Abstract
Microemulsions (MEs) are clear, thermodynamically stable systems. They were used to solubilize drugs and to improve topical drug availability. Salicylic acid (SA) is a keratolytic agent used in topical products with antimicrobial actions. The objective of this work was to prepare and evaluate SA ME systems. Different concentrations of SA were incorporated in an ME base composed of isopropyl myristate, water, and Tween 80: propylene glycol in the ratio of 15:1. Three ME systems were prepared: S2%, S5%, and S10% which contain 2%, 5%, and 10% of SA, respectively. Evaluation by examination under cross-polarizing microscope, measuring of percent transmittance, pH measurement, determination of the specific gravity, assessment of rheological properties, and accelerated stability study were carried out. The data showed that the addition of SA markedly affected the physical properties of the base. All systems were not affected by accelerated stability tests. Stability study for 6 months under ambient conditions was carried out for S10%. No remarkable changes were recorded except a decrease in the viscosity value after 1 month. The results suggested that ME could be a suitable vehicle for topical application of different concentrations of SA.
Key words: microemulsion, salicylic acid, stability
INTRODUCTION
Microemulsions (MEs) are clear, thermodynamically stable, optically isotropic systems. They are formed spontaneously upon mixing a suitable oil, water, and an amphiphile blend (surfactants either alone or in combination with a cosurfactant; (1–4)).
MEs offer advantages over traditional creams and lotions as topical drug delivery. They were used to solubilize drugs and to improve topical drug availability (5). They are able to increase the rate and depth of moisturizing agents into skin. It has been suggested that MEs may dissolve the ordered structure of the stratum corneum lipids, leading to the loss of barrier properties of the skin (6).
Salicylic acid (SA) is a white fluffy crystalline powder, slightly soluble in water (1 in 460 ml). It is used as keratolytic agent in topical products, and it has antimicrobial and fungicidal actions (7–9). It was found that a topical preparation composed of 12% SA and 4% lactic acid in collodion base may be beneficial in speeding resolution of molluscum contagiosum, which is a viral infection spread by contact in children (10). Antibacterial activity of SA was recorded by Park et al. (11) and Kupferwasser et al. (12) as adjuvant therapeutic agent in treatment of Staphylococcus aureus infections. SA is used topically in hyperkeratotic and scaling conditions to enhance the rate of loss of surface scales (13). The combination of a 3% SA combined with a 1% hydrocortisone anti-inflammatory provides the best relief of scalp pruritus (14). SA peels (20–30%) can be combined with traditional acne therapy to speed the resolution of the comedones and inflamed papules (15). They are both safe and efficacious for treatment of acne vulgaris, oily skin, textural changes, and post-inflammatory hyperpigmentation (16), and they were shown to be beneficial in whitening the face of Asian patients with acne (17). Also SA peels were used in combination with 4% hydroquinone and 2% glycolic acid for clearing areas of photodamage on the neck and upper chest (18). In addition, Dainichi et al. (19) findings suggested that chemical peeling with 30% SA in polyethylene glycol may help to prevent as well as to reduce the number of ultraviolet B-induced skin tumors. SA is readily absorbed through the skin, slowly excreted in urine; and systemic toxicity resulting from application to large area of the skin has been reported (8). Klimowicz et al. (20) study confirmed good skin penetration of topical applied 5% SA in Vaseline. However, SA has a fairy strong affinity for polyethylene glycol vehicle as SA is adsorbed to polyethylene glycol, such that there is little release of SA into sebaceous materials, so reducing the potential toxicity of the percutaneously absorption (19). To minimize absorption following topical application, SA should not be used for prolonged periods, in high concentration, on large areas of the body, or on inflamed broken skin. It should also be used with care on the extremities of patients with impaired peripheral circulation and diabetics (8). The side effects of SA include sensitivity, excessive drying, and irritation. Traditional formulations of SA in ointment bases have disadvantages of being greasy and irritant due to free crystals of the drug.
In this study, SA was incorporated with different concentrations in an ME gel base composed of 35% isopropyl myristate (IPM), 20% water, and 45% Tween 80: propylene glycol in ratio of 15:1. Characterization of the prepared systems was carried out by applying different evaluation tests to examine the effect of incorporation of the drug on the ME gel base.
