Abstract
The purpose of the present study was to investigate the potential of nanoemulsions as nanodrug carrier systems for the percutaneous delivery of ropinirole. Nanoemulsions comprised Capryol 90 as the oil phase, Tween 20 as the surfactant, Carbitol as the cosurfactant, and water as an external phase. The effects of composition of nanoemulsion, including the ratio of surfactant and cosurfactant (Smix) and their concentration on skin permeation, were evaluated. All the prepared nanoemulsions showed a significant increase in permeation parameters such as steady state flux (Jss) and permeability coefficient (Kp) when compared to the control (p < 0.01). Nanoemulsion composition (NEL3) comprising ropinirole (0.5% w/w), Capryol 90 (5% w/w), Smix 2:1 (35% w/w), and water (59.5% w/w) showed the highest flux (51.81 ± 5.03 µg/cm2/h) and was selected for formulation into nanoemulsion gel. The gel was further optimized with respect to oil concentration (Capryol 90), polymer concentration (Carbopol), and drug content by employing the Box–Behnken design, which statistically evaluated the effects of these components on ropinirole permeation. Oil and polymer concentrations were found to have a negative influence on permeation, while the drug content had a positive effect. Nanoemulsion gel showed a 7.5-fold increase in skin permeation rate when compared to the conventional hydrogel. In conclusion, the results of the present investigation suggested a promising role of nanoemulsions in enhancing the transdermal permeation of ropinirole.
Key words: Box–Behnken design, flux, nanoemulsion, nanoemulsion gel, permeation, transdermal drug delivery
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized typically by motor features of tremor, rigidity, and bradykinesia due to depletion of dopaminergic nigrostriatal neurons. Non-motor disorder symptoms such as dementia, depression, and falls emerge with the progression of the disease (1). PD results in a significant decline in the quality of life (2) for both patients and family (3) and contributes to significant economic and institutional costs on family and society (4). Because of the aging of the world population, the importance of Parkinson’s disease as a public health issue is expected to increase.
Historically, levodopa has been the standard treatment for PD. Levodopa initially provides a stable therapeutic response but, during long-term treatment, its beneficial effect declines (5). The main problem with the early use of levodopa is not so much a question of its efficacy and its ability to control parkinsonian symptomatology, but rather its tendency to induce motor complications (6). This is a particular problem for young onset PD patients who are at a greater risk of developing motor complications and who have to endure this disability over the course of a long and chronic illness (7). In the management of PD, it is important to minimize the development of motor fluctuations and to postpone them as long as possible (8). For this purpose, it is important to try to delay the initiation of levodopa therapy and to minimize the daily dosage of levodopa. The use of other antiparkinsonian drugs as a primary treatment of early PD before initiation of levodopa therapy offers a treatment option for this purpose.
Ropinirole is a recently introduced dopamine agonist, which stimulates striatal dopamine receptors to produce dopamine, for the treatment of PD (9). It is being increasingly used as monotherapy in the initial treatment of Parkinson’s disease rather than as adjunct to levodopa (10). In addition, ropinirole is also efficacious in the management of more advanced Parkinson’s disease in patients experiencing motor complications after long-term levodopa use (11). The usual dose is 3–9 mg daily and has to be taken in three divided doses owing to the short half-life of the drug. This causes great inconvenience to the patients. When symptoms like slowness of movement, tremor, and rigidity return due to wearing off of the patient’s medication, it can be problematic, causing difficulty with simple activities and movement in patients with PD. Additionally, the drug suffers from low bioavailability of about 50% by oral route due to extensive first-pass metabolism (12).
It was envisaged that the limitations of the conventional oral ropinirole therapy might be addressed by transdermal administration. Ropinirole has a low molecular weight (MW 260), is sufficiently lipophilic (Log p = 3.32), and has a short elimination half-life (6 h), which makes it a suitable candidate for transdermal delivery (12). Besides, therapies that provide more continuous stimulation of dopamine receptors had been found to reduce motor complications in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated monkeys and in patients with Parkinson disease (13). However, continuous infusion is expensive and impractical for most patients. It can be anticipated that the transdermal delivery of ropinirole shall provide continuous, non-pulsatile dopaminergic stimulation, which could be beneficial in delaying the onset of levodopa-related long-term motor complications. Nausea, dizziness, and postural hypotension are the most common adverse events with the drug. Transdermal delivery might help in dosage reduction, which could help in overcoming these side effects. Transdermal formulations would be ideal for the geriatric Parkinson population who may be suffering from dysphagia (difficulty swallowing). Approx. 85% of Parkinson’s patients are over the age of 65, and over 45% have difficulty in swallowing. It is further expected that prolonged drug release shall significantly increase the patient compliance while at the same time reducing the burden of the care giver.
There has been a continuous interest during recent years in a search for new vehicle systems that could modify drug penetration into and through the skin. Many of the topical vehicles contain chemical enhancers and non-friendly solvents to achieve improved permeability (14). These vehicles usually result in various degrees of skin irritancy, especially when chronic treatments are required. Therefore, it is undoubtedly desirous to develop topical vehicles that do not necessitate chemical enhancers to facilitate drug penetration into and through the skin. Particularly, recent attention has been focused on nanoemulsions for topical and transdermal delivery as they exhibit superior stability in comparison to other vesicular carriers likes liposomes, ethosomes, niosomes, etc. Nanoemulsions are clear mixtures consisting of oil, surfactant, cosurfactant, and aqueous phase, which are single optically isotropic and thermodynamically stable with a droplet size typically in the range of 10–100 nm (15,16). Nanoemulsions prepared by spontaneous emulsification method offer several advantages over other drug carriers like lower preparation cost, long shelf life, absence of residual organic solvents, and high drug loading for both hydro- and lipophilic drugs (17–19). Numerous studies had been conducted in the recent past that showed the significance of these systems for dermal and transdermal delivery both in vitro (20–30) and in vivo (31–36). They had been amply demonstrated to improve the transdermal permeation over conventional topical preparations such as emulsions (37–39), gels (40–42), creams (43–45), and ointments (46,47).
Several mechanisms have been proposed to explain the advantages of nanoemulsions for the transdermal delivery of drugs. First, a large amount of drug can be incorporated in the formulation due to the high solubilization capacity (48). Second, the permeation rate of the drug from nanoemulsions may be increased since the affinity of a drug to the internal phase in nanoemulsion can be easily modified to favor partitioning intro stratum corneum using different internal phase or changing its proportion in nanoemulsion. Third, the nanoemulsions can interact with the stratum corneum changing structural organization of its lipid layers and consequently increasing transdermal drug permeation. These systems themselves act as penetration enhancers, and there is no need of incorporation of the conventional chemical enhancers and hence obviate the irritation and compatibility issues posed by them.
In the present study, it was intended to investigate the potential of novel nanoemulsion formulations for the transdermal delivery of ropinirole. O/W nanoemulsions containing ropinirole were developed after screening the oils, surfactants, and cosurfactants, obtaining the components and their concentration range for nanoemulsion formation and optimizing them to achieve maximum skin permeation rate of ropinirole. The optimized nanoemulsion formulation was converted into gel for easy application and better skin adherence. Box–Behnken statistical design was employed for the optimization of nanoemulsion gel and to evaluate the combined effect of the selected variables on ropinirole permeation.
MATERIALS AND METHODS
Components
Ropinirole (RP) was a gift sample from USV (Mumbai, India), while propylene glycol monocaprylate (Capryol 90) was a courtesy from Colorcon Asia (Mumbai, India). Diethylene glycol monoethyl ether (Carbitol) and polyoxyethylene sorbitan monolaurate (Tween 20) and PEG 400 were purchased from Sigma Aldrich Inc. (St. Louis, MO, USA) and S.D Fine Chemicals (Mumbai, India), respectively. High-performance liquid chromatography (HPLC) grade methanol and ammonium acetate were procured from E-Merck (Mumbai, India). Water was obtained from Milli-Q water purification system (Millipore, MA, USA). All other chemicals and solvents were of analytical grade.
