 h later or hatched blastocyst stage. Magnification ×400
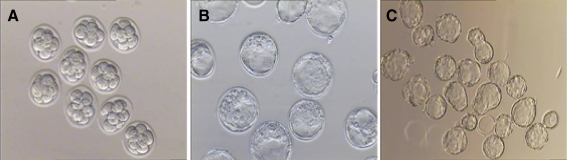
Fig. 4.
Development of mouse vitrified 8-cell embryos. a 2 h later:8-cell stage, Magnification ×200. b 24 h later: blastocyst stage, Magnification ×200. d 48 h later or hatched blastocyst stage. Magnification ×400
Table 1.
Mouse embryonic development after vitrification-warming of early cleavage-stage embryos
| Stage of vitrification | Embryos (n) | Survive rate (%) | Blastocysts formation (%) | Hatched (%) |
|---|---|---|---|---|
| Control | 365 | 100.0% (365/365) | 94.8% (346/365) | 81.6% (298/365) |
| Vitrified 2-cell | 204 | 96.0% (196/204) | 69.4% (136/196)a | 52.6% (103/196)a,b |
| Control | 259 | 100.0% (259/259) | 97.7% (253/259) | 80.3% (208/259) |
| Vitrified 4-cell | 217 | 96.8% (210/217) | 93.3% (196/210)a | 60.0% (126/210)a,b |
| Control | 315 | 100.0% (315/315) | 97.8% (308/315) | 81.9% (258/315) |
| Vitrified 8-cell | 210 | 97.1% (204/210) | 91.1% (186/204)a | 78.4% (160/204) |
acompared to control, P < 0.05
bcompared to vitrified 8-cell stage, P < 0.05
Blastocyst quality was further evaluated by cell number counts after full hatching had occurred (Table 2). Hatched blastocysts from vitrified-warmed embryos generally had lower TCN, TE and ICM compared to the control (P < 0.05).
Table 2.
Numbers of TE and ICM cells in hatched blastocysts grown in vitro for 5 days
| Stage of vitrification | Hatched Blastocysts(n) | TE (mean ± SD) | ICM (mean ± SD | TCN (mean ± SD) | Ratio ICM/TE (mean ± SD) |
|---|---|---|---|---|---|
| 2-cell control | 33 | 67.27 ± 9.36 | 36.24 ± 9.21 | 103.52 ± 12.82 | 0.55 ± 0.15 |
| Vitrified 2-cell | 30 | 54.97 ± 10.48a | 31.10 ± 8.79a | 85.40 ± 17.02a | 0.57 ± 0.13 |
| 4-cell control | 38 | 68.63 ± 11.32 | 36.37 ± 8.57 | 105.00 ± 17.79 | 0.53 ± 0.10 |
| Vitrified 4-cell | 30 | 51.40 ± 10.06a | 29.60 ± 7.06a | 81.00 ± 13.02a | 0.59 ± 0.16 |
| 8-cell control | 37 | 67.89 ± 9.66 | 37.46 ± 7.53 | 105.57 ± 15.00 | 0.55 ± 0.08 |
| Vitrified 8-cell | 34 | 53.44 ± 10.18a | 31.50 ± 7.23a | 84.94 ± 13.75a | 0.60 ± 0.16 |
Means of total cell numbers (TCN), inner cell mass (ICM) and trophectoderm (TE) of blastocysts fixed and stained with propidium iodide and ethanol/Hoechst. Values are represented as mean ± standard deviation
acompare to control group, P < 0.001
Discussion
Vitrification technology has shown great promise for the cryopreservation of human blastocysts. However, blastocyst transfer is currently not widely applied in human ART practice. The majority of human ART laboratories are still practising the transfer and cryopreservation of cleavage-stage embryos. Therefore, vitrification of cleavage-stage embryos still needs to investigated and further optimized. It is well-known that the unique advantage of vitrification is the elimination of mechanical injury caused by intra- or extra-cellular ice crystalization, and reduction of chilling injury, whilst maximizing cooling and warming rates [30]. Currently, the majority of vitrification studies have been focused on either oocytes or blastocyst-stage embryos, with far fewer studies on the vitrification of human cleavage-stage embryos. In the study of Rama Raju et al. [8], there was a statistically significant increase in pregnancy rates after transfer of vitrified/warmed human 8-cell stage embryos, as compared to 8-cell embryos cryopreserved by slow-freezing. Another clinical study reported that following vitrification of human embryos on day 3 of culture at the 6- to 8-cell stage, the subsequent post-vitrification survival rate was 85%, the clinical pregnancy rate was 44%, and the implantation rate was 20% [10]. Balaban et al. [9] also obtained satisfactory results using cryoloop as carrier, and attained a post-vitrification survival rate of 94.8%, blastocyst formation rate of 60.3% and clinical pregnancy rate of 49% for vitrified human day 3 embryos. Upon comparing with conventional slow-freezing techniques, vitrification provided a higher survival rate, minimal adverse effects on post-vitrification embryo morphology and improved clinical outcomes [31]. But up to date, slow freezing still remains the preferred and most common method of cryopreservation in the majority of ART laboratories [32, 33]. Therefore, the development of efficient cryopreservation procedures for human cleavage-stage embryos is of great importance in ART.