MATERIALS AND METHODS
Materials
The materials used were IPM, polyoxyethylene 20 sorbitan mono-oleate (Tween 80), propylene glycol (PG; Merck-Schuchardt, Germany), SA (BP), and double distilled water.
Preparation of the Microemulsion Base and Microemulsion Systems Containing Different Concentrations of Salicylic Acid
An ME gel base composed of 35% IPM, 20% water, and 45% Tween 80: PG as surfactant/cosurfactant mixture (S/CoS) in ratio of 15:1, was prepared. The composition of the ME base was chosen according to preliminary trials explained in a previous work (21). IPM and S/CoS mixture were mixed in the chosen concentrations, and then water was added portion-wise with continuous stirring. The system was stored in a tightly closed glass container and left for 3 days to attain equilibrium before evaluation.
Different concentrations of SA were incorporated in the prepared ME. Three systems were prepared: S2%, S5%, and S10% which contain 2%, 5%, and 10% of SA, respectively. SA was mixed first with IPM by a magnetic stirrer (Wheaton, Rc-2, Japan), then the S/CoS mixture was added, and the mixture was stirred till the drug completely dissolved, then water was added and stirring was continued for about further 10 min.
Evaluation of the Systems
The ME base and the three prepared SA ME systems were subjected to the following tests:
Visual Inspection
The systems were visually inspected for homogeneity, optical clarity, and fluidity.
Examination under Cross-polarizing Microscope
The systems were subjected to examination under cross-polarizing microscope (Olympus, Japan) for the absence of birefringence to exclude liquid crystalline systems (22,23).
Limpidity Test (Percent Transmittance)
The limpidity of the systems was measured spectrophotometrically using spectrophotometer (Shimadzu, UV-160, Japan; (24)).
pH Measurements
The pH of 10% aqueous solution of the base was measured (25). For SA ME systems, it was measured by direct immersion of the electrode of the pH meter (Hanna-213, Portugal) in the system (26).
Determination of the Specific Gravity
The specific gravity of the systems was determined, at ambient conditions, using a specific gravity bottle of 25 ml capacity.
Assessment of the Rheological Properties
The rheological properties of the base were determined by Brookfield digital viscometer (RV-TD, USA). Those of the medicated systems were determined using Brookfield digital viscometer (LV, model DV-III, USA).
Accelerated Stability Tests
Centrifuge Stress Test
The systems were centrifuged at 4,000 rpm (1,073 × g) for 15 min by Centrifuge (Shanghai Surgical Instrument Factory, model 80-2, China), and then they were examined for phase separation.
Freeze-Thaw Cycles (FTC)
The systems were submitted to a total of three complete cycles; each cycle consisting of 24 h at 25°C followed by 24 h at −5°C (27).
Long Term Stability
The ME system containing 10% SA was stored under ambient conditions for 6 months, and the system was examined periodically after 1, 3, and 6 months by visual inspection and measurement of percent transmittance, pH, specific gravity, and rheological evaluation.
RESULTS AND DISCUSSION
Evaluation of the Systems
Visual inspection of the ME base showed homogeneous system of gel consistency. Examination under cross-polarizing microscope showed dark field indicating isotropic system. The percent transmittance measured at 598 nm was 81.6%; pH value of 10% aqueous solution was 4.88. Assessment of rheological properties showed pseudoplastic flow with thixotropic behavior; the calculated Farrow’s number and hysteresis area were 5.54 and 2.33 cm2, respectively. The physical properties of the base are recorded in Table I.
Table I.
Physical Properties of the Microemulsion Base
| Test | Percent transmittance | pH value | Viscosity (cps) | Farrow’s number | |
|---|---|---|---|---|---|
| At a minimum shear ratea (η min; cps × 106) | At a maximum shear rateb (η max; cps × 103) | ||||
| Value | 81.6 | 4.88 | 3.07 | 42.3 | 5.54 |
aMinimum shear rate = 0.105 s−1
bMaximum shear rate = 21 s−1
MEs containing SA showed clear, homogeneous, transparent, and viscous preparations with yellow color. It was observed that the base lost its gel consistency and became viscous liquid. A similar observation was recorded by Kantaria et al. (28) who found that the gelation process in gelatin containing ME based organogel composed of water, IPM, Tween 80 surfactants, and sodium salt of the anionic surfactant sodium dioctylsulfosuccinate was inhibited in the presence of salicylates. Moreover, the degree of inhibition and the extent of gel weakening were proportional to the salicylate concentration. Also, Baudonnet et al. (29) showed that SA addition generated a decrease in their emulsion system viscosity.
