Abstract
Ethyl cellulose microcapsules were developed for use as a drug-delivery device for protecting folic acid from release and degradation in the undesirable environmental conditions of the stomach, whilst allowing its release in the intestinal tract to make it available for absorption. The controlled release folic acid-loaded ethyl cellulose microcapsules were prepared by oil-in-oil emulsion solvent evaporation using a mixed solvent system, consisting of a 9:1 (v/v) ratio of acetone:methanol and light liquid paraffin as the dispersed and continuous phase. Span 80 was used as the surfactant to stabilize the emulsion. Scanning electron microscopy revealed that the microcapsules had a spherical shape. However, the particulate properties and in vitro release profile depended on the concentrations of the ethyl cellulose, Span 80 emulsifier, sucrose (pore inducer), and folic acid. The average diameter of the microcapsules increased from 300 to 448 µm, whilst the folic acid release rate decreased from 52% to 40%, as the ethyl cellulose concentration was increased from 2.5% to 7.5% (w/v). Increasing the Span 80 concentration from 1% to 4% (v/v) decreased the average diameter of microcapsules from 300 to 141 µm and increased the folic acid release rate from 52% to 79%. The addition of 2.5–7.5% (w/v) of sucrose improved the folic acid release from the microcapsules. The entrapment efficiency was improved from 64% to 88% when the initial folic acid concentration was increased from 1 to 3 mg/ml.
Key words: emulsion solvent evaporation, ethyl cellulose, folic acid, microcapsule
INTRODUCTION
Folic acid or folate is a member of the vitamin B family and is essential for the healthy functioning of a variety of physiological processes in humans. Folate plays a crucial role in the biosynthesis of nucleotides that are essential for nucleic acid metabolism, cell division, and gene expression. In recent years, interest in the fortification of food products with folic acid has increased, largely in response to the findings of epidemiological studies that link folate deficiency with neural tube defects (NTDs), coronary heart disease, and megaloblastic anemia (1). According to public health service recommendations, all women who are likely to be or become pregnant should consume 400 µg/day of folic acid to reduce the risk of birth defects (2). Fortification of folic acid in one or more of the commonly consumed dietary items is now regarded as the best method to ensure that women have a sufficient folate intake during pregnancy to reduce the risk of having a fetus affected by NTDs.
Folic acid is typically found in leafy green vegetables, such as spinach and turnip greens, in fruits, such as citrus fruits and juices, and in dried beans, peas, and nuts. Most natural folate derivatives in food are highly sensitive to such parameters as oxygen, temperature, pH, and light. Folic acid is photodegraded in aqueous solution by sunlight, ultraviolet light, and visible light, forming p-aminobenzoyl-L-glutamic acid and pterine-6-carboxylic acid as the major degradation products, along with traces of p-aminobenzoic acid (3,4). Most studies have demonstrated negative effects on the stability of folates in both industrial processing and household preparation of foods, with increasing losses of bioactive folates with higher heating temperatures and longer heating times (5–7). The overall effect, when considering the chemistry of natural folates, is that they are all unstable to a varying degree (8). Folic acid, where the pteridine ring is not reduced, is the cofactor produced synthetically by commercial companies and the form found in supplements (tablets) and supplemented breakfast cereals and flours (8).
There is, therefore, a need to develop new techniques for enhancing the folate content, stability, and bioavailability in foods and supplements. Many studies have demonstrated some ways to protect folic acid from adverse environmental conditions by encapsulating the active ingredient into a polymer matrix coating. For example, incorporation of folic acid into microcapsules using either alginate or combinations of alginate and pectin polymer can improve its stability (9). In addition, the technical feasibility of adding folic acid onto rice by coating it with edible polymers such as locust bean gum, agar, xanthan gum, and pectin has been investigated, which is of interest given the low cost and high consumption of folate-deficient rice-based staple diets in much of the poorer world (1).
Microencapsulation has been used in the pharmaceutical industry for the conversion of liquids to solids, taste-masking of bitter drugs, acquiring prolonged or sustained payload (drug) release, reducing gastric irritation, and environmental protection of labile moieties (10). There are several techniques currently in use to produce sustained release dosage vectors, which include physicochemical processes, such as solvent evaporation or phase separation methods, as well as mechanical processes, such as spray drying, and a non-solvent addition process (11–16). Polymeric microcapsules have received considerable attention as drug-delivery systems in recent years and have been used to modify and retard drug release (17). Ethyl cellulose is a non-biodegradable and biocompatible polymer and is one of the extensively studied encapsulating materials for the controlled release of pharmaceuticals (18). The use of ethyl cellulose-based microcapsules has been reported by several authors for the encapsulation of a variety of drugs, such as the sustained release of amoxicillin (19), theophylline (20), and 5-fluorouracil (21) prepared by solvent evaporation. Fabrication of ethyl cellulose microspheres using chitosan as a stabilizer has been reported (22).
The purpose of the present work was to prepare controlled release microcapsules of folic acid, using ethyl cellulose as a retarding material, by applying the solvent evaporation technique. Various process and formulation parameters, such as the polymer and surfactant concentrations, the amount of pore inducer, and drug content, were all optimized. These microcapsules were then evaluated for their drug entrapment efficiency and in vitro release profile. The physical characteristics were evaluated by scanning electron microscopy (SEM), laser particle size distribution, and infrared spectroscopy.
MATERIALS AND METHODS
Materials
Folic acid (Fluka, Lot No. 455042/1), tetrabutyl ammonium bisulfate, Sorbitan monooleate (Span 80), and ethyl cellulose were purchased from Fluka (UK). Paraffin oil (food grade) was obtained from The Union Chemical 1986 Ltd. (Thailand). Acetone, sodium hydrogen phosphate, potassium dihydrogen phosphate (analytical reagent grade), acetonitrile, and methanol (high-performance liquid chromatography (HPLC) grade) were purchased from Merck (Germany); folic acid tablets were purchased from GPO Pharmacy (Thailand). All the reagents and solvents used were of analytical grade satisfying pharmacopoeial standards.