Preparation of Nanoemulsions
Details of the screening and selection procedure for oils, surfactants, and cosurfactants were presented in our previous report (49). Various nanoemulsions were prepared by aqueous phase titration (spontaneous emulsification) method. The quantitative composition of nanoemulsions was selected on the basis of the pseudoternary phase diagram constructed previously with the same composition by our research group (49). Nanoemulsion formulation of ropinirole was prepared by dissolving 0.5% w/w of ropinirole in 5% w/w Capryol 90. Then, required quantity of surfactant mixture of Tween 20 and Carbitol (Smix) was added to the oil phase with vortex mixing. The final preparation was made up to 100% w/w by a slow addition of distilled water with continuous mixing. The composition of the nanoemulsions is given in Table I. As a control, aqueous solution of 6% ethanol containing 0.5% ropinirole was used in the donor compartment.
Table I.
Composition of the Selected Nanoemulsions, Nanoemulsion Gel, and Conventional Gel
| Formulation code | Ropinirolea | Capryol 90a | S mix a | Watera | Carbopola 934 | Triethanolaminea |
|---|---|---|---|---|---|---|
| NEL1 | 0.5 | 5 | 20b | 74.5 | – | – |
| NEL2 | 0.5 | 5 | 30b | 64.5 | – | – |
| NEL3 | 0.5 | 5 | 35b | 59.5 | – | – |
| NEL4 | 0.5 | 5 | 40b | 54.5 | – | – |
| NEL5 | 0.5 | 5 | 50b | 44.5 | – | – |
| NEL6 | 0.5 | 5 | 60b | 34.5 | – | – |
| NEM1 | 0.5 | 5 | 20c | 74.5 | – | – |
| NEM2 | 0.5 | 5 | 30c | 64.5 | – | – |
| NEM3 | 0.5 | 5 | 40c | 54.5 | – | – |
| NEM4 | 0.5 | 5 | 45c | 49.5 | – | – |
| NEM5 | 0.5 | 5 | 50c | 44.5 | – | – |
| NEM6 | 0.5 | 5 | 60c | 34.5 | – | – |
| NEG | 0.69 | 5 | 35b | 58.05 | 0.75 | 0.5 |
| RPG | 0.69 | – | – | 98.06 | 0.75 | 0.5 |
a% w/w of components
b S mix2:1
c S mix1:1
Thermodynamic Stability Studies
Selected formulations were subjected to different thermodynamic stability tests to assess their physical stability.
Heating–cooling cycle: Six cycles between refrigerator temperature (4°C) and 45°C with storage at each temperature of not less than 48 h were conducted, and the formulations were examined for stability at these temperatures.
Centrifugation test: Formulations were centrifuged at 3,500 rpm for 30 min and looked for phase separation.
Freeze–thaw cycle: Three freeze–thaw cycles between −21°C and +25°C with formulation storage at each temperature for not less than 48 h were performed.
Ex Vivo Permeation Studies
Preparation of Rat Skin
The animal protocol to carry out skin permeation study was reviewed and approved by the Institutional Animal Ethics Committee (approval no. 435, 2008). Abdominal skins were obtained from male albino Wistar rats weighing 200 ± 30 g after killing by diethyl ether aspiration. The skin was carefully excised, and the subcutaneous tissue was removed surgically, and the dermis side was wiped with isopropyl alcohol to remove adhering fat. The hairs on the skin were trimmed using electrical clipper. Then the skins were washed with water and examined for integrity before further use.
Permeation Study
The skin permeation rates of ropinirole from various nanoemulsions were determined to evaluate the effect of the formulation factors. The effect of the Smix on the permeation of ropinirole through excised rat skin was evaluated. The permeation studies were performed using Franz diffusion cell apparatus (FDC-6, LOGAN Instrument Corp., Somerset, NJ, USA). The effective diffusion area of the cell was 0.636 cm2 and receptor volume was 5.0 mL. The skin samples were mounted between the donor and receptor compartments of the diffusion cell with the stratum corneum side facing upward. The receptor compartment was filled with 30% PEG400-phosphate buffer saline (pH 7.4) and magnetically stirred at 600 rpm. The diffusion cell was maintained at 37 ± 1°C using a re-circulating water bath. PEG 400 was added into receptor compartment in order to maintain the sink conditions. It was earlier reported that the skin barrier function is not influenced by using up to 40% PEG 400 (50). The test NE formulations (1 mL) were placed into the donor compartment and sealed with paraffin film to provide occlusive conditions and to prevent the evaporation of water from the formulations. Samples were withdrawn at regular intervals (1, 2, 3, 4, 6, 8, 12, 16, and 24 h), filtered through 0.22-μm membrane filter, and analyzed for drug content by HPLC method as described below in “HPLC Analysis”. The receptor phase was immediately replenished with equal volume of fresh receptor medium.
Data Analysis
The cumulative amount of ropinirole permeated through the skin was plotted as a function of time (t, h). The slope of the steady state portion of the plot represents the flux (Jss, μg/cm2/h), whereas the x-intercept represents the lag time (tL, h). The permeability coefficient (Kp, cm/h) was calculated according to the equation:
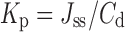 |
where Cd is the concentration of the drug in the donor compartment
The enhancement ratio (ER) was calculated according to the equation:
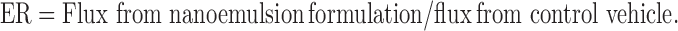 |
HPLC Analysis
Quantitative determination of ropinirole was performed by a validated HPLC method developed in our laboratory (51). A Shimadzu model HPLC equipped with quaternary LC-10A VP pump, variable wavelength programmable UV/VIS detector, SPD-10AVP column oven (Shimadzu), SCL 10AVP system controller (Shimadzu), and Rheodyne injector fitted with a 20 μL loop was used, and the data were recorded and evaluated using Class-VP 5.032 software. Chromatographic separation was achieved on a reversed phase C-18 column, LiChrospher®100 (5 μm, 250 mm × 4.6 mm i.d) using a mobile phase consisting of methanol and 0.05 M ammonium acetate buffer pH 7 (80:20 v/v) at a flow rate of 1 mL/min with UV detection at 250 nm. The mobile phase was filtered through 0.22-µm nylon filter prior to use.
Characterization of Nanoemulsion
Droplet Size Analysis
The droplet size of the nanoemulsion was determined by photon correlation spectroscopy, which analyses the fluctuations in light scattering due to Brownian motion of the particles using a Zetasizer 1000 HS (Malvern Instruments, Worcestershire, UK). Light scattering was monitored at 25°C at a 90° angle. A solid state laser diode was used as light source.
Viscosity
The viscosity of the nanoemulsion was determined by using Brookfield R/S plus rheometer (Brookfield Engineering Laboratories Inc., Middleboro, MA, USA) using a C50-1 spindle in triplicate at 25°C.
Refractive Index and pH Measurements
The refractive index of the system was measured by an Abbe refractometer (Bausch and Lomb Optical Company, Rochester, NY, USA) by placing one drop of the formulation on the slide in triplicate at 25°C. The apparent pH of the formulations was measured by a pH meter (Mettler Toledo MP 220, Greifensee, Switzerland) in triplicate at 25°C.
Transmission Electron Microscopy
Morphology and structure of the nanoemulsion were studied using Morgagni 268D electron microscope (Fei Company, Netherlands) operating at 70 kV capable of point-to-point resolution. Combination of bright field imaging at increasing magnification and of diffraction modes was used to reveal the form and size of the nanoemulsion. In order to perform transmission electron microscopy (TEM) observations, a drop of the nanoemulsion was suitably diluted with water and applied on carbon coated grid, then treated with a drop of 2% phosphotungstic acid and left for 30 s. The coated grid was dried and then taken on a slide and covered with a cover slip and observed under the microscope.
Preparation of Nanoemulsion Gel
The nanoemulsion was insufficiently viscous and could be therefore quickly removed from the skin. Therefore, the optimized nanoemulsion (NEL3) was converted into nanoemulsion gel. Nanoemulsion gel was prepared by dispersing 0.75% w/w of Carbopol 934 in sufficient quantity of distilled water (36). The dispersion was kept in dark for 24 h for complete swelling of carbopol. Then 0.69% w/w of ropinirole was dissolved in 5% w/w of Capryol 90. Ropinirole solution was added slowly to carbopol dispersion. Triethanolamine (0.5% w/w) was added in this mixture to neutralize carbopol. Then 35% w/w mixture of Smix (2:1) was added slowly. Then remaining quantity of distilled water was added to get the final preparation 100% w/w (Table I). The gel was further optimized with respect to polymer concentration, oil concentration, and drug loading by using a three-factor, three-level Box–Behnken statistical design in order to achieve the maximum drug permeation.