Examination under cross-polarizing microscope showed dark field indicating no change in isotropic character, and no crystals of the drug were detected, indicating that the drug was completely dissolved.
Concerning the measurement of percent transmittance, pH, and the specific gravity values, the data are cited in Table II. The percent transmittance increased as the SA concentration increased. It could be noticed that the pH value is lower than that of the plain base, and as SA concentration increased the pH value decreased; this is obviously due to the acidic properties of the drug, a similar observation was recorded by Baudonnet et al. (29) who recorded a decrease in pH of the emulsion system containing SA. The data also showed that as the SA concentration increased, the specific gravity of the system increased.
Table II.
Physical Properties of Microemulsion Systems Containing Different Concentrations of Salicylic Acid
| System | Percent transmittance | pH values | Specific gravity | Viscosity at 25 rpm (cp) | Farrow’s number |
|---|---|---|---|---|---|
| S2% | 73.90 | 3.55 | 0.979 | 3,965 | 1.20 |
| S5% | 79.11 | 3.34 | 0.987 | 730 | 1.80 |
| S10% | 83.54 | 2.56 | 1.003 | 242 | 1.00 |
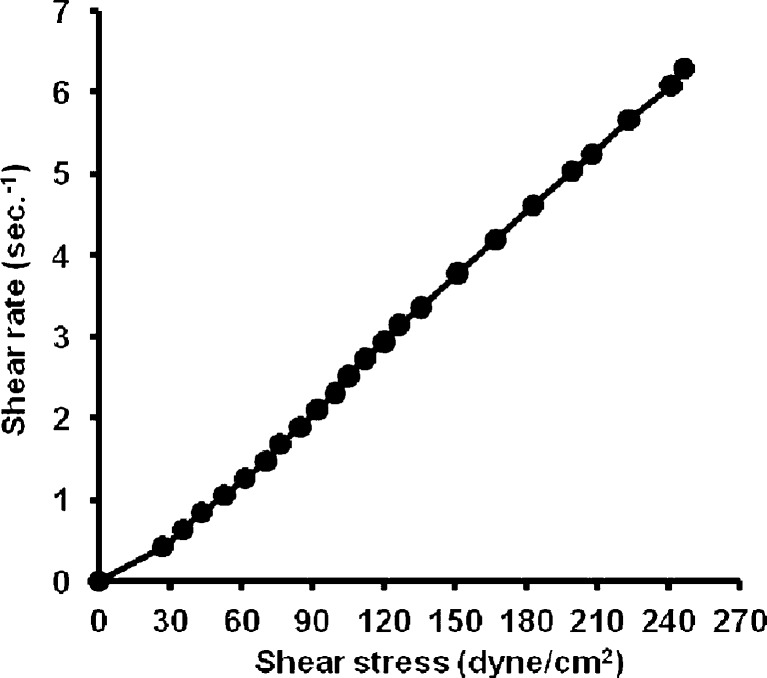
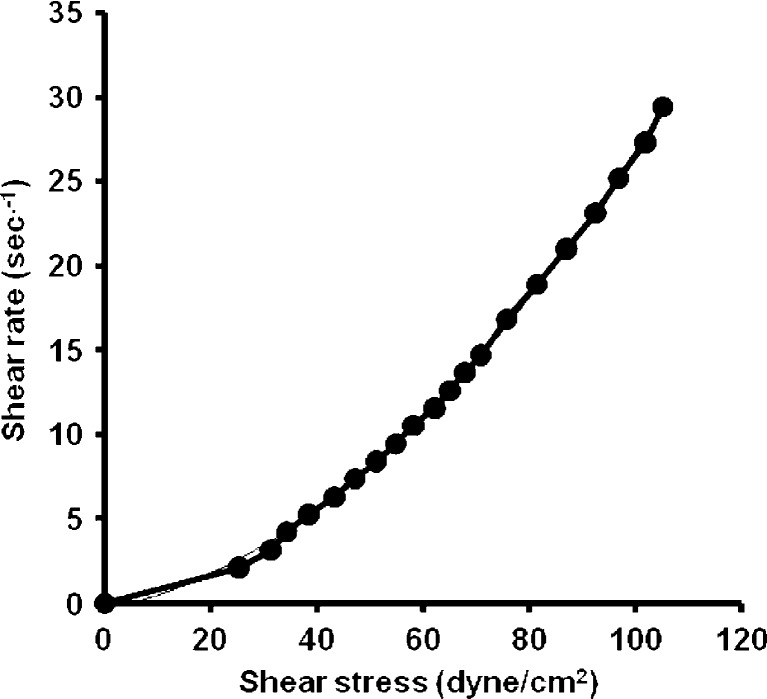
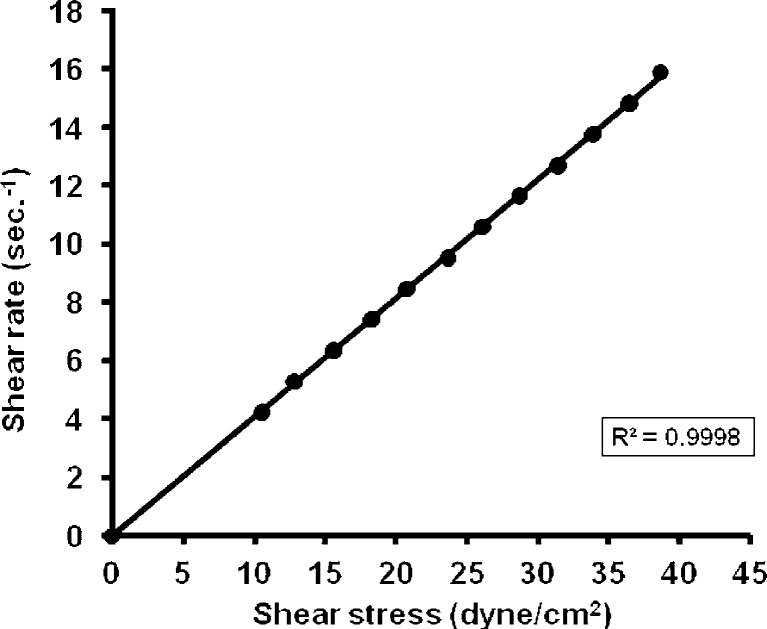
From the data of the rheological properties of the systems, it is obvious that the viscosity decreased with increasing SA concentration in the ME system. It could be noticed that both S2% and S5% were non-Newtonian systems with pseudoplastic flow as the calculated Farrow’s numbers were 1.2 and 1.8, respectively. S10% showed a different pattern of flow which showed a straight line with (R2) value = 0.9998 and a Farrow’s number value of 1 indicating a Newtonian system (Figs. 1, 2, and 3).