Methods
Preparation of Folic Acid Microcapsules
The microcapsules of folic acid were prepared by the solvent evaporation technique. Ethyl cellulose (1.0 g) was dissolved in 20 ml of solvent (1:9 (v/v) methanol:acetone), and 20 mg of folic acid was then dispersed in the ethyl cellulose solution to give a final concentration of 1 mg/ml. The drug-polymer mixture was mixed well and then slowly emulsified into 100 ml of paraffin oil that contained 1 ml of Span 80 as an emulsifier. The whole system was continuously stirred at 2,000 rpm (electric overhead stirrer IKA RW 20) for 5 h at room temperature. Acetone and methanol were then completely removed by evaporation, and the microcapsules were separated from the solution by vacuum filtration. The filtered microcapsules that formed were then washed three times with 50 ml of n-hexane to remove the residual paraffin oil and then collected, dried at room temperature overnight, and stored in a desiccator.
Effect of Concentration of Ethyl Cellulose as the Wall Former
The microcapsules of folic acid were prepared by the above method but with three different final concentrations of ethyl cellulose, namely, 2.5%, 5.0%, and 7.5% (w/v). The other parameters were kept constant, as summarized in Table I.
Table I.
Preparation Parameters for Folic Acid Microcapsules
| Formulation | Polymer concentration % (w/v) | Span 80 concentration % (v/v) | Sucrose concentration % (w/v) | Drug content (mg,% (w/v)) | D50 a µm | SPANb | % EEc |
|---|---|---|---|---|---|---|---|
| F1 | 2.5 | 1 | – | 20, 0.1 | 299 ± 9 | 2.12 ± 0.06 | 80.29 ± 1.46 |
| F2 | 5.0 | 1 | – | 20, 0.1 | 391 ± 2 | 1.86 ± 0.04 | 61.80 ± 1.22 |
| F3 | 7.5 | 1 | – | 20, 0.1 | 447 ± 9 | 1.42 ± 0.04 | 41.85 ± 1.33 |
| F4 | 2.5 | 2 | – | 20, 0.1 | 193 ± 3 | 1.78 ± 0.04 | 65.77 ± 1.39 |
| F5 | 2.5 | 4 | – | 20, 0.1 | 141 ± 2 | 1.65 ± 0.04 | 57.19 ± 1.43 |
| F6 | 2.5 | 1 | 2.5 | 20, 0.1 | 489 ± 9 | 1.73 ± 0.03 | 56.64 ± 3.43 |
| F7 | 2.5 | 1 | 5.0 | 20, 0.1 | 548 ± 7 | 1.66 ± 0.01 | 64.78 ± 3.70 |
| F8 | 2.5 | 1 | 7.5 | 20, 0.1 | 740 ± 9 | 1.33 ± 0.05 | 52.12 ± 3.59 |
| F9 | 2.5 | 1 | 5.0 | 40, 0.2 | 649 ± 18 | 1.82 ± 0.03 | 65.16 ± 2.92 |
| F10 | 2.5 | 1 | 5.0 | 60, 0.3 | 789 ± 13 | 1.30 ± 0.02 | 88.36 ± 1.58 |
Means with a different lowercase superscript letter (a, b, c) within a column differ significantly (p < 0.05)
aD50 = mean particle size (mean ± SD)
bSPAN = polydispersity Index (mean ± SD)
cEE = encapsulation efficiency (mean ± SD)
Effect of Span 80 Emulsifier Concentration
The microcapsules of folic acid were prepared as above except that the Span 80 emulsifier was used at a concentration of 1%, 2%, and 4% (v/v), whilst the other parameters were kept constant, as summarized in Table I.
Effect of Concentration of Pore Inducer
The folic acid microcapsules were prepared as above except that the polymer and folic acid were mixed together with sucrose at 0%, 2.5%, 5.0%, or 7.5% (w/v) as the pore inducer in the internal phase of the emulsion. The other parameters were kept constant, as summarized in Table I.
Effect of Drug Contents
The folic acid microcapsules were prepared with the inclusion of 1, 2, and 4 mg/ml of folic acid in the ethyl cellulose solution, whilst the other parameters were kept constant as summarized in Table I.
Encapsulation Efficiency
Microcapsules (50 mg) were dissolved in 5 ml of dichloromethane, and 20 ml of water was added and mixed well. The mixture was then centrifuged at 4,000 rpm for 10 min, the supernatant was harvested, and ammonium hydroxide was added to 10% (w/v) before being withdrawn and diluted with distilled water. The diluted solution was filtered through a 0.45-µm nylon membrane filter, and 20 µl aliquots of the filtrate were used to determine the free folic acid levels and, by subtraction, the encapsulation efficiency using quantitative HPLC analysis (see below). The encapsulation efficiency was calculated according to the following equation:
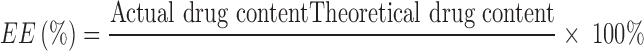 |
Scanning Electron Microscope (SEM)
Samples for SEM analysis were prepared by sprinkling the microcapsule preparation on one side of a double adhesive stub. The stub was then coated by gold under vacuum. The microcapsules were then observed with the scanning electron microscope (JSM-5800 LV, JEOL, Japan).
Particle Size and Size Distribution
The particle size and distribution of microcapsules were evaluated using a laser particle size analysis instrument (Mastersizer S long bed ver. 2.19). Microcapsules were suspended in distilled water with 0.1% (v/v) Nondiet P40. The size distribution (polydispersity) was measured in terms of the SPAN value, expressed as:
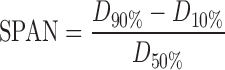 |
where D90%, D10%, and D50% are the diameters where the given percentage of particles are smaller than that stated size. A high SPAN value indicates a wide size distribution and a high polydispersity (23).
In Vitro Drug Release
The dissolution tests of the microcapsules in the simulated intestinal fluid at pH 1.2 and 7.4 were carried out in a dissolution apparatus using the formulation F1 as a representative formulation to investigate the release behavior and the stability of folic acid in the acid environment condition.
The dissolution tests were performed as follows. Accurately weighed aliquots of 100 mg microcapsules were loaded into a bag and then immersed into 250 ml of 0.1 M phosphate buffer saline (pH 7.4) in a conical flask and incubated at 37 ± 1°C under a shaking speed of 100 rpm. At 0.25, 0.5, 1, 2, 3, 4, 5, 6, and 24 h later, triplicate 3 ml aliquots of samples were collected, filtered through a 0.45-µm nylon membrane filter, and the solute folate level was determined in triplicate by quantitative HPLC as described below. Each sample (3 ml) withdrawn was replaced by the same volume of fresh dissolution medium.
