Abstract
The aim of the present study was to develop and evaluate a buccal adhesive tablet containing ondansetron hydrochloride (OH). Special punches and dies were fabricated and used while preparing buccal adhesive tablets. The tablets were prepared using carbopol (CP 934), sodium alginate, sodium carboxymethylcellulose low viscosity (SCMC LV), and hydroxypropylmethylcellulose (HPMC 15cps) as mucoadhsive polymers to impart mucoadhesion and ethyl cellulose to act as an impermeable backing layer. The formulations were prepared by direct compression and characterized by different parameters such as weight uniformity, content uniformity, thickness, hardness, swelling index, in vitro drug release studies, mucoadhesive strength, and ex vivo permeation study. As compared with the optimized formulation composed of OH—5 mg, CP 934—30 mg, SCMC LV—165 mg, PEG 6000—40 mg, lactose—5 mg, magnesium stearate—1.5 mg, and aspartame—2 mg, which gave the maximum release (88.15%), non-bitter (OH) that form namely ondansetron base and complexed ondansetron was used in order to make the selected formulation acceptable to human. The result of the in vitro release studies and permeation studies through bovine buccal mucosa revealed that complexed ondansetron gave the maximum release and permeation. The stability of drug in the optimized adhesive tablet was tested for 6 h in natural human saliva; both the drug and device were found to be stable in natural human saliva. Thus, buccal adhesive tablet of ondansetron could be an alternative route to bypass the hepatic first-pass metabolism and to improve the bioavailability of (OH).
Key words: buccal adhesive tablet, buccal delivery, mucoadhesion, ondansetron hydrochloride, optimized formula
INTRODUCTION
Ondansetron hydrochloride (OH) is a 5HT3 serotonin antagonist used in the prevention of nausea and vomiting associated with emetogenic cancer chemotherapy (1–3). OH is well absorbed and undergoes first-pass metabolism (4). Oral bioavailability of OH is almost 59%, and peak plasma about 0.03–0.04 µg/ml is obtained after 1.5 to 2 h of administration (5). As administering drug by buccal route avoids hepatic first-pass metabolism, thus, delivery of OH to the systemic circulation via the buccal route would improve its bioavailability. Buccal delivery involves the administration of the drug through the buccal mucosal membrane lining of the oral cavity (6). This route has a number of advantages when compared with the oral route. These advantages include the avoidance of first-pass metabolism, the ability to produce systemic effect with a rapid onset of action, easy accessibility, enhanced patient compliance, and rapid cellular recovery following local stress, and the possibility of removal of the dosage form when required (7–12).
Buccal drug delivery necessitates the use of mucoadhesive polymers as a means of prolonging the residence time of the dosage form on the absorbing membrane as well as localizing drugs in a particular region. In this study a large variety of polymers in different combinations were used. The combinations of polymers were preferred because it could offer acceptable adhesion and biocompatibility properties. The selection of polymer and optimization of the formulation both from adhesion and controlled drug release point of view remain an important goal and challenge for development of a buccal dosage form (13).
The objective of the present study was to formulate a bilayered bioadhesive buccal tablet of OH to prolong the residence time of the buccal tablet, which ensures satisfactory drug release in a unidirectional way to the mucosa, thus avoiding loss of drug due to wash out with saliva. The mucoadhesive buccal tablets were evaluated by weight uniformity, content uniformity, thickness, hardness, swelling index, in vitro drug release studies, mucoadhesive strength, and ex vivo permeation study. The mucoadhesive buccal tablet was compared for drug release and mucoadhesive study for both ondansetron hydrochloride with non-bitter taste base ondansetron and complexes of ondansetron.
MATERIALS AND METHODS
Materials
OH (dehydrated) was a gift sample of M/S Brawn Labs., Ltd. (Faridabad, India). Carbopol (CP 934), sodium alginate, sodium, and carboxymethylcellulose low viscosity (SCMC LV) were supplied by CDH. Ltd. (Mumbai, India). Hydroxypropylmethyl cellulose (HPMC 15 cps), polyethylene glycol (PEG 6000), and lactose were supplied by S.D. Fine chem. Ltd. (Mumbai, India). Ethyl cellulose (EC) was purchased from LOBA-Chemia Company (Mumbai, India). All other reagents used were of analytical reagent grade.
Formulation of Drug-Loaded Buccal Tablets
Calculation for the Dose of Drug in the Tablets
The OH is available in conventional tablets in 4- or 8-mg dose level. The amount of OH reaching the systemic circulation from conventional tablets is 59% (oral bioavailability), i.e., 2.36 or 4.72 mg. Therefore dose was reduced, taking into consideration the first-pass effect. The proposed bioavailability through buccal route is 100%. So it was decided to incorporate 5 mg of drug in the mucoadhesive tablets, taking into consideration the amount of drug that reaches the systemic circulation from the conventional tablets.
Preparation of Mucoadhesive Tablets
The tablets were prepared by direct compression method, using different combinations of polymers (Table I). Hydraulic press was used at a pressure of 15 psig using flat-faced punch of 13 mm diameter for compression. The buccal tablets were prepared using CP 934 as primary mucoadhsive polymer and HPMC, SCMC LV, and sodium alginate as a secondary polymer. The effect of secondary polymers on drug release and mucoadhesion was studied. For unidirectional delivery of drug, a solution of ethyl cellulose was used to coat the tablets from all sides except one.
Table I.