Experimental Design
Box–Behnken statistical design was used to statistically optimize the formulation parameters and evaluate the effects of formulation ingredients on the permeation of ropinirole from the nanoemulsion gel formulations. Polymer concentration namely Carbopol 934 (X1), oil concentration namely Capryol 90 (X2), and drug concentration (X3) were three independent variables (factors) selected in the preparation of nanoemulsion gel, while the ropinirole permeation (Y) was taken as the dependent variable (response). The concentration range of X1, X2, and X3 used to prepare the nanoemulsion gel formulations is given in Table II. For each factor, the experimental range based on the result of preliminary experiment was selected, and process variables were studied by conducting the runs at different levels of all factors. Data collected for responses in each run were analyzed using the software Design Expert® 7.1 (Stat-Ease Inc., Minneapolis, MN, USA) and fitted into multiple linear regression model.
Table II.
Variables and Their Corresponding Levels Implemented for the Construction of Box–Behnken Design
| Code | Independent variables (w/w) | Levels | ||
|---|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | ||
| X 1 | Carbopol content | 0.75 | 1.0 | 1.25 |
| X 2 | Capryol 90 concentration | 5.0 | 7.5 | 10 |
| X 3 | Drug concentration | 0.5 | 0.6 | 0.7 |
Preparation of Conventional Ropinirole Gel
Hydrogel with ropinirole was used a classical dermal formulation for comparison of ropinirole action incorporated in nanoemulsion gel. Carbopol 934 (0.75% w/w) was dispersed in sufficient quantity of distilled water (Table I). The dispersion was kept in dark for 24 h for complete swelling of Carbopol 934. Then ropinirole was mixed with the carbopol dispersion, and 0.5% w/w of triethanolamine was added in this mixture to neutralize carbopol, and the remaining water was added to give homogeneously dispersed ropinirole in hydrogel (52,53).
Skin Irritation Test
The animals were kept under standard laboratory conditions, with temperature of 25 ± 1°C and relative humidity of 55 ± 5%. They were housed in polypropylene cages with free excess to a standard laboratory diet (Lipton feed, Mumbai, India) and water ad libitum. The hairs on the abdominal side of albino Wistar rats were removed by clipping 1 day before the experiment (54). The rats were divided into three groups (n = 6). Group I served as the control, group II received nanoemulsion gel, and group III received 0.8% v/v aqueous solution of formalin as a standard irritant (55). After 24 h, the gel was removed, and the application sites were graded according to a visual scoring scale.
Statistical Analysis
All skin permeation experiments were conducted in triplicate over 24-h time period. Data were expressed as mean value ± SD. The data were compared for statistical significance by the one-way analysis of variance followed by Dunnett multiple comparisons test using GraphPad Instat software (GraphPad Software Inc., CA, USA). The data were considered to be significant at p < 0.05.
RESULTS AND DISCUSSION
Preparation of Nanoemulsions
Nanoemulsions containing 0.5% w/w ropinirole were prepared using Capryol 90 as the oil phase, Tween 20 as the surfactant, and Carbitol as the cosurfactant using phase titration (spontaneous emulsification) method (Table I). The excipients selected were pharmaceutically acceptable ingredients and fall into the generally regarded as safe category. The construction of phase diagrams makes it easy to find out the concentration range of components for the existence range of nanoemulsions. No change was found in the phase behavior of the pseudoternary phase diagram when ropinirole was loaded in the formulations, which might be attributed to the fact that the formation and stability of nanoemulsions consisting of non-ionic surfactants are not affected by the change in pH or ionic strength (56,57). The ratio of Tween 20 and carbitol (Smix) was selected at 2:1 and 1:1 since the largest nanoemulsion regions were obtained at these ratios (nanoemulsion area with Smix 2:1 was however greater than Smix 1:1) when compared with the other Smix ratios, i.e., 1:0, 1:1, 3:1, 1:2, and 1:3 (49). Large nanoemulsion region would give the formulator flexibility to manipulate with the concentration range of the ingredients to obtain maximum drug permeation and at the same time would also facilitate the selection of formulation with low surfactant and cosurfactant concentration, desirable for preparing non-irritating formulations. The prepared nanoemulsions were found to be transparent and easily flowable and were subjected to thermodynamic stability studies.
Thermodynamic Stability Studies
The selected nanoemulsion formulations (Table I) were subjected to various thermodynamic stability tests, which included heating–cooling cycle, centrifugation, and freeze–thaw cycle tests. No phase separation, creaming, or drug precipitation was observed while performing these tests (data not shown), and all the formulations survived these stress tests. The results showed that all the formulations had a good physical stability. Very low interfacial tension between oil and water and small droplet size made these systems thermodynamically stable (58). It is the thermostability which differentiates nanoemulsion from emulsions that have kinetic stability and will eventually phase separate (57). The nanoemulsions were then evaluated for their permeation characteristics.
Ex Vivo Skin Permeation Studies
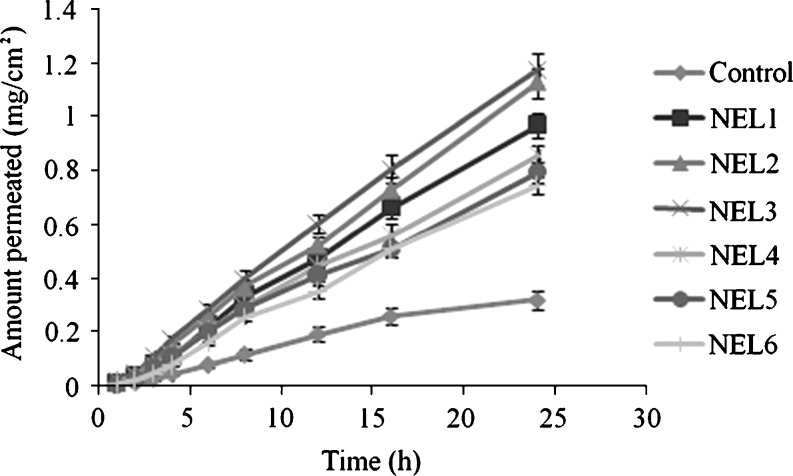
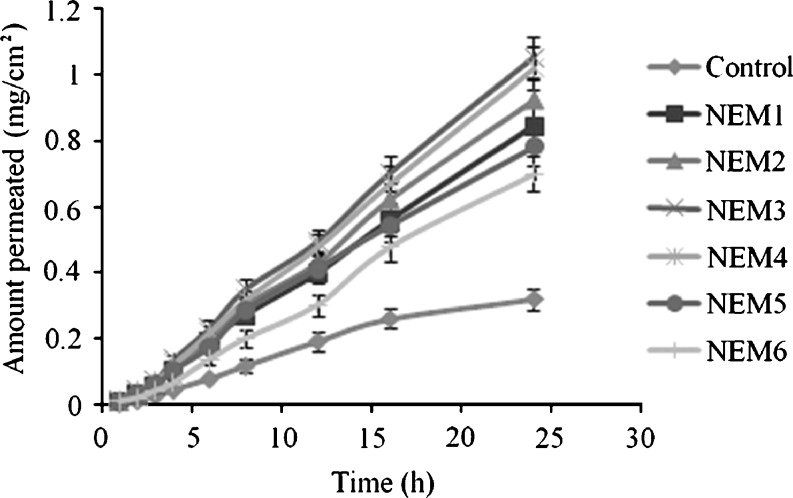
The effect of the Smix on the skin permeation of ropinirole was evaluated by varying the content of Smix in the nanoemulsion formulations from 20% to 60%. Twelve nanoemulsions were prepared for the purpose with two different Smix ratios essentially 2:1 and 1:1. The detailed composition of nanoemulsions is shown in Table I. The permeation parameters of the tested nanoemulsion formulations are presented in Table III. The permeation profiles of ropinirole through rat skin from various vehicles are shown in Figs. 1 and 2.