HPLC Assay of Folic Acid
The folic acid contents in the microcapsules was evaluated utilizing a HPLC-based method that has been thoroughly described and validated by Andrisano et al. (24) Briefly, the chromatographic separation was conducted on a reverse phase Pinnacle II C18 column (C18, 5.0 µm particle size, 250 × 46 mm ID) preceded by a guard column (5.0 µm particle size, 20 × 4.0 mm ID). The mobile phase was acetonitrile-0.01 M phosphate buffer (pH 5.0) containing 4 mM tetrabutylammonium hydrogensulfate (24:76, (v/v)) at a flow rate of 1 ml/min and a detection wavelength of 280 nm. All chromatographic experiments were conducted on a Thermo-Finnigan P4000 HPLC (Thermo-Finnigan, San Jose, CA, USA) consisting of a Spectra SYSTEM with a pump (P4000), UV detector (UV 6000 LP), and an automatic injector.
Statistical Analysis
All statistical analyses were evaluated using Statistical Package for the Social Sciences (SPSS) version 9.0 for Windows Operating System (version 9.0, SPSS Inc., Chicago, IL, USA). A p value of less than 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
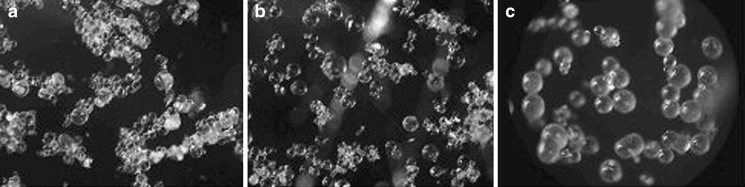
This study sought to investigate the feasibility of preparing folic acid-loaded microcapsules using the oil-in-oil emulsion solvent evaporation technique. One of the features of this process is the use of two solvents, termed as a mixed solvent system (25), consisting of a dispersed medium and a suitable non-aqueous processing medium, to enable the formation of an oil-in-oil emulsion. The mixed solvent of acetone and methanol was selected as the dispersed medium where microcapsules are formed by the following steps. When the polymeric solution is poured into the continuous phase, emulsion droplets are formed. Acetone quickly diffuses out from each emulsion droplet, drastically reducing its size, and the remaining methanol is removed from the system, causing the droplets to solidify and form polymeric microcapsules. The effect of the solvent ratio on the morphology of microcapsules was investigated using methanol:acetone ratios (v/v) of 1:2, 1:4, and 1:9 and was found to play a key role in the formation of ethyl cellulose microcapsules. The higher the proportion of methanol in the mixed solvent system, such as at a methanol ratio (v/v) of 33% and 20%, the smaller the size of the obtained microcapsules were, including with irregular shapes (data not shown). When the amount of methanol was reduced to 10% (v/v), the resulting microcapsules were well-shaped spherical particles (Fig. 1). Therefore, the dispersal medium in the mixed solvent system used for these studies was 1:9 (v/v) methanol:acetone.
Fig. 1.
Representative microphotographs (×10) of F1 microcapsules prepared with various methanol:acetone ratios (v/v) of a 1:2, b 1:4, and c 1:9
In addition to the solvent system used, generally, the characteristics of the microcapsules, such as their particle size, morphology, and drug content, are also affected by the different experimental conditions. In the present study, several parameters, such as the polymer and emulsifier concentrations, the amount of the pore inducer (sucrose), and folic acid loading contents, were investigated to determine their effect on the characteristics of the folic acid-loaded microcapsules. The preparation parameters used in this study, referred to as formulations F1 to F10, are listed in Table I.
Microcapsule Morphology
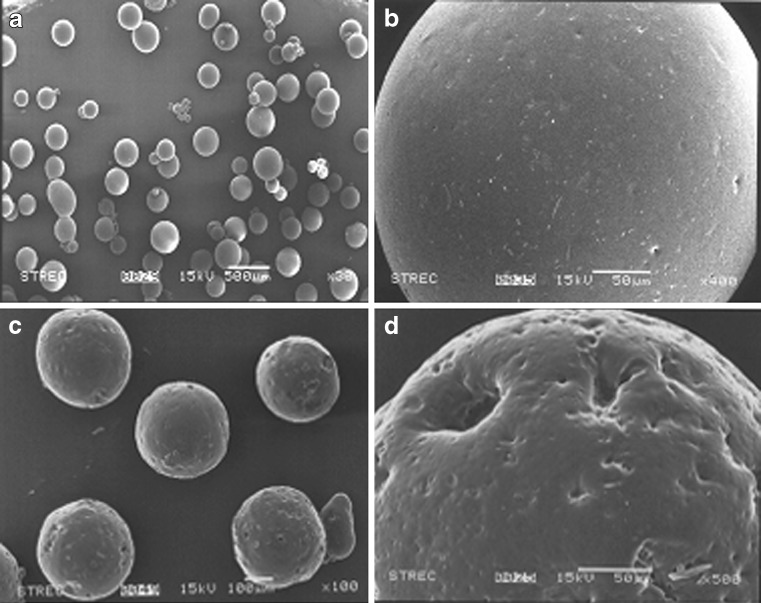
SEM photographs of folic acid-loaded microcapsules revealed that the surface structure of F1 microcapsules produced by the oil-in-oil emulsion solvent evaporation appeared spherical and not aggregated (Fig. 2a, b). With different concentrations of polymer and emulsifier in the system, no differences in the morphology of the resultant microparticles were observed (data not shown). However, the microcapsules prepared with sucrose as the pore inducer (F6) showed a highly porous and coarser surface (Fig. 2c, d), suggesting that the addition of sucrose effected the morphology of the resultant microcapsules, potentially forming pores.
Fig. 2.
Representative scanning electron microscopy photographs of folic acid-loaded microcapsules for a overall view of F1 microcapsules, b surface morphology of a typical F1 microcapsule, c overall view of F6 microcapsules (with 2.5% (w/v) pore inducer), and d surface morphology of a typical F6 microcapsule
Effect of an Increase in Concentration of the Ethyl Cellulose Wall Former
The concentration of ethyl cellulose in the dispersed phase exerted a significant impact upon the microencapsulation process. Ethyl cellulose solutions of 2.5%, 5.0%, and 7.5% (w/v) were used to prepare folic acid microcapsules whilst keeping all the other factors constant (F1–F3; Table I).