Fig. 1.
Rheogram of microemulsion system containing 2% salicylic acid
Fig. 2.
Rheogram of microemulsion system containing 5% salicylic acid
Fig. 3.
Rheogram of microemulsion system containing 10% salicylic acid
All systems of SA MEs and the base showed no changes in physical appearance after subjecting to centrifugation and freeze-thaw cycles.
Long Term Stability
Storage of ME containing 10% SA for 6 months at ambient conditions showed no change in appearance of the system. Also, there were no remarkable changes in the values of percent transmittance or specific gravity, while there was a very slight decrease in pH value during storage. Concerning the rheological properties, there was no change in the type of flow, while the viscosity decreased after 1 month, and then no changes were recorded. The data is recorded in Table III.
Table III.
Physical Properties of 10% Salicylic Acid Microemulsion Stored at Ambient Conditions for 6 Months
| Storage period (months) | pH | Specific gravity | Viscosity (cp) | Farrow’s number |
|---|---|---|---|---|
| 0 | 2.56 | 1.003 | 242 | 1.00 |
| 1 | 2.7 | 1.005 | 169.2 | 0.99 |
| 3 | 2.55 | 1.005 | 160.2 | 0.999 |
| 6 | 2.48 | 1.002 | 169.9 | 1.00 |
CONCLUSION
Incorporation of different concentrations of SA into an ME gel base remarkably decreased the viscosity of the base without affecting its isotropic nature, and the drug was completely dissolved. As the concentration of SA increased, the percent transmittance and the specific gravity of the systems increased, while viscosity and pH decreased. ME containing 10% SA (S10%) retained its characters upon storage at ambient conditions for 6 months. The results suggested that ME could be a suitable vehicle for topical application of different concentrations SA.