Composition of Various Mucoadhesive Buccal Tablets of Ondansetron
| Batch code | 5% CP 934 (mg) | 10% CP 934 (mg) | 15% CP 934 (mg) | 20% CP 34 (mg) | HPMC 15 cps (mg) | SCMC LV (mg) | Sod. alg. (mg) | OH (mg) | Magnesium stearate (mg) | Aspartame (mg) |
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 10 | 185 | 5 | 1.5 | 2 | |||||
| C2 | 20 | 175 | 5 | 1.5 | 2 | |||||
| C3 | 30 | 165 | 5 | 1.5 | 2 | |||||
| C4 | 40 | 155 | 5 | 1.5 | 2 | |||||
| C5 | 10 | 185 | 5 | 1.5 | 2 | |||||
| C6 | 20 | 175 | 5 | 1.5 | 2 | |||||
| C7 | 30 | 165 | 5 | 1.5 | 2 | |||||
| C8 | 40 | 155 | 5 | 1.5 | 2 | |||||
| C9 | 10 | 185 | 5 | 1.5 | 2 | |||||
| C10 | 20 | 175 | 5 | 1.5 | 2 | |||||
| C11 | 30 | 165 | 5 | 1.5 | 2 | |||||
| C12 | 40 | 155 | 5 | 1.5 | 2 |
OH ondansetron hydrochloride, CP carbopol-934, SCMC LV sodium carboxymethyl cellulose low viscosity, HPMC 15cps hydroxypropylmethylcellulose 15cps, sod. alg. sodium alginate
Optimization of Ondansetron Release from the Prepared Bioadhesive Buccal Tablets
PEG 6000 and lactose in different concentrations were incorporated into the formula, which gave maximum release in order to further enhance drug release (Table II). The results were evaluated by in vitro drug release and ex vivo permeation studies. The formula that gave the maximum drug release of (88.15%) in 8 h was selected as the optimized formulation.
Table II.
Composition of Various Formula of Ondansetron with Different Release Enhancers
| Ingredients (mg) | Formula composition | |||||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | |
| OH | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| (CP 934) 15% | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| SCMC LV | 165 | 165 | 165 | 165 | 165 | 165 | 165 | 165 |
| PEG 6000 | 10 | 20 | 30 | 40 | 40 | 40 | 40 | 40 |
| Lactose | – | – | – | – | 5 | 10 | 15 | 20 |
| Magnesium stearate | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Aspartame | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
OH ondansetron hydrochloride, CP 934 carbopol-934, SCMC LV sodium carboxymethyl cellulose low viscosity, PEG 6000 polyethyleneglycol 6000
Preparation of Ondansetron Base and Complexed Ondansetron
Isolation of Ondansetron Base from Ondansetron Hydrochloride
For isolation of OH base, 100 mg of OH HCl was dissolved in 5 ml of water, to which a dilute ammonia solution was added drop wise until it turned milky. The solution was kept untouched for few hours. The crystals obtained were then filtered, washed with demineralized water two to three times, and subsequently dried. The crystals were found to be tasteless with a melting point of 225°C. Isolation of ondansetron base from ondansetron hydrochloride was confirmed by IR spectral analysis and by differential scanning calorimetry (DSC). Comparison of IR spectrum and DSC chromatogram of ondansetron base with that of ondansetron hydrochloride showed different peaks, which confirmed base formation (14).
Preparation of Complex at pH 6.6
OH is a bitter drug; therefore, in order to make the optimized formulation acceptable to human use, ondansetron base and complexes of ondansetron were prepared (Table III). β cyclodextrin is a cyclic oligosaccharide capable of forming complexes with many drugs by taking up a whole drug molecule, or the same non-polar part of the drug molecule, into its cavity. This is possible because of the hydrophilic outer surface and the hydrophobic central cavity of cyclodextrin molecule. The non-covalent complexes between drug and cyclodextrin are advantageous in improving the bitter taste of the drugs (15).
Table III.
Composition of the Optimized Mucoadhesive Buccal Tablets of Ondansetron
| Ingredients (mg/tablet) | Formulation X with ondansetron hydrochloride | Formulation Y with complexed ondansetron | Formulation Z with ondansetron base |
|---|---|---|---|
| CP 934 (15%) | 30 | 30 | 30 |
| SCMC LV | 165 | 165 | 165 |
| PEG 6000 | 40 | 40 | 40 |
| Lactose | 5 | 5 | 5 |
| Mag. stearate | 1.5 | 1.5 | 1.5 |
| Aspartame | 2 | 2 | 2 |
CP 934 carbopol-934, SCMC LV sodium carboxymethyl cellulose low viscosity, PEG 6000 polyethyleneglycol 6000
For the preparation of complex, 250 mg of OH was dissolved in 10 ml distilled water. Separately, 750 mg of β cyclodextrin was suspended in 12.5 ml of distilled water and adjusted to pH 6.6 using 2% sodium hydroxide solution. Drug solution was added drop wise to the β cyclodextrin suspension with continuous stirring by magnetic stirrer, and finally, the pH was adjusted to 6.6 with 2% sodium hydroxide solution. The solution was stirred at 1,000 rpm for 6 h followed by filtration using Whatman filter paper to obtain the residues, which were washed two to three times with water to remove free drug (if any). The residues were further dried, tasted, and found to be tasteless. Assay of the resulting residue revealed that more than 99.9% of the drug was complexed (5).
Formation of drug complex was confirmed by IR spectral analysis and by DSC. Comparison of IR spectrum and DSC chromatogram of complexed drug with that of OH showed different peaks, which confirmed complex formation.
Determination of Physiochemical Parameters
Weight Variation
Twenty tablets were weighed individually and then collectively, average weight of the tablets was calculated, then weight variation was calculated. The hardness of the tablets was determined using a Monsanto hardness tester.