As the concentration of ethyl cellulose was increased from 2.5% to 7.5% (v/v), the average diameter of microcapsules increased significantly, reaching up to 1.5-fold larger. This is likely to be due to the increase in the viscosity of the medium at higher polymer concentrations resulting in an enhanced interfacial tension. At a fixed stirring shear force, it is difficult for small emulsion droplets to form because higher shear forces are necessary for droplet disruption, resulting in the formation of larger particles. In addition, the SPAN values, an indicator of the particle size distribution, correspondingly decreased with increasing concentrations of ethyl cellulose. Thus, the size distribution of the microcapsules attained was narrower as the ethyl cellulose concentration increased, presumably because the microcapsule particles stably separated without aggregation once the solvent was completely evaporated.
Finally, the encapsulation efficiency, expressed as a percentage of the actual loading to the theoretical loading, decreased from 80.29 ± 1.46% to 41.85 ± 1.33% (p < 0.05) with increasing ethyl cellulose concentrations. The increasing viscosity of the polymer solution at higher concentrations likely yielded the formation of larger polymer/solvent droplets, causing the drug to diffuse out of the particles and so decreasing the loading efficiency due to the slower hardening of larger particles (26).
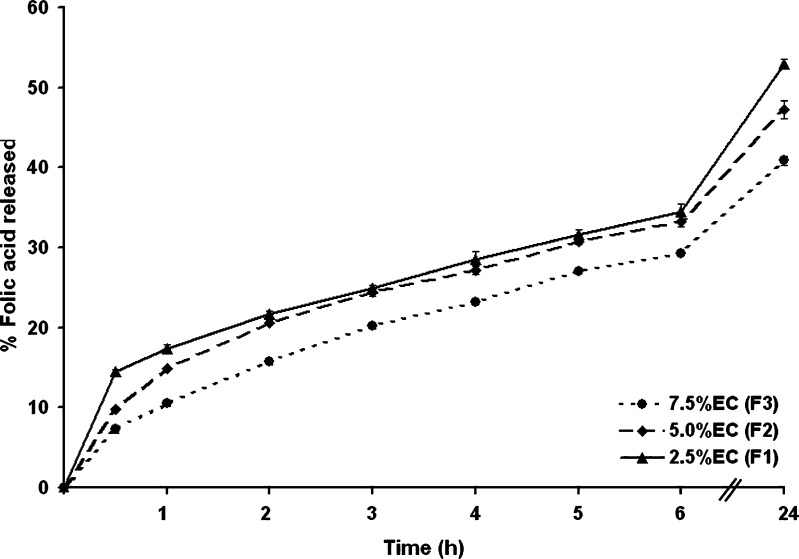
The effect of varying the concentration of ethyl cellulose during microcapsule synthesis, upon the folic acid release behavior of the resultant microcapsules, revealed a slight but significant dose-dependent effect upon the folic acid release profile (Fig. 3). Ethyl cellulose microcapsules significantly retarded the folic acid release at all the examined time points over a 24-h period, with an essentially constant rate of release. However, the microcapsules prepared from a higher concentration of ethyl cellulose gave a lower release rate. Retardation of folic acid release could be a result of the increase in the microcapsule size, as well as the increase in the matrix density, as the concentration of ethyl cellulose was increased. Furthermore, larger microcapsules were formed at higher polymer concentrations leading to a smaller surface area being exposed to the dissolution medium and hence to a reduced release. After 24 h, only about 52%, 47%, and 40% of the folic acid was released compared with the initial amount loaded (derived from the loading efficiencies) for the formulations F1, F2, and F3, respectively. Since ethyl cellulose is a non-water-soluble polymer, the release of folic acid will mainly be driven by permeation of the folic acid through the hydrophobic polymer membrane within water-filled pores. Therefore, as the amount of ethyl cellulose increased, the folic acid diffusion reduced correspondingly.
Fig. 3.
The release profile of folic acid from microcapsules prepared with increasing concentrations of ethyl cellulose in SIF (pH 7.4 phosphate buffer). Data are shown as the mean ± SD and are derived from three independent repeats. Means with a different lower case letter differ significantly (p < 0.05)
Effect of Increasing the Emulsifier Concentration
The effect of different emulsifier concentrations on the properties of folic acid microcapsules was studied by comparing the size distribution and release of folic acid from microcapsules prepared with different concentrations of sorbitan monooleate (Span 80), whilst all the other synthesis factors were kept constant (F1, F4, and F5; Table I). The mean diameter of the microcapsules prepared using 1%, 2%, and 4% (v/v) Span 80 decreased significantly with increasing concentrations of Span 80 in the continuous phase. Span 80, a nonionic surface-active emulsifying agent that is frequently used in oil-in-oil solvent evaporation, plays an important role in emulsion stability where it stabilizes the interface between the two phases to form an emulsion droplet by reducing the surface tension. An increase in the Span 80 concentration allows the stabilization of a greater interfacial surface area, leading to a smaller particle size. In addition, the SPAN value, used as an indicator of particle size distribution, correspondingly decreased with increasing concentrations of Span 80 emulsifier, suggesting that the microcapsule size distribution became narrower with an increase in the concentration of the emulsifier.
However, varying the emulsifier concentration between 1% and 4% (v/v) reduced the encapsulation efficiency of microcapsules from 80% to 57%, respectively (F1, F4, and F5; Table I; p < 0.1), reflecting a decrease in the loading efficiency of folic acid with increasing concentrations of Span 80. This may be due to the reduction in the particle size of the emulsion droplets. The miscibility of solvent with liquid paraffin oil was increased with increasing emulsifier concentrations, which might increase the diffusion of folic acid into the continuous phase, a notion which was supported by SEM analysis which showed the presence of folic acid particles on the surface of microcapsules (data not shown).