REFERENCES
- 1.Collier K, Matalonis S, Owen AJ. Evaluation of permeability enhancement by microemulsion in a caco-2 cell system. Proc Int Symp Control Release Bioact Mater. 1999;26:5444. [Google Scholar]
- 2.Alany RG, Rades T, Agatonovic-Kustrin S, Dvies NM, Tucker IG. Effects of alcohols and diols on the phase behaviour of quaternary systems. Int J Pharm. 2000;196:141–145. doi: 10.1016/S0378-5173(99)00408-1. [DOI] [PubMed] [Google Scholar]
- 3.Trotta M, Pattarino F, Grosa G. Formation of lecithin-based microemulsions containing n-alkanol phosphocholines. Int J Pharm. 1998;174:253–259. doi: 10.1016/S0378-5173(98)00273-7. [DOI] [Google Scholar]
- 4.Marti-Mestres G, Nielloud F. Emulsions in health care applications—an overview. J Dispers Sci Technol. 2002;23:419–439. [Google Scholar]
- 5.Osborne DW, Ward AJ, O’Neill KJ. Microemulsions as topical drug delivery vehicles: in-vitro transdermal studies of a model hydrophilic drug. J Pharm Pharmacol Comm. 1991;43:451–454. [PubMed] [Google Scholar]
- 6.Jachowicz J, Berthiaume MD. Microemulsions vs. macroemulsions in hair care products. Cosmet Toiletries. 1993;108:65–72. [Google Scholar]
- 7.Orth DS, Widjaja J, Ly L, Cao N, Shapiro WB. Stability and skin persistence of topical products: evaluating the effect of a hydroalcoholic hydroquinone vehicle. Cosmet Toiletries. 1998;113(10):51–63. [Google Scholar]
- 8.Reynolds JEF, editor. Martindale, the extra pharmacopoeia, 30th edition. London: The Pharmaceutical Press; 1993. [Google Scholar]
- 9.Bashir SJ, Dreher F, Chew AL, Zhai H, Levin C, Stern R, et al. Cutaneous bioassay of salicylic acid as a keratolytic. Int J Pharm. 2005;292:187–194. doi: 10.1016/j.ijpharm.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Leslie KS, Dootson G, Sterling JC. Topical salicylic acid gel as a treatment for molluscum contagiosum in Children. J Derm Treat. 2005;16:336–340. doi: 10.1080/09546630500430521. [DOI] [PubMed] [Google Scholar]
- 11.Park WB, Kim SH, Cho JH, Bang JH, Kim HB, Kim NJ, et al. Effect of salicylic acid on invasion of human vascular endothelial cells by Staphylococcus aureus. FEMS Immunol Med Microbiol. 2007;49:56–61. doi: 10.1111/j.1574-695X.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- 12.Kupferwasser LI, Yeaman MR, Nast CC, Kupferwasser D, Xiong YQ, Palma M, et al. Salicylic acid attenuates virulence in endovascular infections by targeting global regulatory pathways in Staphylococcus aureus. J Clin Invest. 2003;112(2):222–233. doi: 10.1172/JCI16876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.British Medical Association and the Royal Pharmaceutical Society of Great Britain. British National Formulary, No. 26; 1993. p. 408–10.