Friability Test
The tablets were tested for friability testing using Roche friabilator. For this test, six tablets were weighed and subjected to combined effect of abrasion and shock in the plastic chamber of friabilator revolving at 25 rpm for 4 min, and the tablets were then dusted and reweighed.
Content Uniformity
Ten tablets were accurately weighed and powder-crushed in a glass pestle mortar. An accurately weighed amount equivalent to 5 mg of pure drug was taken, and the assay was performed spectrophotometrically at 249 nm in triplicates.
Surface pH
The surface pH of the tablets was determined in order to investigate the possibility of any irritation in the oral cavity. The tablets were kept in contact with simulated saliva solution for 2 h, and pH was noted by bringing the electrode in contact with surface of formulations (16).
Swelling Studies
Buccal tablets (n = 3) were weighed individually (W1) and placed separately in petri dishes containing 5 ml of isotonic phosphate buffer (pH 6.5) solution. At regular intervals (0.5, 1, 2, 3, 4, 5, and 6 h), the tablets were removed from the petri dishes, and excess surface water was removed carefully using the filter paper. The swollen tablet was then reweighed (W2), and swelling index (SI) was calculated using the following formula (17): SI = W2 − W1/W1.
In Vitro Drug Release
The in vitro drug release study was conducted using Hanson Research-SR8 Plus apparatus (Los Angeles, CA, USA). Isotonic phosphate buffer (IPB) pH 6.5 (500 ml) was used as the release medium. The release media were used to simulate the physiological in vivo condition of the buccal cavity. The release was performed at 37.5 ± 0.5°C, at a rotation speed of 50 rpm. Samples (5 ml) were withdrawn at intervals of 15, 30, 60, 90, 120, 180, 240, 300, 360, 420, and 480 min. The samples were filtered using Whatman filter paper, and the drug concentration was measured by UV spectrophotometer at 249 nm. The dissolution medium was replaced with fresh buffer to maintain its constant volume and sink condition, when the sample was withdrawn each time (18). Teflon block was used in order to cover all sides of the tablet except one, which is used as only the side of drug released to ensure unidirectional release.
In Vitro Drug Permeation
Approval to carry out in vivo animal studies was obtained from the Institutional Animal Ethics Committee (IAEC) Jamia Hamdard, New Delhi, India. For the permeation studies across bovine buccal mucosa, the animals were killed in the slaughterhouse, and the bovine buccal mucosa was surgically removed from the oral cavity. It was washed thoroughly and then dipped in 0.2 M ammonia solution. This treatment leads to the separation of buccal membrane from its underlying tissues. The buccal membrane so obtained was washed with IPB pH 7.4 and mounted at the junction between the two chambers of Franz diffusion cell with mucosal side upward. The two chambers were then tied securely with the help of silica gum and rubber bands. A measured volume of IPB pH 7.4 was added to the lower chamber of the cell, such that there was no bubble between the membrane and the buffer. The assembly was placed on a magnetic stirrer. The temperature was maintained at 37 ± 0.5°C by circulating water in the outer jacket of the cell with the help of peristaltic pump (19–21). The buccal mucosa was allowed to stabilize for 24 h by continuously replacing the buffer in the lower chamber with fresh buffer until no absorbance was obtained at λmax of the drug. This was done to allow the removal of soluble components in the membrane as they may interfere with the analysis of the drug. The upper side of the membrane was kept moistened by IPB pH 6.5. As in the buccal oral cavity, the pH varies from 5.5 to 6.8; therefore, an average of pH 6.5 was used. Since after permeation the drug is in the systemic circulation; therefore, to simulate it, a pH of 7.4 was used. Therefore during the permeation study, the upper compartment simulated the buccal cavity pH 6.5, and the lower compartment simulated the physiological pH of 7.4 for blood. After stabilization, the bioadhesive tablet was mechanically stuck on the mucosal membrane. Samples (3 ml) were withdrawn at fixed interval for 8 h. The samples were analyzed, and percentage drug permeated was determined (8,22,23).
Mucoadhesive Study
Texture analyzer TX-XT2i, Stable Micro Systems (Surrey, England) was used to determine the bioadhesive strength of the tablets. Bovine buccal mucosa membrane was used as model membrane. It was hydrated with simulated saliva solution and tied to the lower probe of the assembly. The simulated saliva solution was prepared by using disodium hydrogen orthophosphate (dihydrate) 38 g, potassium dihydrogen orthophosphate (anhydrous) 0.18 g, sodium chloride 8.0 g, and demineralized water (for a volume of up to 1,000 ml). The tablet was attached to the upper probe of the assembly using an adhesive. The upper probe was allowed to fall on the lower probe with a test speed of 0.5 mm/s and a post-test speed of 1 mm/s. The tablet was allowed to adhere to the bovine buccal mucosa membrane with applied force 150 g, return distance 10 mm, and contact time 15 s (24). Mucoadhesive force was than calculated according to the following equation:
 |
RESULTS AND DISCUSSION
The average weight of the tablet was found to be between 243.5 and 248.5 mg. The maximum variation from average was found to be ±0.8% from all the formulations. Hardness of the tablets for all the formulations was found to be between 7.0 and 7.5 kg/cm2 with an average of 7.18 kg/cm2. The percentage deviation in hardness was 0.02 to 0.18. Percentage loss in weight was found to be between 0.045 and 0.055 mg with an average of 0.05 mg. Percentage drug content for all formulation was found to be between 107.4% and 99.15%. Surface pH of all the formulations was found to be between 6.98 and 5.8, which were within the acceptable salivary pH range (5.5–7.0). It was conducted that the tablets would produce no local irritation to the mucosal surface.