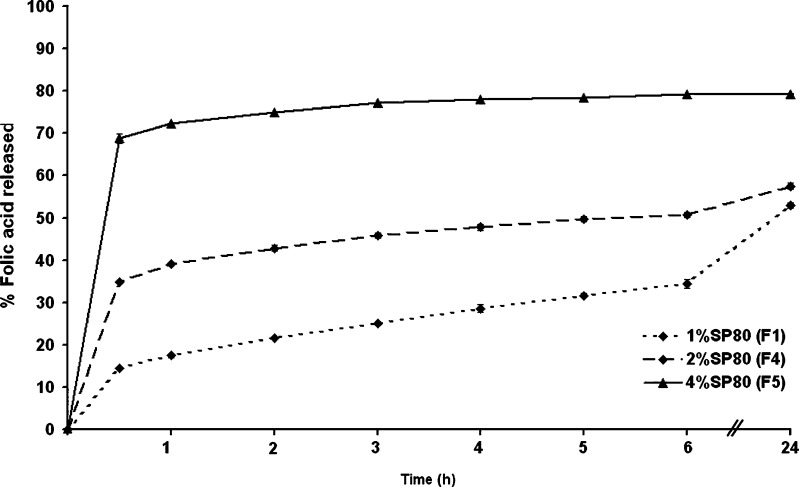
The amount of folic acid released from microcapsules varied significantly at all time points assayed between the microcapsules made from the three different Span 80 levels, decreasing markedly as the amount of emulsifier decreased (Fig. 4). For example, about 14%, 34%, and 68% of folic acid were released from the microcapsules prepared with 1%, 2%, and 4% (v/v) emulsifier after 30 min, respectively, and about 52%, 57%, and 79% after 24 h, respectively. Thus, the folic acid release rate increased as the emulsifier concentration increased.
Fig. 4.
The release profile of folic acid from microcapsules prepared with increasing concentrations of sorbitan monooleate in SIF (pH 7.4 phosphate buffer). Data are shown as the mean ± SD and are derived from three independent repeats. Means with a different lower case letter differ significantly (p < 0.05)
In summary, increasing the emulsifier concentration decreased the encapsulation efficiency and increased the burst effect, which has also been reported by others (26,27), where the burst release (within 30 min) was considered to be due to the release of the drug adsorbed on the surface of the particles and the development of a high concentration gradient across the particle surface. Given that increasing the emulsifier concentration decreased the size of the microcapsules, resulting in an increase in the interface area between the microcapsules and the release medium, then smaller microcapsules with a greater surface area would yield a faster drug release rate compared to larger microcapsules (19).
Effect of Varying the Pore Inducer (Sucrose) Concentration
Typically, the release mechanism of a drug depends on diffusion though the porous matrix. Thus, the addition of an excipient as a pore inducer to the microcapsules during formulation may increase the drug release rate profile. Sucrose, in the form of a water-soluble powder or solvated liquid, can be added to the microcapsule formulation by the emulsion solvent evaporation process to obtain a more desirable release rate of the active ingredients. This is because the dissolution of pore inducers into the release media induced pores in the surface and channels in the matrix of the microcapsules, which then ease the release of the active ingredients (28). In this study, different amounts of sucrose (2.5%, 5.0%, and 7.5% (w/w)), as the pore inducer, were added to the formulation of folic acid microcapsules to study the effect on the particulate properties and drug release profiles.
Microcapsules prepared from increasing concentrations of sucrose yielded particles with an increasing average size (F1, F6–F8; Table I), attaining up to approximately 2.5-fold increase in particle size (p < 0.01), in a dose-dependent manner. Presumably, the inclusion of sucrose increased the matrix of the emulsion droplet and, therefore, the size of the microcapsules depended on the size of the emulsion droplets formed during homogenization. The size distribution (polydispersity), recorded as the SPAN value, of the prepared formulations correspondingly decreased in a dose-dependent manner as the sucrose concentration increased, supporting the increasing size uniformity of the microcapsules.
The folic acid encapsulation efficiency within the ethyl cellulose microcapsules was significantly reduced by the inclusion of sucrose (formulations F1 and F6–F8; Table I). Although no simple clear dose-dependent trend was evident between the sucrose level and the resultant encapsulation efficiency, there was a net trend of decreased encapsulation efficiency with the inclusion of sucrose, and especially at higher concentrations (p > 0.1). If so, this might be due to the fact that as more encapsulating materials are included in the assembly, then more competition of materials for the inside of microcapsules will lead to competitive exclusion of folic acid. Alternatively, the migration of folic acid through the microcapsule pores into the outer oil phase during droplet hardening may increase which will in turn result in a greater loss of encapsulated folic acid.
Although it is well known that additives are able to modify the release rate of drugs from microcapsules, the effect of these substances can be quite different. Therefore, the actual effect of sucrose addition upon the encapsulation and release rates of folic acid from the different ethyl cellulose microcapsules were evaluated.
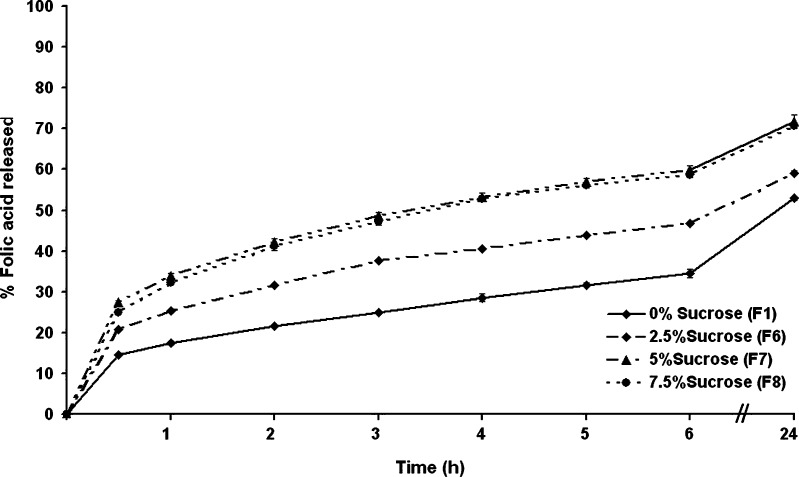
The folic acid release profiles of the microcapsules prepared with different amounts of sucrose revealed clear dose-dependent differences (Fig. 5). Within the first hour, the cumulative folic acid release was lowest in microcapsules prepared without sucrose (formulation F1), at about 17% of the total encapsulated amount, whilst it was higher, in a dose-dependent manner, with those prepared with increasing sucrose levels, attaining about 25%, 33%, and 32% folic acid release from microcapsules prepared with 2.5%, 5%, and 7.5% (w/v) of sucrose, respectively. The same trend was visible at all time points up to 24 h with a marked increase in the cumulative folic acid release level from microcapsules with 2.5% or 5% (w/v) sucrose compared to that from microcapsules prepared without sucrose. However, the folic acid release rate from microcapsules prepared from 7.5% (w/v) sucrose was numerically only slightly higher than that from microcapsules prepared with the inclusion of 5% (w/v) sucrose. Thus, for example, after 6 h, the microcapsules prepared with 0%, 2.5%, 5%, and 7.5% (w/v) of sucrose had released about 33%, 46%, 59%, and 58% of the total folic acid, respectively, and after 24 h, this was about 52%, 59%, 71%, and 70%, respectively. That the addition of sucrose up to 5% (w/w) can promote a higher release of folic acid from the microcapsules is likely to be mediated via opened pores and percolation channels, where as the porosity of the formulation increased, the amount of drug released also increased. However, the release profiles of folic acid from microcapsules containing 5% and 7.5% (w/w) are similar, suggesting that the capability of microcapsules for encapsulating sucrose was already maximal at 5% (w/v) sucrose loading.