Table III.
Permeability Parameters of Nanoemulsion Formulations (Mean ± SD, n = 3)
| S. no. | Formulation code | J ss (µg/cm2/h) | K p (×10−2 cm/h) | t L (h) | ER |
|---|---|---|---|---|---|
| 1 | NEL1 | 42.64 ± 5.21 | 0.853 ± 0.132 | 1.43 ± 0.16 | 2.71 |
| 2 | NEL2 | 48.47 ± 5.15 | 0.969 ± 0.116 | 1.31 ± 0.11 | 3.08 |
| 3 | NEL3 | 51.81 ± 5.03 | 1.036 ± 0.152 | 1.21 ± 0.13 | 3.29 |
| 4 | NEL4 | 37.98 ± 4.91 | 0.759 ± 0.141 | 1.48 ± 0.15 | 2.41 |
| 5 | NEL5 | 34.27 ± 4.45 | 0.685 ± 0.108 | 1.69 ± 0.20 | 2.18 |
| 6 | NEL6 | 32.93 ± 4.32 | 0.658 ± 0.126 | 1.98 ± 0.31 | 2.09 |
| 7 | NEM1 | 36.62 ± 5.44 | 0.732 ± 0.121 | 1.54 ± 0.19 | 2.32 |
| 8 | NEM2 | 40.25 ± 4.92 | 0.805 ± 0.133 | 1.37 ± 0.14 | 2.55 |
| 9 | NEM3 | 45.51 ± 5.14 | 0.910 ± 0.149 | 1.28 ± 0.12 | 2.89 |
| 10 | NEM4 | 44.13 ± 4.46 | 0.883 ± 0.135 | 1.41 ± 0.17 | 2.80 |
| 11 | NEM5 | 34.58 ± 3.78 | 0.692 ± 0.123 | 1.88 ± 0.28 | 2.19 |
| 12 | NEM6 | 30.76 ± 3.94 | 0.615 ± 0.102 | 2.26 ± 0.39 | 1.95 |
| 13 | Control | 15.76 ± 2.83 | 0.326 ± 0.092 | 1.12 ± 0.11 | – |
Fig. 1.
Permeation profiles of ropinirole through excised rat skin from nanoemulsions formulated with S mix 2:1 (mean ± SD, n = 3)
Fig. 2.
Permeation profiles of ropinirole through excised rat skin from nanoemulsions formulated with S mix 1:1 (mean ± SD, n = 3)
The content of Smix in the nanoemulsion formulation was found to affect the skin permeation rate of ropinirole directly. As the content of Smix (2:1) increased from 20% to 60% (NEL1 to NEL6), the skin permeation increased from 20% to 35% then decreased up to 60% Smix concentration (Fig. 1). This might be due to a decreased thermodynamic activity of the drug in the nanoemulsion at the higher content of surfactant (59). The thermodynamic activity of a drug in the formulation is a significant driving force for the release and penetration of the drug into the skin. With increased surfactant concentration, affinity to the vehicle became greater, and there is a slow release of the drug and/or a poor transfer from the vehicle to the skin. Another plausible reason that could have an additive effect was the hydration effect of water. On the parallel side, when the water content was increased from 34.5% to 74.5% in the formulation (NEL6 to NEL1), then the hydration of stratum corneum increased. It is because the water in nanoemulsion could hydrate the skin and caused the corneous cell to swell, thus making the drug channels wide; therefore, with the increasing amount of the water in the system, the cumulative permeation amount might have improved. As some lipid chains in the stratum corneum are covalently attached to the corneocytes, hydration of these proteins will also lead to the disorder of the lipid bilayers. The findings were consistent with the previous studies (28,60,61). Nanoemulsions are known to provide increase permeation rates and decreased lag times compared to conventional formulations (42) by altering both the lipophilic and the polar pathway by synergestic interactions of vehicle components with the stratum corneum (62). Similarly, permeation increased when the Smix content was increased from 20% to 40% and then decreased in case of nanoemulsions formulated with Smix 1:1. Thus, the composition played an important role in permeation enhancement of nanoemulsions as also demonstrated by previous studies (63–65).
Permeation of ropinirole was also considered from the neat oil phase as Capryol 90 has permeation enhancing properties. However, a lower flux value (9.32 ± 2.03 μg/cm2/h) was obtained as compared to nanoemulsion formulations, which might be ascribed to the greater affinity of the drug for the oil phase because of its lipophilic nature and therefore lesser partitioning of the drug from the vehicle to the skin (p < 0.01). Permeation was also carried out from neat surfactant (Tween 20) in order to investigate whether the nanoemulsions had any superior effect in comparison to the surfactant per se. It was found that comparatively far lower flux (11.68 ± 2.74 μg/cm2/h) could be attained with Tween 20 in contrast with the nanoemulsions and this was statistically significant (p < 0.01). Again, drug release from the vehicle might have played a crucial role.
Among the formulations tested, the formulation NEL3, which was composed of 0.5% w/w ropinirole, 5% w/w Capryol 90, and 35% w/w Smix (2:1), showed the highest permeation profile. The skin permeation rate of ropinirole from this nanoemulsion was 51.81 ± 5.03 μg/cm2/h. In comparison of the permeation of all nanoemulsions for 24 h from Smix (2:1), an average efficiency in promoting the percutaneous permeation of ropinirole tended to be in the following order NEL3>NEL2>NEL1>NEL4>NEL5>NEL6. The decreasing order of permeation of nanoemulsion formulations obtained with Smix (1:1) is given as NEM3>NEM4>NEM2>NEM1>NEM5>NEM6. From the results, permeation enhancing efficacy of nanoemulsions was found to be dependent on the amount of surfactant mixture.
The lag time is a permeation parameter depending mainly on the diffusivity of the drug through the skin and ranged from 1.21 to 2.26 h. On the other hand, fluxes ranged from 51.81 ± 5.03 to 30.76 ± 4.94 μg/cm2/h. The flux values obtained for all the nanoemulsions were significantly higher than the control (p < 0.01), indicating that the permeation parameters of ropinirole from nanoemulsions were markedly influenced by the composition of the formulations. Since all the nanoemulsions carried equal drug load it could, therefore, be concluded that the concentration gradient is not the only factor governing the permeation process, and other probable mechanisms were also involved. The shortest lag time (1.21 ± 0.13 h) was obtained with nanoemulsion NEL3. Overall, short lag times were observed for nanoemulsions. It is assumed that by incorporation into the colloidal carrier, better contact with the skin is achieved, which could be one of the reasons why the effect appeared sooner, resulting in shorter lag times. Surfactants present in the nanoemulsions may cause increased membrane fluidity, solubilization, or extraction of lipids present in the stratum corneum and cause alterations in the tight junction properties (66) leading to improved permeation. The nanosized droplets in nanoemulsions lead to an enormous increase in the interfacial area, which influences transport properties of the drug (56). It is assumed that the low interfacial tension and the continuously and spontaneously fluctuating interfaces of nanoemulsions are supposed to facilitate transfer of the drug to the skin. This assumption is further corroborated by the NMR studies of Krielgaard et al. (45) and Hua et al. (67). They found that the transdermal flux was related to drug mobility resulting from a certain nanoemulsion structure. Another probable mechanism could be attributed to the permeation of loaded drug directly from the nanoemulsion droplets to the stratum corneum without nanoemulsion fusion at the stratum corneum, which indicates that enhancement effect of nanoemulsions is caused by the nanosized droplets dispersed in the continuous phase, which can move easily into the stratum corneum and carry the drug through the skin barrier.
A trend was observed of decreasing flux and increasing lag time on increasing the amount of Smix after an optimum level (for both Smix 1:1 and 2:1). The observed results might be due to a decreased thermodynamic activity of the drug in the nanoemulsion at higher concentration of Smix (22,48). Higher content of surfactant is also not useful as it might cause skin irritation, and the safety of the transdermal delivery vehicle is an important factor in formulation development.