The bioadhesive performance of different formulations is given in (Table IV). The strength was dependent on the property of bioadhesive polymers, which on hydration, adhere to mucosal surface, as well as on the concentration of the polymer used. The tablets containing a higher proportion of CP showed higher mucoadhesive strength for 15 s contact time. This high bioadhesive strength of CP may be due to the formation of secondary bioadhesion bonds with mucin and interpenetration of the polymer chains in the interfacial region, as compared to other polymers that only undergo superficial bioadhesion. Tablets containing CP 20% and SCMC LV (C8) exhibited the highest bioadhesive strength 145.09 g, which however decreased with increasing amount of SCMC LV and decreasing amount of CP (C8–C5). As SCMC LV, owing to its solubility in water, resulted in lower mucoadhesion strength, however, SCMC LV has high viscosity when compared with HPMC 15 cps and sodium alginate polymers; this viscosity makes the SCMC group have more mucoadhesive strength level than HPMC group and sodium alginate group; however, all the tablets exhibited good mucoadhsive strength with bovine buccal mucosa.
Table IV.
Physiochemical Properties of Mucoadhesive Buccal Tablets of Ondansetron
| Batch code | % loss in weight | Hardness | % Drug content | Surface pH | Swelling index after (8 h) | Mucoadhesive strength (g) |
|---|---|---|---|---|---|---|
| C1 | 0.045 ± 0.05 | 7.0 ± 0.15 | 99.15 ± 0.5 | 6.12 ± 0.02 | 60.70 ± 0.85 | 47.597 ± 1.20 |
| C2 | 0.055 ± 0.08 | 7.0 ± 0.16 | 100.62 ± 0.4 | 6.98 ± 0.08 | 63.35 ± 0.80 | 79.022 ± 1.00 |
| C3 | 0.053 ± 0.07 | 7.50 ± 0.18 | 100.25 ± 0.6 | 6.69 ± 0.05 | 64.11 ± 2.55 | 116.18 ± 1.25 |
| C4 | 0.055 ± 0.001 | 7.25 ± 0.30 | 99.27 ± 0.8 | 6.98 ± 0.03 | 66.10 ± 1.88 | 130.97 ± 2.34 |
| C5 | 0.065 ± 0.005 | 7.30 ± 0.25 | 100.55 ± 0.6 | 6.93 ± 0.02 | 70.05 ± 1.20 | 135.51 ± 3.25 |
| C6 | 0.052 ± 0.006 | 7.0 ± 0.20 | 100.45 ± 0.4 | 5.81 ± 0.07 | 71.40 ± 1.13 | 137.16 ± 2.10 |
| C7 | 0.046 ± 0.002 | 7.0 ± 0.22 | 99.84 ± 0.7 | 5.95 ± 0.07 | 73.16 ± 0.95 | 141.51 ± 1.23 |
| C8 | 0.060 ± 0.001 | 7.25 ± 0.23 | 99.35 ± 0.8 | 5.85 ± 0.05 | 79.72 ± 0.70 | 145.09 ± 2.37 |
| C9 | 0.070 ± 0.003 | 7.40 ± 0.24 | 100.25 ± 0.2 | 5.80 ± 0.08 | 66.06 ± 0.85 | 19.123 ± 064 |
| C10 | 0.065 ± 0.002 | 7.15 ± 0.19 | 100.16 ± 0.5 | 5.90 ± 0.09 | 67.56 ± 1.15 | 20.855 ± 0.90 |
| C11 | 0.059 ± 0.004 | 7.0 ± 0.16 | 99.38 ± 0.4 | 6.88 ± 0.03 | 68.82 ± 0.65 | 22.167 ± 0.65 |
| C12 | 0.070 ± 0.015 | 7.0 ± 0.21 | 107.48 ± 0.3 | 6.92 ± 0.02 | 70.06 ± 0.90 | 30.832 ± 1.10 |
Appropriate swelling behavior of a buccal adhesive system is an essential property for uniform and prolonged release of the drug and effective mucoadhesion. All the tablets swelled gradually, reaching a plateau after 8 h and remaining constant till the end of experiment. Most of the tablets exhibited 65% swelling within 1 h except group I (HPMC 15 cps) that showed maximum swelling after 2 h. Faster swelling rate was obtained from groups II (sodium alginate) and III (SCMC LV) that swell above 65% within 1 h. These findings can be correlated with the hydrophillicity of cellulose derivatives. This usually varies according to the degree of substitution and to some extent with the polymer viscosity grade. The hydrophilic polymer SCMC LV increased the surface wettability and consequently water penetration within the matrix. As SCMC LV is hydrophilic polymer and has the ability to absorb water, so maximum swelling was seen with formulations containing a high proportion of SCMC LV to CP 934, so C5 formula at the CP 934 percentage of 5% exhibited the highest SI.