Fig. 5.
The release profile of folic acid from microcapsules prepared with increasing concentrations of sucrose in SIF (pH 7.4 phosphate buffer). Data are shown as the mean ± SD and are derived from three independent repeats. Means with a different lower case letter differ significantly (p < 0.05)
Effect of Folic Acid (Drug) Concentration
Folic acid-loaded microcapsules were prepared using three different amounts of folic acid, that is 1, 2, and 3 mg/ml, whilst keeping all the other factors constant (F7, F9, and F10; Table I), but note in this assay, in contrast to the previous ones, the sucrose concentration was maintained at 5% (w/v). The mean particle size increased significantly with increasing concentrations of folic acid in a dose-dependent manner (Table I). This could be explained by the fact that an increased amount of folic acid resulted in a more viscous dispersed phase, leading to a larger droplet formation. The size distribution (polydispersity), recorded as the SPAN value, in prepared formulations showed no clear pattern with respect to folic acid concentration and varied within the range of 1.30 to 1.82 (Table I).
The effect of folic acid contents on its encapsulation efficiency within ethyl cellulose microcapsules showed no significant change with increasing folate levels from 1 to 2 mg/ml, but increased significantly at 3 mg/ml, resulting in a significantly higher folic acid encapsulation level per unit polymer than in the other formulations (Table I).
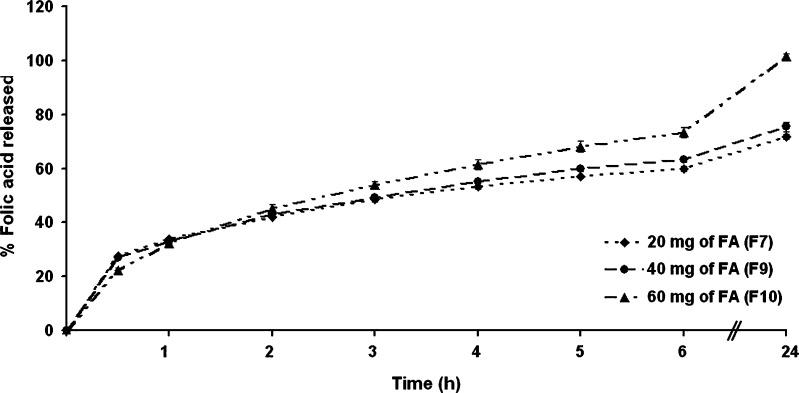
These microcapsules with different folic acid loadings were then evaluated for folic acid release. From the release profile of microcapsules prepared with different amounts of folic acid, the folic acid entrapment efficiency was found to be a relatively minor but nevertheless a significant factor influencing drug release (Fig. 6). The initial (30 min) and early (2 h) cumulative release of folic acid from microcapsules prepared with 1, 2, and 3 mg/ml of folic acid were essentially the same, but by 3 h revealed a slight dose-dependency, and at 6 and 24 h, the microcapsules prepared with 1, 2, and 3 mg/ml of folic acid had released a cumulative total of 58%, 63%, and 69% and 71%, 75%, and 100% folic acid, respectively. Thus, the cumulative release of folic acid increased with increasing amounts of folic acid in the formulation. Given a higher level of folic acid will correspond to a lower level of polymer in the microcapsules, this will result in an increase in the cumulative percentage release since as more folic acid is released from the microcapsules, more channels were produced, contributing to a faster drug release rate. In addition, higher folic acid levels in the microcapsule formulations produced a higher drug concentration gradient between the microcapsules and dissolution medium and, therefore, the cumulative release of drug was increased (29).
Fig. 6.
The release profile of folic acid from microcapsules prepared with increasing amounts of folic acid in SIF (pH 7.4 phosphate buffer). Data are shown as the mean ± SD and are derived from three independent repeats. Means with a different lower case letter differ significantly (p < 0.05)
Folic Acid Release Behavior in pH 1.2
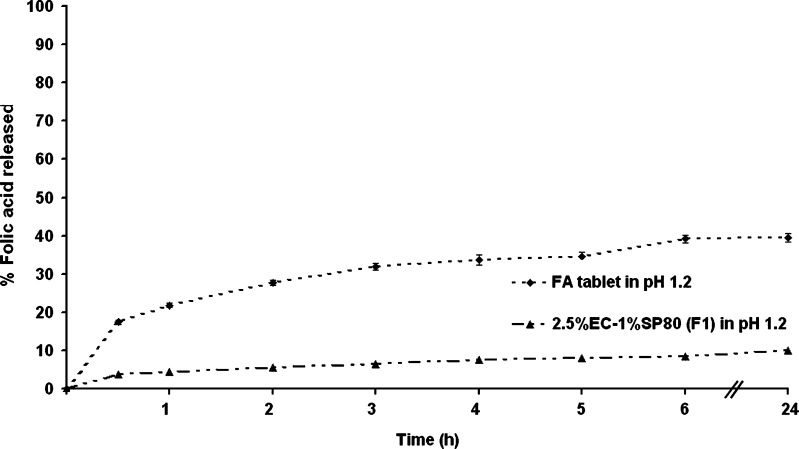
Upon ingesting, any folate-carrying vector would be subject to both the time delay and the low pH that residence in the stomach entails prior to release into the small intestine, the site of folate absorption. Thus, folate delivery vectors must be acid resistant and sustain the folate for several hours. To this end, the release of folic acid from these ethyl cellulose vectors was evaluated at pH 1.2 using formulation F1 as a representation formulation. Comparison of the release behavior of folic acid-loaded microcapsules (formulation F1) and a commercial folic acid tablet in an acidic medium at pH 1.2 (0.1 N HCl) revealed a much lower release rate (better retention) of folic acid by the ethyl cellulose microcapsules (Fig. 7). The cumulative drug release of the commercial tablet reached nearly 40% within 6 h, whilst that for the F1 microcapsules was only approximately 6% and reached only 10% within 24 h. Thus, ethyl cellulose microcapsules can retard folic acid release in an acidic medium, possibly due to the hydrophobicity of the polymer that provides a good stability at varying pH values and so provides relatively stable folic acid-loaded microcapsules capable of preventing the drug release in acidic medium. This advantage of the hydrophobic polymer is that it should provide a higher release, and so absorption into the body, of folic acid in the upper small intestine, its site of absorption.