In this study, we vitrified 2-cell, 4-cell and 8-cell stage mouse embryos by the Cryotop method. The data obtained demonstrated the different cryotolerance of mouse embryos at various pre-implantation developmental stages.The overall survival rate of vitrified embryos in the present study was 96.7%. Despite the high survival rate, the blastocyst formation rate of vitrified embryos was still lower than that of the non-vitrified control group (p < 0.01) indicating that vitrification had some adverse effects on embryonic developmental potential. The blastocyst formation rate of vitrified 2-cell stage embryos was significantly lower than that of 4-cell or 8-cell stage embryos. It must be noted that for mouse embryos, cleavage arrest often occurs at the 2-cell stage, due to the onset of embryonic genome activation. This is commonly referred to as the “two-cell block” phenomenon. In this study, the “arrested” embryos continued to be further cultured for 72 h, but remained at the 2-cell stage with intact blastomere morphology (Fig. 5). This suggests that the developmental potential of 2-cell stage mouse embryos is more sensitive to vitrification, as compared to either the 4-cell or 8-cell stage. In a similar study, Lane et al. [34] demonstrated that vitrification of hamster 2-cell embryos affected the activity of both the Na+/ H+ antiporter and HCO−/ Cl− exchanger. Inhibiting the activity of both the Na+/ H+ antiporter and HCO−/ Cl− exchanger reduced subsequent embryonic development. Since these two transport proteins are responsible for regulation of intracellular pH which plays a key regulatory role in metabolism, energy production, and cell division, vitrification of hamster embryos often resulted in cleavage arrest at the 2-cell stage. In contrast, Graves-Herring et al. [35] demonstrated that neither the blastocyst formation rate nor live-birth rate of mouse 2-cell embryos were significantly different upon comparing vitrification and slow-freezing techniques. Yan et al. [28] investigated vitrification of IVF embryos from the 2-cell stage up to the early blastocyst stage with the open-pulled straw (OPS) method, and attained similar blastocyst formation (71.8 ∼ 89.5%) and hatching (61.1 ∼ 69.6%) rates. However, contrary to our findings, Yan et al. [28] demonstrated that embryos at the 2-cell stage had the best tolerance for vitrification.
Fig. 5.
2-cell embryos that have arrested after vitrification-warming. a 24 h later b 48 h later c 72 h later: d 96 h later Magnification ×200
In this study, the high rate of blastocyst formation attained with vitrified 4-cell embryos was similar to 8-cell embryos, but the hatching rate was significantly lower (p < 0.001) than that of the control and 8-cell embryo group, which could indicate that either the cryoprotectants themselves or the vitrification process induced zona pellucida hardening [36]. Vitrified 8-cell stage embryos had the highest blastocyst formation and hatching rates, which implied that this stage is the most appropriate for vitrification. This could be due to higher permeability to cryoprotectants at the 8-cell stage, as compared with earlier developmental stages [37]. Our results correlate with previous successful reports on the vitrification of human cleavage-stage embryos [8–10, 31].
The total cell number (TCN) and the ICM/TE ratio are well-established parameters for evaluating blastocyst developmental competence. We had differentially stained hatched embryos in order to analyse blastocyst implantation potential. Under our present experimental conditions, differences in the TCN counts between fresh and vitrified-warmed blastocysts were observed after full hatching had occurred. TCN, ICM and TE cell numbers of blastocysts obtained from vitrified cleavage-stage embryos were significantly lower than that of the control group. The decrease in cell numbers of day 5 blastocysts obtained from vitrified cleavage-stage embryos is a consistent observation in all three replicate experiments. These differences in the total cell numbers between fresh and vitrified-warmed blastocysts are consistent with the results of previous studies [38]. The decreased cell numbers of vitrified-warmed blastocysts might be due to delayed cleavage as a result of some degree of cryoinjury sustained by the embryonic blastomeres during the vitrification process.
In conclusion, the present data demonstrated that the Cryotop method is suitable for vitrification of mouse cleavage-stage embryos from the 2-cell stage to the 8-cell stage without a significant loss of survival. Embryos at the 8-cell stage had the best tolerance for cryotop vitrification and would ensure the highest degree of post-vitrification developmental competence among early cleavage-stage embryos under our experimental conditions. Further studies should focus on the in vivo development comptence upon transfer of the vitrified-warmed embryos at various preimplantation cleavage stages, as well as elucidate the mechanism of any observed differences. Although it is unclear how our findings can be extrapolated to human embryos, it is hoped that these findings provide a reference point for investigating which developmental stage is most suited for the vitrification of human cleavage-stage embryos.