Nanoemulsion Characterization
The physicochemical parameters of the developed nanoemulsion (NEL3) are depicted in Table IV. The average droplet size of the nanoemulsion was determined to be 40.25 nm. The nanodroplets were nearly 1.4-fold larger than those without the drug. Polydispersity index was determined as the ratio of standard deviation to the mean droplet size of the formulation. The low polydispersibility value observed for the formulation indicated uniformity of droplet size within the formulation. It was obvious from the physicochemical data that the developed nanoemulsion had a low viscosity (39.94 ± 1.52 mPa s). Low viscosity is one of the characteristic features of the nanoemulsions, which exhibit Newtonian flow behavior (57). pH value (5.71 ± 0.05) obtained was within the physiological range and suitable for topical application. The droplets in the nanoemulsion appeared dark, and the surroundings were bright, and a “positive” image was seen using TEM (Fig. 3).
Table IV.
Characteristics of the Optimized Nanoemulsion (NEL3) (Mean ± SD, n = 3)
| S. no. | Parameter | Value |
|---|---|---|
| 1 | Droplet size (nm) | 40.25 |
| 2 | Polydispersity | 0.079 |
| 3 | Viscosity (mPa s) | 39.94 ± 1.52 |
| 4 | pH | 5.71 ± 0.05 |
| 5 | Refractive index | 1.460 ± 0.021 |
Fig. 3.
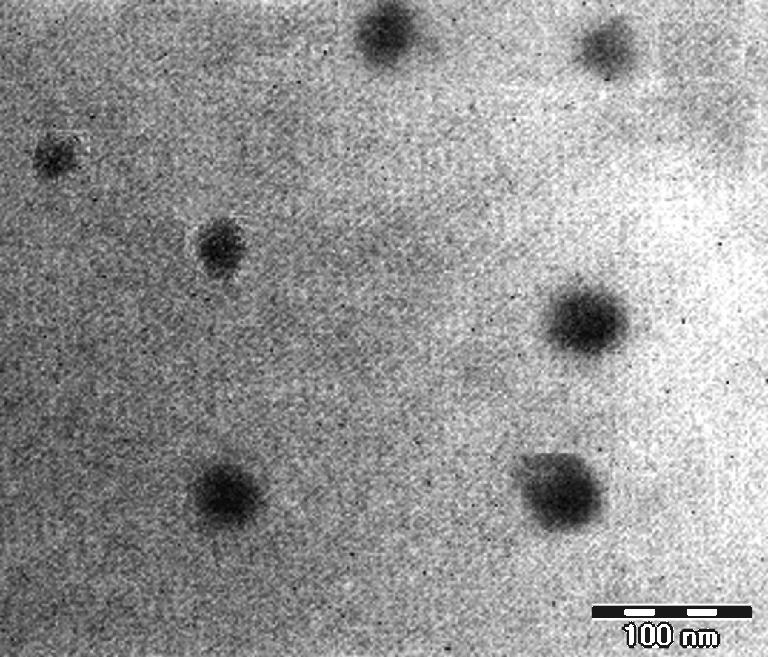
Transmission electron micrograph of nanoemulsion NEL3
Statistical Optimization of Nanoemulsion Gel
Recently, the gel matrices formed by carbomers, xantham gum, and carrageenan have been used to prepare the nanoemulsion gel for improving the viscosity of the nanoemulsion systems (68–71). In the present work, we have selected Carbopol 934 as the gel forming polymer. In contrast to xantham gum and carrageenan, a small amount of neutralizing agent like sodium hydroxide or triethanolamine is added to carbopol, which is a polycarboxylic acid, to start a partial ionization that in turn promotes the gel formation (72). In our preliminary study, gel containing 0.5% w/w carbopol had a relatively high fluidity. So, the minimum concentration taken for the preparation of nanoemulsion gel was 0.75%. It has been previously reported that when the polymer concentration is close to 1%, the viscoelastic and flow properties of the nanoemulsions are suitable to ensure an adequate performance during topical and transdermal administration (73). At such a polymer concentration, a weak gel behavior is exhibited by the aqueous carbopol system and the corresponding nanoemulsion.
The nanoemulsion NEL3 exhibiting higher flux was converted into nanoemulsion gel and taken for further optimization. To identify the optimum levels of different process variables influencing the response, i.e., transdermal permeation of ropinirole, an experimental design was made according to the Box–Behnken design for the three selected variables. The individual and interactive effects of these process variables were studied by conducting the process at different levels of all the factors. The results of the experimental data and simulated values are listed in Table V.
Table V.
Box–Behnken Design Showing the Various Experiments To Be Performed Along with the Obtained Results
| Runs | Formulation code | X 1 | X 2 | X 3 | Cumulative amount of ropinirole permeated at 24 h (mg/cm2) | |
|---|---|---|---|---|---|---|
| Actual | Predicted | |||||
| 1 | NG1 | + | 0 | + | 0.751 ± 0.025 | 0.75 |
| 2 | NG2 | 0 | + | + | 0.811 ± 0.032 | 0.81 |
| 3 | NG3 | 0 | 0 | 0 | 0.836 ± 0.029 | 0.84 |
| 4 | NG4 | − | 0 | − | 0.943 ± 0.034 | 0.94 |
| 5 | NG5 | 0 | 0 | 0 | 0.825 ± 0.026 | 0.82 |
| 6 | NG6 | − | 0 | + | 0.975 ± 0.037 | 0.97 |
| 7 | NG7 | + | − | 0 | 0.793 ± 0.031 | 0.79 |
| 8 | NG8 | + | + | 0 | 0.722 ± 0.028 | 0.72 |
| 9 | NG9 | 0 | 0 | 0 | 0.845 ± 0.037 | 0.84 |
| 10 | NG10 | 0 | − | + | 0.866 ± 0.035 | 0.87 |
| 11 | NG11 | + | 0 | − | 0.734 ± 0.033 | 0.73 |
| 12 | NG12 | 0 | + | − | 0.772 ± 0.027 | 0.77 |
| 13 | NG13 | − | − | 0 | 0.988 ± 0.041 | 0.99 |
| 14 | NG14 | 0 | 0 | 0 | 0.832 ± 0.035 | 0.83 |
| 15 | NG15 | − | + | 0 | 0.931 ± 0.039 | 0.93 |
| 16 | NG16 | 0 | − | − | 0.842 ± 0.034 | 0.84 |
| 17 | NG17 | 0 | 0 | 0 | 0.822 ± 0.037 | 0.82 |
The model proposed the following polynomial equation for the cumulative amount of ropinirole permeated:
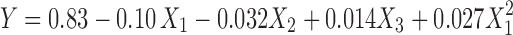 |
1 |
where Y is the cumulative amount of drug permeated at the end of 24 h, X1 is the carbopol concentration, X2 is the oil concentration (i.e., Capryol 90 concentration), and X3 is the drug content. Only the terms with statistical significance were included in Eq. 1. A positive value represents an effect that favors the optimization (synergestic effect), while a negative value indicates an inverse relationship between the factor and the response. Coefficients with higher order terms or more than one factor term in the regression equation represent quadratic relationships or interaction terms, respectively.
The model F value of 164.81 implied that the model is significant (p < 0.0001). The “lack of fit F value” of 0.57 implied that the lack of fit is not significant. Non-significant lack of fit is good for the model to fit. Regression analysis for the response (Y) was performed. The “predicted R-squared” of 0.9725 was in reasonable agreement with the “adjusted R-squared” of 0.9893. % CV was found to be 0.98. “Adequate precision” measures the signal-to-noise ratio. A ratio greater than 4 is desirable. The obtained ratio of 43.137 indicated an adequate signal. Therefore, this model could be used to navigate the design space.
In our case, X1, X2, X3, and 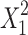 were the significant model terms, and X1, i.e., carbopol concentration had a more pronounced effect on drug permeation than any other variables. It is thus evident that the drug concentration had a positive effect on the response, while carbopol and Capryol 90 concentration had a negative effect. Additionally, no significant interactions between the factors were found. The relationship between the dependent and independent variables was further elucidated using contour and response surface plots.
were the significant model terms, and X1, i.e., carbopol concentration had a more pronounced effect on drug permeation than any other variables. It is thus evident that the drug concentration had a positive effect on the response, while carbopol and Capryol 90 concentration had a negative effect. Additionally, no significant interactions between the factors were found. The relationship between the dependent and independent variables was further elucidated using contour and response surface plots.