Fig. 7.
The release profile of a commercial folic acid tablet compared with folic acid-loaded microcapsules (formulation F1) in 0.1 N HCl (pH 1.2). Data are shown as the mean ± SD and are derived from three independent repeats. Means with a different lower case letter differ significantly (p < 0.05)
Comparative Evaluation of the In Vitro Release Performance of the Commercial Folic Acid Tablets and the Fabricated Controlled Release Folic Acid Microcapsules
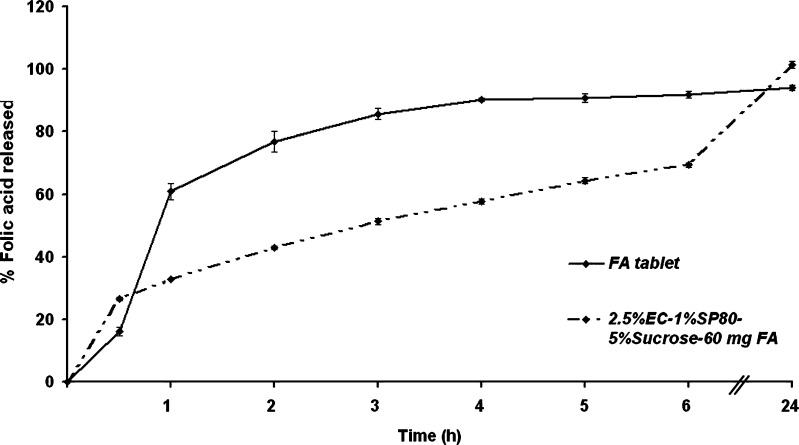
However, for absorption in the upper small intestine to occur, any folic acid delivery vesicle would be required not only to be acid resistant but also to unload the folic acid at this mildly alkaline pH and within a few hours prior to movement into the large intestine and loss from the absorption region. To ascertain this, the release profile of folic acid in SIF pH 7.4 was evaluated. From the above results, folic acid-loaded formulation F10 microcapsules showed the most appropriate sustained release profile of folic acid, rather than the F1 preparation. Therefore, the comparative dissolution of folic acid between formulation F10 and the commercial tablet was studied (Fig. 8).
Fig. 8.
The release profile of a commercial folic acid tablet compared with folic acid-loaded microcapsules (formulation F10) in SIF (pH 7.4 phosphate buffer). Data are shown as the mean ± SD and are derived from three independent repeats. Means with a different lower case letter differ significantly (p < 0.05)
The release profile of the commercial folic acid tablet showed an extremely high burst effect with about 60% and 90% of the entrapped folic acid being released after 1 and 5 h, respectively. In contrast, the microcapsules of formulation F10 showed an ability to delay the folic acid release with a cumulative drug release of 32% and 70% after 1 and 6 h, respectively. However, although the complete release of drug from formulation F10 microcapsules was not observed until 24 h, which will likely lay outside the time window for upper small intestine absorption, some 70% release was still attained within 6 h which is important so as to minimize the amount of voided unabsorbed folic acid that is not released until the large intestine or not at all.
CONCLUSIONS
Preparation conditions for an oil-in-oil emulsion solvent evaporation method to encapsulate folic acid into ethyl cellulose microcapsules using Span 80 (emulsifier) and sucrose (pore former) were optimized to obtain a relatively high entrapment efficiency (up to 88%) with a relatively long release period. The addition of small amounts of the water-soluble pore inducer, sucrose, could be used to modify the release of folic acid from the microcapsule matrix. The formulation F10 showed a suitable sustained release profile which occurred by a diffusion process as in the case of insoluble polymer microcapsules.
ACKNOWLEDGEMENTS
We gratefully acknowledge the Graduate Research Grant of Chulalongkorn University; the National Center of Excellence for Petroleum, Petrochemicals, and Advanced Materials (NCE-PPAM), Thailand; the Thailand Research Fund (TRF); and the A1-B1 Project, for financial support and PCU center.