- 14.Draelos ZD. An evaluation of topical 3% salicylic acid and 1% hydrocortisone in the maintenance of scalp pruritus. J Cosmet Dermatol. 2005;4:193–197. doi: 10.1111/j.1473-2165.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee HS, Kim IH. Salicylic acid peels for the treatment of acne vulgaris in Asian patients. Dermatol Surg. 2003;29:1196–1199. doi: 10.1111/j.1524-4725.2003.29384.x. [DOI] [PubMed] [Google Scholar]
- 16.Pearl E, Grimes MD. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg. 1999;25(1):18–22. doi: 10.1046/j.1524-4725.1999.08145.x. [DOI] [PubMed] [Google Scholar]
- 17.Ahn HH, Kim IH. Whitening effect of salicylic acid peels in Asian patients. Dermatol Surg. 2006;32:372–375. doi: 10.1111/j.1524-4725.2006.32075.x. [DOI] [PubMed] [Google Scholar]
- 18.Gladstone HB, Nguyen BA, Williams R, Ottomeyer T, Wortzman M, Jeffers M, et al. Efficacy of hydroquinone cream (USP 4%) used alone or in combination with salicylic acid peels in improving photodamage on the neck and upper chest. Dermatol Surg. 2000;26(4):333–377. doi: 10.1046/j.1524-4725.2000.99233.x. [DOI] [PubMed] [Google Scholar]
- 19.Dainichi T, Ueda S, Isoda M, Koga T, Kinukawa N, Nose Y, et al. Chemical peeling with salicylic acid in polyethylene glycol vehicle suppresses skin tumour development in hairless mice. Br J Dermatol. 2003;148:906–912. doi: 10.1046/j.1365-2133.2003.05282.x. [DOI] [PubMed] [Google Scholar]
- 20.Klimowicz A, Farfał S, Bielecka-Grzela S. Evaluation of skin penetration of topically applied drugs in humans by cutaneous microdialysis: acyclovir vs. salicylic acid. J Clin Pharm Ther. 2007;32:143–148. doi: 10.1111/j.1365-2710.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 21.Badawi AA, Nour SA, Sakran WS, El-Mancy SS. Formulation and evaluation of new emulsion systems as dosage forms. MD thesis submitted to faculty of pharmacy, Egypt: Cairo University; 2006. [Google Scholar]
- 22.Malcolmson C, Lawrence M. Three-component non-ionic oil-in-water microemulsions using polyoxyethylene ether surfactants. Colloids Surf., B Biointerfaces. 1995;4:97–109. doi: 10.1016/0927-7765(94)01160-7. [DOI] [Google Scholar]
- 23.Constantinides PP, Scalart JP, Lancaster C, Marcello J, Marks G, Ellens H, et al. Formulation and intestinal absorption enhancement evaluation of water-in-oil microemulsions incorporating medium-chain glycerides. Pharm Res. 1994;11(10):1385–1390. doi: 10.1023/A:1018927402875. [DOI] [PubMed] [Google Scholar]
- 24.Constantinides PP, Welzel G, Ellens H, Smith PL, Sturgis S, Yiv SH, et al. Water-in-oil microemulsions containing medium-chain fatty acids/salts: formulation and intestinal absorption enhancement evaluation. Pharm Res. 1996;13(2):210–215. doi: 10.1023/A:1016030812272. [DOI] [PubMed] [Google Scholar]
- 25.Nour SA, Shalaby SH, Afify NN, Abd El-Aal S, Mekhael MK. Formulation and evaluation of econazole nitrate emulgels. J Drug Res Egypt. 2002;24(1–2):63–71. [Google Scholar]
- 26.Lucero MJ, Vigo J, Leon MJ. A study of shear and compression deformations on hydrophilic gels of tretinoin. Int J Pharm. 1994;106:125–133. doi: 10.1016/0378-5173(94)90310-7. [DOI] [Google Scholar]
- 27.Brime B, Moreno MA, Frutos G, Ballesteros MA, Frutos P. Amphotericin B in oil-water lecithin-based microemulsions: formulation and toxicity evaluation. J Pharm Sci. 2002;91(4):1178–1185. doi: 10.1002/jps.10065. [DOI] [PubMed] [Google Scholar]
- 28.Kantaria S, Rees GD, Lawrence MJ. Gelatin-stabilized microemulsion-based organogels: rheology and application in iontophoretic transdermal drug delivery. J Control Release. 1999;60:355–365. doi: 10.1016/S0168-3659(99)00092-9. [DOI] [PubMed] [Google Scholar]
- 29.Baudonnet L, Grossiord J-L, Rodriguez F. Physicochemical characterization and in vitro release of salicylic acid from o/w emulsions prepared with Montanov 68®: effect of formulation parameters. Drug Dev Ind Pharm. 2004;30(11):975–984. doi: 10.1081/DDC-200037251. [DOI] [PubMed] [Google Scholar]