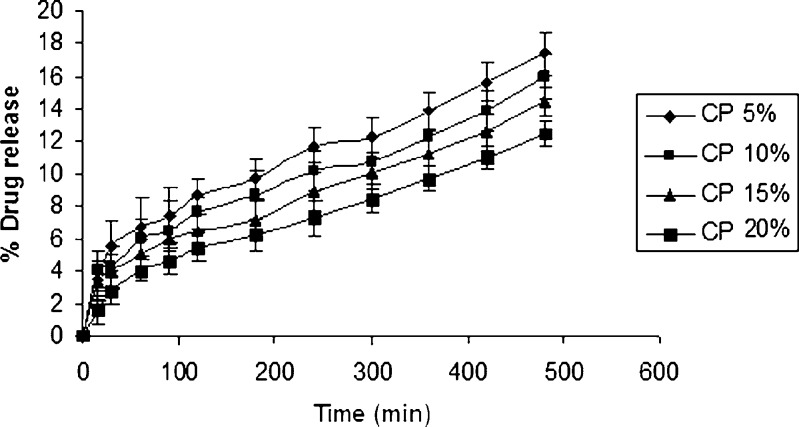
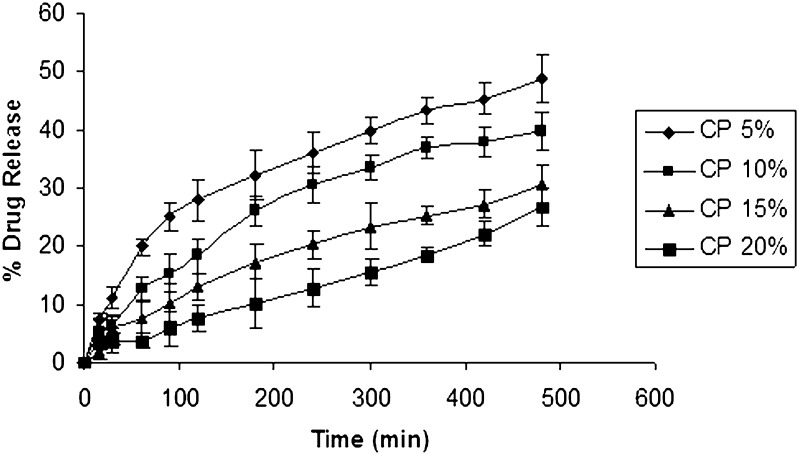
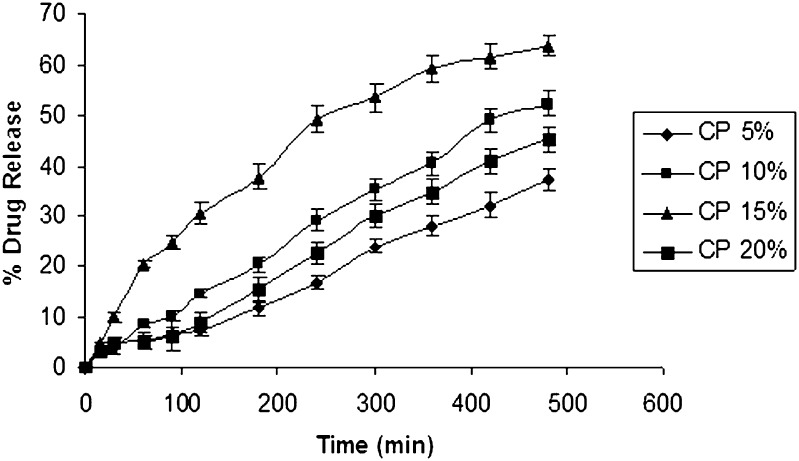
Using IPB pH 6.5 (500 ml) as the dissolution medium to simulate the oral environment, the percentage drug release from formulations containing different polymers is given in (Figs. 1, 2, and 3). Tablets from HPMC group showed a percentage drug release that is less than other groups, and the highest release from this group was found to be 17.40% (Fig. 1). These results were due to slower swelling rate and low viscosity of HPMC 15 cps. Tablets from sodium alginate group showed a higher percentage drug release compared to HPMC group. The maximum drug release from this group was found to be 48.893% (Fig. 2). Tablets from SCMC group showed a maximum percentage drug release 63.7% (Fig. 3), which was attributed to the higher percentage of swelling of SCMC. This finding was also supported by the results of swelling studies where the highest SI was also exhibited by a formulation containing SCMC LV and 5% CP 934 (Fig. 3). It is anticipated that the high amount of water uptake by SCMC LV may lead to considerable swelling of the polymer matrix, allowing the drug to diffuse out at a faster rate.
Fig. 1.
In vitro release curve of ondansetron with varying concentration of carbopol and HPMC 15cps using isotonic phosphate buffer pH 6.5 (500 ml) as the release medium
Fig. 2.
In vitro release curve of ondansetron with varying concentration of carbopol and sodium alginate using isotonic phosphate buffer pH 6.5 (500 ml) as the release medium
Fig. 3.