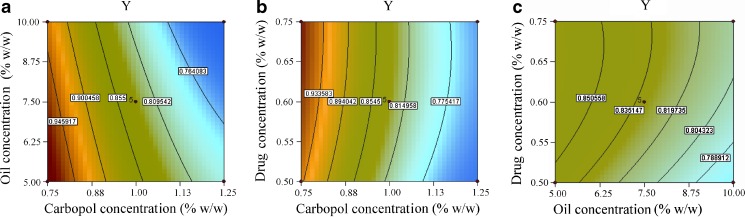
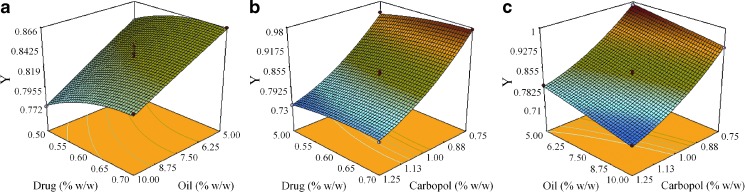
Contour Plots and Response Surface Analysis
Two-dimensional contour plots and three-dimensional response surface plots are presented in Figs. 4 and 5. These types of plots are useful in the study of the effects of two factors/variables on the response at one time. In all the presented figures, the third factor was kept at a constant level. Figure 4a, b exhibited a nearly linear relationship of factor X1 with factors X2 and X3 in the form of almost straight lines. Factors X2 and X3 also had a near linear relationship at all levels of the two variables (Fig. 4c). Response surface plots show the relationship between these factors even more distinctly (Fig. 5). The results showed that when the carbopol concentration was increased the response decreases, i.e., ropinirole permeation decreases on increasing the polymer concentration. This observation might be ascribed to the increased viscosity of the system. Permeation was observed to be inversely related to the viscosity of the medium, and this had also been reported previously (67). Also, increasing the oil level (i.e., Capryol 90) from 5% w/w to 10% w/w led to a decrease in the transdermal permeation. Since the drug is lipophilic, its affinity to the oil phase might have increased, resulting in a decrease of thermodynamic activity of the drug and hence the release was compromised. Improvement in drug permeation was observed when the drug load was increased. Linear correlation between the actual and the predicted response variable was obtained (R2 = 0.9953), which indicated excellent goodness of fit and thus proving the high prognostic ability of the response surface methodology.
Fig. 4.
Contour plot showing effect of a carbopol concentration (X 1) and oil concentration (X 2) on response Y, b carbopol concentration (X 1) and drug concentration (X 3) on response Y, and c oil concentration (X 2) and drug concentration (X 3) on response Y
Fig. 5.
Response surface plot showing effect of a oil concentration (X 2) and drug concentration (X 3) on response Y, b carbopol concentration (X 1) and drug concentration (X 3) on response Y, and c carbopol concentration (X 1) and oil concentration (X 2) on response Y
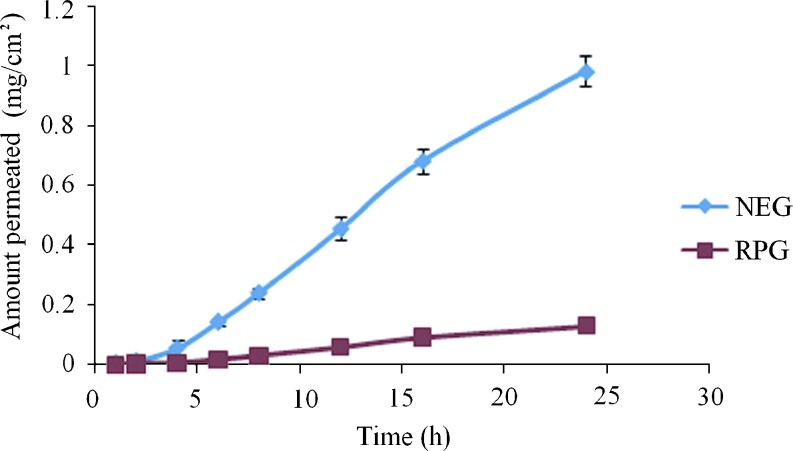
Point prediction of the Design Expert® software was used to determine the optimum values of the factors for maximum drug permeation. Eventually, the optimum values of carbopol (0.75%w/w), Capryol 90 (5%w/w), and drug content (0.692%w/w) were obtained. These values predicted 0.997 mg/cm2 permeation of ropinirole at the end of 24 h. predicted value of permeation was validated by further performing the studies with the previously optimized parameters and an average of 0.983 ± 0.038 mg/cm2 permeation was achieved. This showed 98.6% validity of the predicted model for drug permeation. Figure 6 shows the comparative permeation profiles of ropinirole obtained with the nanoemulsion gel (NEG) optimized with Box–Behnken design and the conventional ropinirole gel (RPG). The permeation parameters of NEG and RPG are presented in Table VI. The optimized nanoemulsion gel showed a 7.5- fold enhancement of ropinirole flux as compared to the conventional hydrogel. The obtained results could be ascribed to the presence of efficient permeation enhancers in the NEG like Capryol 90, Tween 20, and carbitol.
Fig. 6.
Permeation profiles of ropinirole from nanoemulsion gel (NEG) and conventional hydrogel (RPG) across rat skin under ex vivo conditions (mean ± SD, n = 3)
Table VI.
Permeation Parameters of Ropinirole Through Excised Rat Skin from Nanoemulsion Gel (NEG) and Conventional Hydrogel (RPG) (Mean ± SD, n = 3)
| S. no. | Formulation | J ss (µg/cm2/h) | K p (×10−2 cm/h) | ER |
|---|---|---|---|---|
| 1 | NEG | 45 ± 5.12 | 0.650 ± 0.103 | 7.5 |
| 2 | RPG | 6 ± 0.67 | 0.087 ± 0.004 | – |
Skin Irritation Study
Various formulations, when applied topically, might elicit primary skin irritation. Rat skin irritation experiments were, therefore, conducted in order to assess the potential irritant effects of the developed nanoemulsion formulation (NEG). The test formulation (50 µl) was applied to an area of about 0.786 cm2. The transdermal nanoemulsion formulation showed a skin irritation score (erythema and edema) of less than 2, which was significant when compared with formalin (p < 0.05). According to Draize et al., compounds producing scores of 2 or less are considered negative (no skin irritation) (74). Hence, from the results of the preliminary skin irritation study, the optimized nanoemulsion formulation appeared to be safe for transdermal delivery.
Conclusion
An appropriate combination of the oil, surfactant, cosurfactant, and water is a major formulation consideration in nanoemulsion preparation for the transdermal drug delivery. The optimized nanoemulsion formulation, which exhibited highest drug permeation, consisted of 0.5% w/w ropinirole, 5% w/w Capryol 90, 35% w/w Smix (2:1), and 59.5% w/w water. Subsequently, ropinirole nanoemulsion gels were formulated successfully using Box–Behnken statistical technique, which helped in finding the optimum formulation for the transdermal delivery of ropinirole. The results revealed that the oil, polymer, and drug concentration significantly affect the drug permeation across the skin from the nanoemulsion gels. Oil and polymer concentrations were found to have a negative influence on drug permeation while the drug content had a positive influence. The permeation studies with nanoemulsions were carried out under occlusive conditions. However, under in-use conditions, the water in the formulation evaporates quickly, and the oil/surfactant remains on skin for much longer period of time. This might alter not only the thermodynamic activity of the drug but also the micro-structure of the nanoemulsion. Overall, the results of the present investigation suggested a promising role of nanoemulsions in enhancing the percutaneous penetration of ropinirole.
Acknowledgments
The authors are indebted to the University Grants Commission, Govt. of India for providing the financial assistance to the project (project no. F.31-74/2005 SR). Adnan Azeem thanks the Colocon Asia for providing gift samples of surfactants and oils (Gattefosse, France) and USV (Mumbai, India) for the gift sample of ropinirole. The authors also wish to thank the Sophisticated Analytical Instruments Facilities (SAIF) of All India Institute of Medical Sciences (AIIMS), New Delhi, India for carrying out the TEM analysis.