Acknowledgements
We thank all our team members for giving technical support and valuable suggestions. This research is supported from grants of 07NMUZ026, ZKX08006 and 200801092.
Footnotes
Capsule Vitrification of early cleavage stage mouse embryos results in better retention of developmental potential when applied to later (4-8 cell) rather than earlier (2-cell) stages of development suggesting that this method of cryopreservation may require stage-specific applications in a species-specific context.
References
- 1.Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at −196°C by vitrification. Nature. 1985;313:573–5. [DOI] [PubMed]
- 2.Vajta G, Nagy ZP. Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod Biomed Online. 2006;12:779–96. [DOI] [PubMed]
- 3.Gordts S, Roziers P, Campo R, Noto V. Survival and pregnancy outcome after ultrarapid freezing of human embryos. Fertil Steril. 1990;53:469–72. [PubMed]
- 4.Kuwayama M. Highly efficient vitrification for cryopreservationof human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67:73–80. [DOI] [PubMed]
- 5.Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11:300–8. [DOI] [PubMed]
- 6.Al-Hasani S, Osmen B, Koutlaki N, Schoepper B, Diedrich K, Schultze-Mosgau A. Three years of routine vitrification of human zygotes: is it still fair to advocate slow-rate freezing? Reprod Biomed Online. 2007;14:288–93. [DOI] [PubMed]
- 7.Kumasako Y, Kumon M, Utsunomiya T, Araki Y. Successful pregnancy after the vitrification of zygotes using commercial vitrification solutions and conventional straws to protect against infections in liquid nitrogen. J Assist Reprod Genet. 2005;22:33–5. [DOI] [PMC free article] [PubMed]
- 8.Rama Raju GA, Haranath GB, Krishna KM, Prakash GJ, Madan K. Vitrification of human 8-cell embryos, a modified protocol for better pregnancy rates. Reprod Biomed Online. 2005;11:434–7. [DOI] [PubMed]
- 9.Balaban B, Urman B, Ata B, Isiklar A, Larman MG, Hamilton R, et al. A randomized controlled study of human day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Hum Reprod. 2008;23:1976–82. [DOI] [PubMed]
- 10.Desai N, Blackmon H, Szeptycki J, Goldfarb J. Cryoloop vitrification of human day 3 cleavage stage embryos: post vitrification development, pregnancy outcomes and live births. Reprod Biomed Online. 2007;14:208–13. [DOI] [PubMed]
- 11.Rama Raju GA, Jaya Prakash G, Murali Krishna K, Madan K. Neonatal outcome after vitrified day3 embryo transfer: a preliminary study. Fertile steril. 2009;92(1):143–8. [DOI] [PubMed]
- 12.Hiraoka K, Hiraoka K, Kinutani M, Kinutani K. Blastocoele collapse by micropipetting prior to vitrification gives excellent survival and pregnancy outcomes for human day 5 and 6 expanded blastocysts. Hum Reprod. 2004;19:2884–8. [DOI] [PubMed]
- 13.Zech NH, Lejeune B, Zech H, Vanderzwalmen P. Vitrification of hatching and hatched human blastocysts: effect of an opening in the zona pellucida before vitrification. Reprod Biomed Online. 2005;11:355–61. [DOI] [PubMed]
- 14.Youssry M, Ozmen B, Zohni K, Diedrich K, Al-Hasani S. Current aspects of blastocyst cryopreservation. Reprod Biomed Online. 2008;16:311–20. [DOI] [PubMed]
- 15.Park SP, Kim EY, Oh JH, Nam HK, Leek S, Park Y, et al. Ultra-rapid freezing of human multipronuclear zygotes using electronmicroscope grids. Hum Reprod. 2000;15:1787–90. [DOI] [PubMed]
- 16.Son WY, Yoon SH, Park SJ, Yoon HJ, Lee WD, Lim JH. Ongoingtwin pregnancy after vitrification of blastocysts produced by in-vitro matured oocytes retrieved from a woman with polycysticovary syndrome: Case report. Hum Reprod. 2002;17:2963–6. [DOI] [PubMed]
- 17.Vanderzwalmen P, Bertin G, Debauche C, Standaert V, Bollen N, Van Roosendal E, et al. Vitrification of human blastocysts with theHemi-Straw carrier: application of assisted hatching after thawing. Hum Reprod. 2003;18:1504–11. [DOI] [PubMed]
- 18.Liebermann J, Tucker MJ, Graham JR, Han T, Davis A, Levy MJ. Blastocyst development after vitrification of multipronuclear zygotes using the Flexipet denuding pipette. Reprod Biomed Online. 2002;4:146–50. [DOI] [PubMed]
- 19.Mukaida T, Nakamura S, Tomiyama T, Wada S, Oka C, Kasai M, et al. Vitrification of human blastocysts using cryoloops: clinicaloutcome of 223 cycles. Hum Reprod. 2003;18:384–91. [DOI] [PubMed]
- 20.Mukaida T, Takahashi K, Kasai M. Blastocyst cryopreservation: ultrarapid vitrification using CryoLoop technique. Reprod Biomed Online. 2003;6:221–5. [DOI] [PubMed]
- 21.Kono T, Suzuki O, Tsunoda Y. Cryopreservation of rat blastocysts by vitrification. Cryobiology. 1988;25:170–3. [DOI] [PubMed]
- 22.Anzai M, Nakagata N, Matsumoto K, Takahashi A, Takahash Y, Miyata K. Cryopreservation of in vitro fertilized embryos from transgenic rats by ultrarapid freezing. Exp Anim. 1994;43:247–50. [PubMed]
- 23.Hirabayashi M, Chaya N. Low temperature storage of rat 2-cell embryos by vitrification. J Mamm Ova Res. 1990;7:72–7.