In vitro release curve of ondansetron with varying concentration of carbopol and SCMC low viscosity using isotonic phosphate buffer pH 6.5 (500 ml) as the release medium
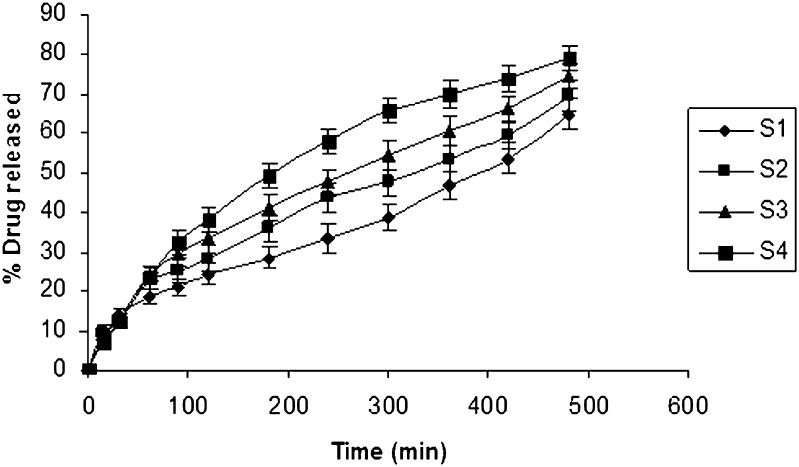
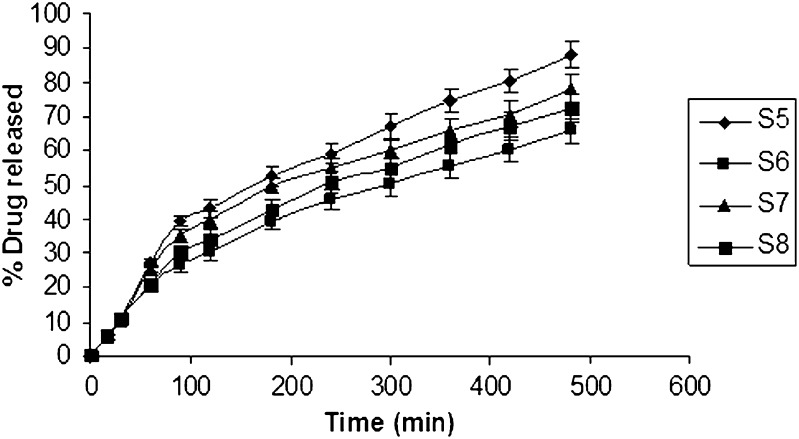
PEG 6000 in different concentrations 10, 20, 30, and 40 mg was incorporated as a release enhancer in the chosen formulation in Table II. The results of the dissolution studies revealed that formulation S4 comprising PEG 6000 (40 mg) exhibited a maximum in vitro release of 79.24% in 8 h (Fig. 4). The release pattern of formulation containing PEG 6000 is S4 > S3 > S2 > S1>. PEG 6000 is reported to increase porosity of the matrix and produce channels, which in turn facilitate the dissolution medium to penetrate the matrix and dissolve the drug more rapidly, thereby enhancing drug release with increased PEG 6000 concentration (25). The second release enhancer lactose was incorporated in different concentrations in the formulation for further drug release. Five, 10, 15, and 20 mg lactose were used, respectively. The release rate was increased to 88.148% with 5 mg lactose. Lactose enhances the release of a drug by the formation of pores and channels in the matrix due to its rapid and high solubility in water. The release of the drug is mainly through the channels formed in the matrix, which allow the dissolution medium to penetrate the deeper sites of matrix and dissolve the drug more rapidly (26). Since an increase in the concentration of lactose incorporated in the formula did not influence the rate of release remarkably (Fig. 5), it was decided to use a combination of PEG 6000 and lactose as release modifiers.
Fig. 4.
In vitro release curve of ondansetron with varying concentration of PEG 6000 using isotonic phosphate buffer pH 6.5 (500 ml) as the release medium
Fig. 5.
In vitro release curve of ondansetron with varying concentration of PEG 6000 and lactose using isotonic phosphate buffer pH 6.5 (500 ml) as the release medium
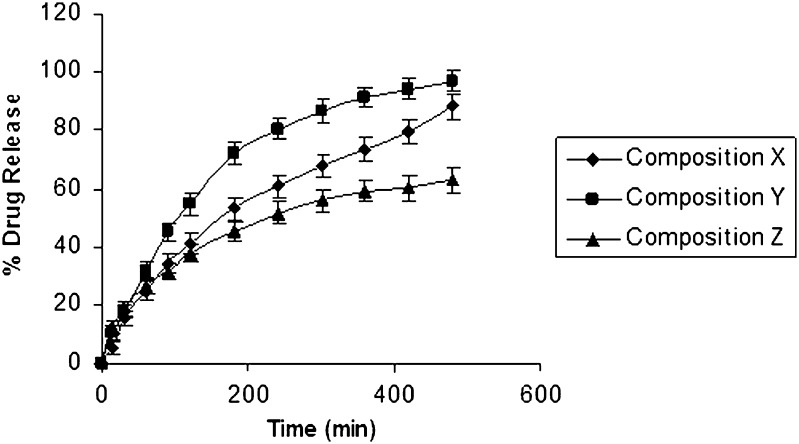
In order to mask the bitter taste of the optimized formulation X, OH was substituted with an equivalent amount of ondansetron base and complexes of ondansetron to obtain the formulations Y and Z (Table III). The dissolution study of the optimized formula X, complexes ondansetron Y, and based ondansetron Z revealed rapid release of drug from formula Y in dissolution media (96.83%) as compared with formula X (88.148%) and formula Z (62.95%; Fig. 6). It was postulated that the dissolution process might have involved solubilization of β cyclodextrin, which is used as complexing agent in formula Y. The profile of formulation X, Y, and Z was found significant (P > 0.05) using GraphPadInStat [DATASET1.ISD].
Fig. 6.
In vitro release rate curve of composition X (pure ondansetron), Y (complexed ondansetron), and Z (ondansetron base) using isotonic phosphate buffer pH 6.5 (500 ml) as the release medium
The release mechanism was analyzed using the well-known semi-empirical equation (27). Mt/M∞ = Ktn, where Mt/M∞ is fractional release of the drug; t denotes the release time; and k represents a constant, incorporating structural and geometrical exponent and characterizes the type of release mechanism during the dissolution process. For non-Fickian release, the value of n falls between 0.5 and 1.0; while in case of Fickian diffusion, n = 0.5; for zero order release (case II), n = 1; and for (super case II), n > 1. The obtained values of k (kinetic constant), n (diffusional exponent), and r2 (correlation coefficient) are depicted in Table V. The values of n are estimated by linear regression of log (Mt/M∞) versus log (t), and these values were between 0.5 and 1.0, indicating that the release of OH was found to be a non-Fickian diffusion. In the kinetics study, the order of drug release from all the formulations was studied by plotting the log percentage cumulative retained versus time curve, and it followed first-order kinetics.
Table V.