REFERENCES
- 1.Shrestha AK, Arcot J, Paterson JL. Edible coating materials—their properties and use in the fortification of rice with folic acid. Food Res Intern. 2003;36:921–928. doi: 10.1016/S0963-9969(03)00101-7. [DOI] [Google Scholar]
- 2.Amitai Y, Fisher N, Meiraz H, Baram N, Tounis M, Leventhal A. Precoceptional folic acid utilization in Israel: five years after the guidelines. Prev Med. 2008;46:166–169. doi: 10.1016/j.ypmed.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Akhtar MJ, Khan MA, Ahmad I. Identification of photoproducts of folic acid and its degradation pathways in aqueous solution. J Pharm Biomed Anal. 2003;31:579–588. doi: 10.1016/S0731-7085(02)00724-0. [DOI] [PubMed] [Google Scholar]
- 4.Off MK, Steindal AE, Porojnicu AC. Ultraviolet photodegradation of folic acid. J Photochem Photobiol B: Biol. 2005;80:47–55. doi: 10.1016/j.jphotobiol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Williams PG, Ross H, Miller B. Ascorbic acid and 5-methyltetrahydrofolate losses in vegetables with cook/chill or cook/hot-hold food service systems. J Food Sci. 1995;60:541–546. doi: 10.1111/j.1365-2621.1995.tb09822.x. [DOI] [Google Scholar]
- 6.Wigertz K, Svensson UK, Jagerstad M. Folate and folate binding protein content in dairy products. J Dairy Res. 1997;64:239–252. doi: 10.1017/S002202999700215X. [DOI] [PubMed] [Google Scholar]
- 7.Vahteristo L, Lehikoinen K, Ollilainen V, Koivistoinen PE, Varo P. Ovenbanking and frozen storage affect folate vitamer retention. Lebensm -Wiss Technol. 1998;31:329–333. doi: 10.1006/fstl.1997.0362. [DOI] [Google Scholar]
- 8.Scott J, Rebeille F, Fletcher J. Review—folic acid and folates: the feasibility for nutritional enhancement in plant foods. J Sci Food Agric. 2000;80:795–824. doi: 10.1002/(SICI)1097-0010(20000515)80:7<795::AID-JSFA599>3.0.CO;2-K. [DOI] [Google Scholar]
- 9.Madziva H, Kailasapathy K, Phillips M. Alginate-pectin microcapsules as a potential for folic acid delivery in foods. J Microencapsul. 2005;22:343–351. doi: 10.1080/02652040500100931. [DOI] [PubMed] [Google Scholar]
- 10.Bakan JA, Swarbrick J, Boylan J. Encyclopedia of pharmaceutical technology. New York: M. Dekker; 1994. pp. 423–441. [Google Scholar]
- 11.Suzuki K, Price JC. Microencapsulation and dissolution properties of a neuroleptic in a biodegradable poly (d, 1-Lactide) J Pharm Sci. 1985;74:21–24. doi: 10.1002/jps.2600740106. [DOI] [PubMed] [Google Scholar]
- 12.Sprockel OL, Prapaitrakul W. A comparison of microencapsulation by various emulsion techniques. Int J Pharm. 1990;58:123–127. doi: 10.1016/0378-5173(90)90249-4. [DOI] [Google Scholar]
- 13.Bosela AA. Bioavailability and in vitro evaluation of microencapsulated nitrofurantoin. J Biomed Sci Ther. 1991;7:423–438. [Google Scholar]
- 14.Thanoo BC, Sunny MC, Jayakrishnan A. Oral sustained-release drug delivery systems using polycarbonate microspheres capable of floating on the gastric fluid. J Pharm Pharmacol. 1993;45:21–24. doi: 10.1111/j.2042-7158.1993.tb03672.x. [DOI] [PubMed] [Google Scholar]
- 15.Cheu SJ, Chen RRL, Chen PF, Lin WJ. In vitro modified release of acyclovir from ethyl cellulose microspheres. J Microencapsul. 2001;18:559–565. doi: 10.1080/02652040010018155. [DOI] [PubMed] [Google Scholar]
- 16.Rafienia M, Orang F, Emami SH. Preparation and characterization of polyurethane microspheres containing theophylline. J Bioact Compat Polym. 2006;21:341–349. doi: 10.1177/0883911506066931. [DOI] [Google Scholar]
- 17.Zhang WF, Chen G, Li PW, He QZ, Zhou HY. Chitosan and chitosan/β-cyclodextrin microspheres as sustained-release drug carriers. J Appl Polym Sci. 2006;103:1183–1190. doi: 10.1002/app.25373. [DOI] [Google Scholar]
- 18.Sanchez LC, Teresa FM, Fernandez AM, Alvarez FJ, Rabasco AM, Mura P. Development of sustained release matrix tablets of didanosine containing methacrylic and ethylcellulose polymers. Int J Pharm. 2002;234:213–221. doi: 10.1016/S0378-5173(01)00962-0. [DOI] [PubMed] [Google Scholar]
- 19.Das MK, Rao KR. Evaluation of zidovudine encapsulated ethylcellulose microspheres prepared by water-in-oil-in-oil (W/O/O) double emulsion solvent diffusion technique. Acta Pol Pharm. 2006;63:141–148. [PubMed] [Google Scholar]
- 20.Zinutti C, Kedzierewicz F, Hoffman M, Maincent P. Preparation and characterization of ethylcellulose microspheres containing 5-fluorouracil. J Microencapsul. 1994;11:555–563. doi: 10.3109/02652049409034994. [DOI] [PubMed] [Google Scholar]
- 21.El-Bagory IM, Hosny EA, Al-Suwayeh SA, Mahrous GM, Al-Jenoobi FI. Effects of sphere size, polymer to drug ratio and plasticizer concentration on the release of theophyline from ethyl cellulose microspheres. Saudi Pharm J. 2007;15:213–217. [Google Scholar]
- 22.Li XW, Yang TF. Fabrication of ethyl cellulose microspheres: chitosan solution as a stabilizer. Korean J Chem Eng. 2008;25:1201–1204. doi: 10.1007/s11814-008-0198-8. [DOI] [Google Scholar]
- 23.Torrado JJ, Illum L, Davis SS. Particle size and size distribution of albumin microspheres produced by heat and chemical stabilization. Int J Pharm. 1989;51:85–93. doi: 10.1016/0378-5173(89)90079-3. [DOI] [Google Scholar]
- 24.Andrisano V, Bartolini M, Bertucci V, Cavrini V, Luppi B, Cerhiara T. Analytical methods for the determination of folic acid in a polymeric micellar carrier. J Pharm Biomed Anal. 2003;32:983–989. doi: 10.1016/S0731-7085(03)00200-0. [DOI] [PubMed] [Google Scholar]
- 25.Viswanathan NB, Thomas PA, Pandit JK, Kulkarni MG, Mashelkar RA. Preparation of non-porous microspheres with high entrapment efficiency of proteins by a (water-in-oil)-in-oil emulsion technique. J Control Release. 1999;58:9–20. doi: 10.1016/S0168-3659(98)00140-0. [DOI] [PubMed] [Google Scholar]
- 26.Bodmeier R, Wang II, Hermann J. Microencapsulation of chlorpheniramine maleate, a drug with intermediate solubility properties, by a non-aqueous solvent evaporation technique. STP Pharma Sci. 1994;4:275–281. [Google Scholar]
- 27.Kyo M, Hyon SH, Ikada Y. Effect of preparation conditions of cisplatin-loaded microspheres on the in vitro release. J Control Release. 2007;35:73–82. doi: 10.1016/0168-3659(95)00030-C. [DOI] [Google Scholar]
- 28.Song M, Li N, Sun S, Tiedt LR, Liebenberg W, Villiers MM. Effect of viscosity and concentration of wall former, emulsifier and pore-inducer on the properties of amoxicillin microcapsules prepared by emulsion solvent evaporation. II Farmaco. 2005;60:261–267. doi: 10.1016/j.farmac.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Das MK, Rao KR. Microencapsulation of zidovudine by double emulsion solvent diffusion technique using ethylcellulose. Indian J Pharm Sci. 2007;69:244–250. doi: 10.4103/0250-474X.33151. [DOI] [Google Scholar]