- 24.Tada N, Sato M, Mizorogi T, Kasai K, Ogawa S. Efficient cyropreservation of hairless mutant and normal Wistar rat embryos by vitrification. Lab Anim Sci. 1995;45:323–5. [PubMed]
- 25.Leibo SP, Martino A, Kobayashi S, Pollard JW. Stage-dependent sensitivity of oocytes and embryos to low temperatures. Anim Reprod Sci. 1996;42:45–53. [DOI]
- 26.Han M-S, Niwa K, Kasai M. Vitrification of rat embryos at various developmental stages. Theriogenology. 2003;59:1851–63. [DOI] [PubMed]
- 27.Zhou GB, Zhu SE, Hou YP, Jin F, Yang QE, Yang ZQ, et al. Vitrification of mouse embryos at various stages by Open-pulled Straw (OPS) method. Anim Biotechnol. 2005;16:153–63. [DOI] [PubMed]
- 28.Chang-Liang YAN, Qi-En YANG, Guang-Bin ZHOU, Yun-Peng HOU, et al. Open-pulled straw (OPS) vitrification of in vitro fertilised mouse embryos at various stages. Acta Vet Hung. 2008;56(2):245–53. [DOI] [PubMed]
- 29.Ling XF, Zhang JQ, Tong GQ, et al. Effect of cryotop vitrification on preimplantation developmental competence of murine morula and blastocyst stage embryos. Reprod Biomed Online 2009; 19(5):708–13. [DOI] [PubMed]
- 30.Lane M, Schoolcraft WB, Gardner DK. Vitrification of mouse and human blastocysts using a novel CryoLoop container-less technique. Fertil Steril. 1999;72:1073–8. [DOI] [PubMed]
- 31.Valojerdi MR, Eftekhari-Yazdi P, Karimian L, et al. Vitrification versus slow freezing gives excellent survival, post warming embryo morphology and pregnancy outcomes for human cleaved embryos. J Assist Reprod Genet Online. 2009;10. [DOI] [PMC free article] [PubMed]
- 32.Loutradi KE, Kolibianakis EM. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril. 2008;90(1):186–92. [DOI] [PubMed]
- 33.Kolibianakis EM, Venetis CA, Tarlatzis BC. Cryopreservation of human embryos by vitrification or slow freezing: which one is better? Curr Opin Obstet Gynecol. 2009;21:270–4. [DOI] [PubMed]
- 34.Lane M, Lyons EA, Bavister BD. Cryopreservation reduces the ability of hamster 2-cell embryos to regulate intracellular pH. Hum Reprod. 2000;15(2):389–94. [DOI] [PubMed]
- 35.Graves-Herring JE, Boone WR. Blastocyst rate and live births from vitrification and slow-cooled two-cell mouse embryos. Fertil Steril. 2009;91(3):920–4. [DOI] [PubMed]
- 36.Sheehan CB, Lane M, Gardner DK. The CryoLoop facilitates re-vitrification of embryos at four successive stages of development without impairing embryo growth. Hum Reprod. 2006;21(11):2978–84. [DOI] [PubMed]
- 37.Pedro PB, Yokoyama E, Zhu SE, et al. Permeability of mouse oocytes and embryos at various developmental stages to five cryoprotectants. J Reprod Dev. 2005;51(2):235–46. [DOI] [PubMed]
- 38.Cuello C, Sanchez-Osorio J, Almiñana C, Gil MA, Perals ML, Lucas X, et al. Effect of the cryoprotectant concentration on the in vitro embryo development and cell proliferation of OPS-vitrified porcine blastocysts. Cryobiology. 2008;56:189–94. [DOI] [PubMed]