Values of r 2 (Correlation Coefficient), n (Diffusional Exponent), k (Kinetic Constant) Following Linear Regression of Log (M t/M ∞) Versus Log (t) for Optimized Formula
| Code no. | n | k | r 2 |
|---|---|---|---|
| X | 0.7047 | 1.363 | 0.9606 |
| Y | 0.6415 | 1.531 | 0.9824 |
| Z | 0.5688 | 1.732 | 0.9855 |
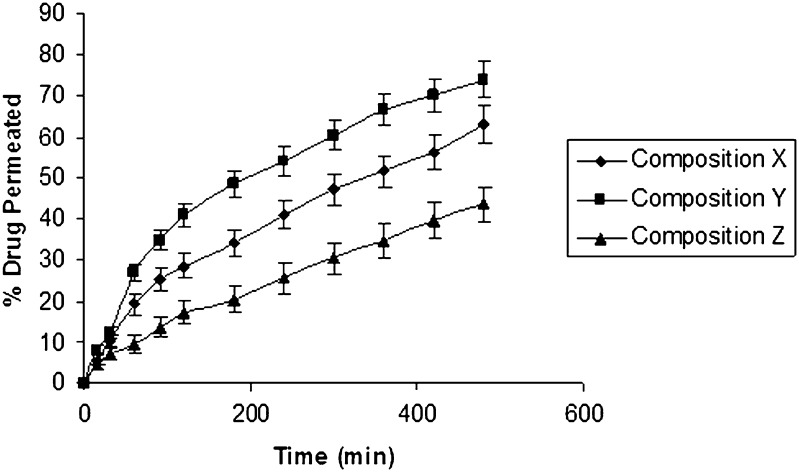
All the three formulations (X, Y, and Z) exhibited almost similar and satisfactory bioadhesion values. Permeation studies were also carried out through bovine buccal mucosa using modified Franz diffusion cell. Drug (62.96%, 73.96%, and 43.46%) was obtained to permeate through mucosal membrane from composition X, Y, and Z, respectively (Fig. 7). The results of the permeation study were in good correlation with the result of the dissolution study (Table VI).
Fig. 7.
Permeation rate curve of composition X (pure ondansetron), Y (complexed ondansetron), and Z (ondansetron base) through bovine buccal mucosa
Table VI.
Comparison of the Three Ondansetron Forms of Optimized Formulae
| Parameters | Formulae of ondansetron hydrochloride | Formulae of complexed ondansetron | Formulae of based ondansetron |
|---|---|---|---|
| Hardness | 7.0 ± 0.15 | 7.50 ± 0.18 | 7.30 ± 0.25 |
| % drug content | 99.45 ± 0.5 | 100.52 ± 0.4 | 100.23 ± 0.6 |
| % loss in weight | 0.065 ± 0.005 | 0.070 ± 0.003 | 0.066 ± 0.008 |
| Surface pH | 6.95 ± 0.08 | 6.88 ± 0.07 | 6.00 ± 0.05 |
| % swelling index (8 h) | 68.50 ± 0.80 | 70.55 ± 0.50 | 64.50 ± 0.80 |
| Bioadhesive strength (g) | 134.05 ± 2.50 | 132.05 ± 1.80 | 130.05 ± 2.50 |
| In vitro drug release (8 h) | 88.148 ± 3.65 | 96.83 ± 2.88 | 62.95 ± 2.23 |
| In vitro buccal permeation (8 h) | 62.96 ± 1.52 | 73.96 ± 1.73 | 43.46 ± .88 |
CONCLUSION
Transmucosal buccal route of delivery for OH is one of the best alternatives as it would bypass the pre-systemic metabolism and thus the dose can also be reduced.
Formulation Y comprising complex ondansetron exhibited a good balance between in vitro release, in vitro drug permeation, and mucoadhesion strength and was therefore selected as the optimized formulation. The optimized formulation was also satisfactory in terms of surface pH and physical parameters.
References
- 1.Lachaine J, Laurier C, Langleben A, Vaillant L. Cost-effectiveness and quality of life evaluation of ondansetron and metoclopramide for moderately emetogenic chemotherapy regimens in breast cancer. J Oncol Hema. 1999;32:105–12. doi: 10.1016/s1040-8428(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 2.Mohan KC, Ravikumar K. Ondansetron hydrochloride: a competitive serotonin 5-HT3 receptor blocker. Acta Cryst Sect C Cryst Struct Comm. 1995;C51:2627–9. doi: 10.1107/S0108270195008043. [DOI] [PubMed] [Google Scholar]
- 3.Milap PhDNC, Leanna HN, John PhDK. Efficacy and safety of ondansetron in pediatric patients undergoing bone marrow transplantation. Clinical Therapeutics. 1996;18:466–76. doi: 10.1016/S0149-2918(96)80027-0. [DOI] [PubMed] [Google Scholar]
- 4.Alur HH, Pather SI, Mitra AK, Johnston TP. Transmucosal sustained-delivery of chlorpheniramine maleate in rabbits using a novel natural mucoadhesive gum as excipients in buccal tablets. Int J Pharm. 1999;188:1–10. doi: 10.1016/S0378-5173(99)00211-2. [DOI] [PubMed] [Google Scholar]
- 5.Salem II, Lopez JMR, Galan AC. Ondansetron hydrochloride. In: Brittan HG, Academic Press an Imprint of Elsevier, editor. Analytical profiles of drug substances and excipients, vol. 27. California: San Diego; 2001. pp. 301–339. [Google Scholar]
- 6.Miller NS, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Del. 2005;57:1666–91. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Jain CB, Aungs J, Adeyeye MC. Development and in vivo evaluation of buccal tablets prepared using danazol-, sulfobutylether 7ß-cyclodextrin (SBE7) complexes. J Pharm Sci. 2002;91:1659–68. doi: 10.1002/jps.10163. [DOI] [PubMed] [Google Scholar]
- 8.Patel VM, Prajapati BG, Patel MM. Formulation, evaluation, and comparison of bilayered and multilayered mucoadhesive buccal devices of propranolol hydrochloride. AAPS PharmSciTech. 2007;8:E22. doi: 10.1208/pt0804089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park CR, Munday DL. Evaluation of selected polysaccharide excipients in buccoadhesive tablets for sustained release of nicotine. Drug Dev Ind Pharm. 2004;30:609–17. doi: 10.1081/DDC-120037492. [DOI] [PubMed] [Google Scholar]