References
- 1.Hely MA, Morris Reid WGJ. Sydney multicenter study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20:190–199. doi: 10.1002/mds.20324. [DOI] [PubMed] [Google Scholar]
- 2.Schrag A, Jahanshahi M, Quinn NP. What contributes to quality of life in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2000;69:308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Parkinson’s disease Survey Steering Committee Factors impacting on quality of life in Parkinson’s disease: results from an international survey. Mov Disord. 2002;17:60–67. doi: 10.1002/mds.10010. [DOI] [PubMed] [Google Scholar]
- 4.Findley L, Aujla M, Bain PG, Baker M, Beech C, Bowman C, Holmes J, Kingdom WK, MacMohan DG, Peto V, Playfer JR. Direct economic impact of Parkinson’s disease: a research survey in the United Kingdom. Mov Disord. 2003;18:1139–1145. doi: 10.1002/mds.10507. [DOI] [PubMed] [Google Scholar]
- 5.Brandabur MM. Current therapy in Parkinson’s disease. Surg Neurol. 1999;52:318–322. doi: 10.1016/S0090-3019(99)00091-9. [DOI] [PubMed] [Google Scholar]
- 6.Ball J. Current advances in Parkinson’s disease. Trends Neurosci. 2001;24:367–369. doi: 10.1016/S0166-2236(00)01850-6. [DOI] [PubMed] [Google Scholar]
- 7.Golbe LI. Young-onset Parkinson’s disease: a clinical review. Neurology. 1991;41:168–173. doi: 10.1212/wnl.41.2_part_1.168. [DOI] [PubMed] [Google Scholar]
- 8.Rinne UK. Treatment of early Parkinson disease. Parkinsonism Relat Disord. 2001;7:59–62. doi: 10.1016/S1353-8020(00)00040-7. [DOI] [PubMed] [Google Scholar]
- 9.Kaye CM, Nicholls B. Clinical pharmacokinetics of ropinirole. Clin Pharmacokinet. 2000;39:243–254. doi: 10.2165/00003088-200039040-00001. [DOI] [PubMed] [Google Scholar]
- 10.Standaert DG, Young AB. In: Bruton LL, Lazo JS, Parker KL, editors. Goodman Gilman’s the pharmacological basis of therapeutics, 11th edn. International Edition. New York: McGraw-Hill; 2006. p. 527–538.
- 11.Matheson AJ, Spencer CM. Ropinirole: a review of its use in the management of Parkinson’s disease. Drugs. 2000;60:115–137. doi: 10.2165/00003495-200060010-00007. [DOI] [PubMed] [Google Scholar]
- 12.Dollery C (ed). Therapeutic drugs. 2nd ed., vol. 2. Edinburgh: Churchill Livingstone; 1999. p. R50–R54.
- 13.Olanow CW, Agid Y, Mizuno Y, Albanese A, Bonucelli U, Damier P, Yebenes JD, Gershanik O, Guttman M, Grandas F, Hallett M, Hornykiewicz O, Jenner P, Katzenschlager R, Langston WJ, Lewitt P, Melamed E, Mena MA, Michel PP, Mytilineou C, Obeso JA, Poewe W, Quinn N, Raisman-Vozari R, Rajput AH, Rascol O, Sampaio C, Stocchi F. Levodopa in the treatment of Parkinson's disease: current controversies. Mov Disord. 2004;19:97–1005. doi: 10.1002/mds.20243. [DOI] [PubMed] [Google Scholar]
- 14.Walters KA. Penetration enhancers and their use in transdermal therapeutic systems. In: Hadgraft J, Guy RH, editors. Transdermal drug delivery, development issues and research initiatives. New York: Marcel Dekker; 1989. pp. 197–246. [Google Scholar]
- 15.Kim BS, Won M, Lee KM, Kim S. In vitro permeation studies of nanoemulsions containing ketoprofen as a model drug. Drug Del. 2008;15:465–469. doi: 10.1080/10717540802328599. [DOI] [PubMed] [Google Scholar]
- 16.Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Design and development of oral oil in water ramipril nanoemulsion formulation: in vitro and in vivo assessment. J Biomed Nanotech. 2007;3:1–17. doi: 10.1166/jbn.2007.008. [DOI] [Google Scholar]
- 17.Azeem A, Khan ZI, Aqil M, Ahmad FJ, Khar RK, Talegaonkar S. Microemulsions as a surrogate carrier for dermal drug delivery. Drug Dev Ind Pharm. 2009;35:525–547. doi: 10.1080/03639040802448646. [DOI] [PubMed] [Google Scholar]
- 18.Azeem A, Rizwan M, Ahmad FJ, Khan ZI, Khar RK, Aqil M, Talegaonkar S. Emerging role of microemulsions in cosmetics. Recent Pat Drug Deliv Formul. 2008;2:275–289. doi: 10.2174/187221108786241624. [DOI] [PubMed] [Google Scholar]
- 19.Heuschkel S, Goebel A, Neubert RHH. Microemulsions—modern colloidal carrier for dermal and transdermal drug delivery. J Pharm Sci. 2008;97:603–631. doi: 10.1002/jps.20995. [DOI] [PubMed] [Google Scholar]
- 20.El Maghraby GM. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: effects of cosurfactants. Int J Pharm. 2008;355:285–292. doi: 10.1016/j.ijpharm.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Shevachman M, Garti N, Shani A, Sintov AC. Enhanced percutaneous permeability of diclofenac using a new U-type dilutable microemulsion. Drug Dev Ind Pharm. 2008;34:403–412. doi: 10.1080/03639040701662479. [DOI] [PubMed] [Google Scholar]
- 22.Huang YB, Lin YH, Lu TM, Wang RJ, Tsai YH, Wu PC. Transdermal delivery of capsaicin derivative-sodium nonivamide acetate using microemulsions as vehicles. Int J Pharm. 2008;349:206–211. doi: 10.1016/j.ijpharm.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Yuan JS, Ansari M, Samaan M, Acosta EJ. Linker-based lecithin microemulsions for transdermal delivery of lidocaine. Int J Pharm. 2008;349:130–143. doi: 10.1016/j.ijpharm.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 24.Biruss B, Kählig H, Valenta C. Evaluation of an eucalyptus oil containing topical drug delivery system for selected steroid hormones. Int J Pharm. 2007;328:142–151. doi: 10.1016/j.ijpharm.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Biruss B, Valenta C. The advantage of polymer addition to a non-ionic oil in water microemulsion for the dermal delivery of progesterone. Int J Pharm. 2008;349:269–273. doi: 10.1016/j.ijpharm.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Kamal MA, Iimura N, Nabekura T, Kitagawa S. Enhanced skin permeation of diclofenac by ion-pair formation and further enhancement by microemulsion. Chem Pharm Bull. 2007;55:368–371. doi: 10.1248/cpb.55.368. [DOI] [PubMed] [Google Scholar]
- 27.Kantarci G, Ozgüney I, Karasulu HY, Arzi S, Güneri T. Comparison of different water/oil microemulsions containing diclofenac sodium: preparation, characterization, release rate, and skin irritation studies. AAPS Pharm Sci Tech. 2007;8:E91. doi: 10.1208/pt0804091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan Y, Li SM, Mo FK, Zhong DF. Investigation of microemulsion system for transdermal delivery of meloxicam. Int J Pharm. 2006;321:117–123. doi: 10.1016/j.ijpharm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Lee PJ, Langer R, Shastri VP. Novel microemulsion enhancer formulation for simultaneous transdermal delivery of hydrophilic and hydrophobic drugs. Pharm Res. 2003;20:264–269. doi: 10.1023/A:1022283423116. [DOI] [PubMed] [Google Scholar]
- 30.Junyaprasert VB, Boonsaner P, Leatwimonlak S, Boonme P. Enhancement of the skin permeation of clindamycin phosphate by Aerosol OT/1-butanol microemulsions. Drug Dev Ind Pharm. 2007;33:874–880. doi: 10.1080/03639040600975097. [DOI] [PubMed] [Google Scholar]