- 10.Nagai T, Konishi R. Buccal/gingival drug delivery systems. J Control Release. 1987;6:353–60. doi: 10.1016/0168-3659(87)90088-5. [DOI] [Google Scholar]
- 11.Junginger HE, Hoogstraate JA, Verhoef JC. Recent advances in buccal drug delivery and absorption: in vitro and In vivo studies. J Control Release. 1999;62:149–59. doi: 10.1016/S0168-3659(99)00032-2. [DOI] [PubMed] [Google Scholar]
- 12.Pramodkumar TM, Desai KGh, Shivakumar HG. Buccal permeation enhancers. Ind J Pharm Edu. 2002;36:147–51. [Google Scholar]
- 13.Munasur P, Pillay V, Chetty DJ, Govender T. Statistical optimization of the mucoadhesivity and characterization of multipolymeric propranolol matrices for buccal therapy. Int J Pharm. 2006;323:43–51. doi: 10.1016/j.ijpharm.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 14.Sohi H, Khar RK, Singh S. Development of controlled release tablets of anti-migraine agent. Proceeding of the 31st Annual Meeting and Exposition of the Controlled Release Society. 2004, June 12–16, Honolulu, Hawaii, USA; 290
- 15.Jug M, Becirevic-Lacan M. Influence of hydroxypropyl-β-cyclodextrin complexation on piroxicam release from buccoadhesive tablets. Eur J Pharm Sci. 2004;21:251–260. doi: 10.1016/j.ejps.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Bottenberg P, Cleymaet R, Muynck CD, Remon JP, Coomans D, Slop D. Development and testing of bio-adhesive, fluoride-containing slow release tablets for oral use. J Pharm Pharmacol. 1991;43:457–64. doi: 10.1111/j.2042-7158.1991.tb03514.x. [DOI] [PubMed] [Google Scholar]
- 17.Parodi B, Russo E, Caviglioli G, Cafaggi S, Bignardi G. Development and characterization of a buccoadhesive dosage form of oxycodone hydrochloride. Drug Dev Ind Pharm. 1996;22:445–50. doi: 10.3109/03639049609069353. [DOI] [Google Scholar]
- 18.Okamoto H, Taguchi H, Iida K, Danjo K. Development of polymer film dosage forms of lidocaine for buccal administration I. penetration rate and release rate. J Control Release. 2001;77:253–60. doi: 10.1016/S0168-3659(01)00509-0. [DOI] [PubMed] [Google Scholar]
- 19.Sandri G, Rossi S, Bonferoni MC, Ferrari F, Zambito Y, Colo GD, Caramella C. Buccal penetration enhancement properties of N-trimethyl chitosan: influence of quaternization degree on absorption of a high molecular weight molecule. Int J Pharm. 2005;297:146–55. doi: 10.1016/j.ijpharm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Ceschel GC, Bergamante V, Calabrese V, Biserni S. Design and evaluation in vitro of controlled release mucoadhesive tablets containing chlorhexidene. Drug Dev Ind Pharm. 2006;32:53–61. doi: 10.1080/03639040500388300. [DOI] [PubMed] [Google Scholar]
- 21.Sandri G, Rossi S, Ferrari F, Bonferoni MC, Muzzarelli C, Caramella C. Assessment of chitosan derivatives as buccal and vaginal penetration enhancers. Eur J Pharm Sci. 2004;21:351–9. doi: 10.1016/j.ejps.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Patel VM, Prajapati BG, Patel MM. Design and characterization of chitosan-containing mucoadhesive buccal patches of propranolol hydrochloride. Acta Pharm. 2007;57:61–72. doi: 10.2478/v10007-007-0005-9. [DOI] [PubMed] [Google Scholar]
- 23.Jain SK, Jain NK, Gupta Y, Jain A, Jain D, Chaurasia M. mucoadhesive chitosan microspheres for non-invasive and improved nasal delivery of insulin. Ind J Pharm Sci. 2007;69:498–504. doi: 10.4103/0250-474X.36933. [DOI] [Google Scholar]
- 24.Eouani C, Piccerelle Ph, Prinderre P, Bourret E, Joachim J. In vitro comparative study of buccal mucoadhesive performance of different polymeric films. Eur J Pharm Biopharm. 2001;52:45–55. doi: 10.1016/S0939-6411(01)00146-1. [DOI] [PubMed] [Google Scholar]
- 25.Feng-Qian LI, Jin-Hong HU, Deng JX, Su H, Xu S, Liu JY. In vitro controlled release of sodium ferulate from Compritol 888 ATO-based matrix tablets. Int J Pharm. 2006;324:152–7. doi: 10.1016/j.ijpharm.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Obaidat AA, Obaidat RM. Controlled release of tramadol hydrochloride from matrices prepared using glyceryl behenate. Eur J Pharm Biopharm. 2001;52:231–5. doi: 10.1016/S0939-6411(01)00173-4. [DOI] [PubMed] [Google Scholar]
- 27.Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–11. [PubMed] [Google Scholar]