- 31.Talegaonkar S, Akhter S, Jain GK, Ahmad FJ, Khar RK, Jain N, Khan ZI. Investigation of nanoemulsion system for transdermal delivery of domperidone: ex-vivo and in-vivo studies. Curr Nanosci. 2008;4:381–390. doi: 10.2174/157341308786306071. [DOI] [Google Scholar]
- 32.Ambade KW, Jadhav SL, Gambhire MN, Kurmi SD, Kadam VJ, Jadhav KR. Formulation and evaluation of flurbiprofen microemulsion. Curr Drug Deliv. 2008;5:32–41. doi: 10.2174/156720108783331032. [DOI] [PubMed] [Google Scholar]
- 33.Shakeel F, Baboota S, Ahuja A, Ali J, Aqil M, Shafiq S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech. 2007;8:E104. doi: 10.1208/pt0804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao X, Liu JP, Zhang X, Li Y. Enhancement of transdermal delivery of theophylline using microemulsion vehicle. Int J Pharm. 2006;327:58–64. doi: 10.1016/j.ijpharm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 35.Paolino D, Ventura CA, Nisticò S, Puglisi G, Fresta M. Lecithin microemulsions for the topical administration of ketoprofen: percutaneous adsorption through human skin and in vivo human skin tolerability. Int J Pharm. 2002;244:21–31. doi: 10.1016/S0378-5173(02)00295-8. [DOI] [PubMed] [Google Scholar]
- 36.Shakeel F, Baboota S, Ahuja A, Ali J, Shafiq S. Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion. J Nanobiotech. 2008;6:8. doi: 10.1186/1477-3155-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolzinger MA, Briancon S, Pelletier J, Fessi H, Chevalier Y. Percutaneous release of caffeine from microemulsion, emulsion and gel dosage forms. Eur J Pharm Biopharm. 2008;68:446–451. doi: 10.1016/j.ejpb.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Ktistis G, Niopas I. A study on the in-vitro percutaneous absorption of propranolol from disperse systems. J Pharm Pharmacol. 1998;50:413–419. doi: 10.1111/j.2042-7158.1998.tb06881.x. [DOI] [PubMed] [Google Scholar]
- 39.Gasco MR, Gallarate M, Pattarino F. In vitro permeation of azelaic acid from viscosized microemulsions. Int J Pharm. 1991;69:193–196. doi: 10.1016/0378-5173(91)90361-Q. [DOI] [Google Scholar]
- 40.Abramovi Z, Sustarsic U, Teskac K, Sentjurc M, Kristl J. Influence of nanosized delivery systems with benzyl nicotinate and penetration enhancers on skin oxygenation. Int J Pharm. 2008;359:220–227. doi: 10.1016/j.ijpharm.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Trotta M. Influence of phase transformation on indomethacin release from microemulsions. J Control Rel. 1999;60:399–405. doi: 10.1016/S0168-3659(99)00094-2. [DOI] [PubMed] [Google Scholar]
- 42.Kriwet K, Muller-Goymann CC. Diclofenac release from phospholipid drug systems and permeation through excised human stratum corneum. Int J Pharm. 1995;125:231–242. doi: 10.1016/0378-5173(95)00130-B. [DOI] [Google Scholar]
- 43.Zhu W, Yu A, Wang W, Dong R, Wu J, Zhai G. Formulation design of microemulsion for dermal delivery of penciclovir. Int J Pharm. 2008;360:184–190. doi: 10.1016/j.ijpharm.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Teichmann A, Heuschkel S, Jacobi U, Presse G, Neubert RHH, Sterry W, Lademann J. Comparison of stratum corneum penetration and localization of a lipophilic model drug applied in an o/w microemulsion and an amphiphilic cream. Eur J Pharm Biopharm. 2007;67:699–706. doi: 10.1016/j.ejpb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Kreilgaard M, Pedersen EJ, Jaroszewski JW. NMR characterization and transdermal drug delivery potential of microemulsion systems. J Control Rel. 2000;69:421–433. doi: 10.1016/S0168-3659(00)00325-4. [DOI] [PubMed] [Google Scholar]
- 46.Fini A, Bergamante V, Ceschel GC, Ronchi C, De Moraes CA. Control of transdermal permeation of hydrocortisone acetate from hydrophilic and lipophilic formulations. AAPS Pharm Sci Tech. 2008;9:762–768. doi: 10.1208/s12249-008-9107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolzinger MA, Carduner TC, Poelman MC. Bicontinuous sucrose ester microemulsion: a new vehicle for topical delivery of niflumic acid. Int J Pharm. 1998;176:39–45. doi: 10.1016/S0378-5173(98)00292-0. [DOI] [Google Scholar]
- 48.Rhee Y-S, Choi E-S, Park S-C. Transdermal delivery of ketoprofen using microemulsions. Int J Pharm. 2001;228:161–170. doi: 10.1016/S0378-5173(01)00827-4. [DOI] [PubMed] [Google Scholar]
- 49.Azeem A, Rizwan M, Ahmad FJ, Iqbal Z, Khar RK, Aqil M, Talegaonkar S. Nanoemulsion components screening and selection: a technical note. AAPS PharmSci Tech. 2009;10:69. doi: 10.1208/s12249-008-9178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tojo K, Chiang CC, Chien YW. Influence of donor solution upon skin permeation of drug. J Chem Eng Jpn. 1986;19:153–155. doi: 10.1252/jcej.19.153. [DOI] [Google Scholar]
- 51.Azeem A, Iqbal Z, Ahmad FJ, Khar RK, Talegaonkar S. Development and validation of a stability indicating method for determination of ropinirole in the bulk drug and in pharmaceutical dosage forms. Acta Chromat. 2008;20:95–107. doi: 10.1556/AChrom.20.2008.1.8. [DOI] [Google Scholar]
- 52.Bergamante V, Ceschel GC, Marazzita S. Effect of vehicles on topical application of aloe vera and arnica montana components. Drug Del. 2007;14:427–432. doi: 10.1080/10717540701202960. [DOI] [PubMed] [Google Scholar]
- 53.Desai KGH. Enhanced skin permeation of rofecoxib using topical microemulsion gel. Drug Dev Res. 2004;63:33–40. doi: 10.1002/ddr.10386. [DOI] [Google Scholar]
- 54.Namdeo A, Jain NK. Liquid crystalline pharmacogel based enhanced transdermal delivery of propranolol hydrochloride. J Control Rel. 2002;82:223–236. doi: 10.1016/S0168-3659(02)00106-2. [DOI] [PubMed] [Google Scholar]
- 55.Mutalik S, Udupa N. Glibenclamide transdermal patches: physicochemical, pharmacodynamic, and pharmacokinetic evaluations. J Pharm Sci. 2004;93:1577–1594. doi: 10.1002/jps.20058. [DOI] [PubMed] [Google Scholar]
- 56.Eccleston J. Microemulsions. In: Swarbrick J, Boylan JC, editors. Encyclopedia of pharmaceutical technology. New York: Marcel Dekker; 1994. pp. 375–421. [Google Scholar]
- 57.Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45:89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 58.Langevin D. Microemulsions. Acc Chem Res. 1988;21:255–260. doi: 10.1021/ar00151a001. [DOI] [Google Scholar]
- 59.Shah VP. Skin penetration enhancers: scientific perspectives. In: Hsieh DS, editor. Drug permeation enhancement; theory and applications. New York: Marcel Dekker; 1994. pp. 19–24. [Google Scholar]
- 60.Alvarez-Figueroa MJ, Blanco-Mendez J. Transdermal delivery of methotrexate: iontophoretic delivery from hydrogels and passive delivery from microemulsions. Int J Pharm. 2001;215:57–65. doi: 10.1016/S0378-5173(00)00674-8. [DOI] [PubMed] [Google Scholar]
- 61.Tracharodi D, Rao KP. Transdermal absorption of nifedipine from microemulsions of lipophilic skin penetration enhancers. Int J Pharm. 1994;111:235–241. doi: 10.1016/0378-5173(94)90346-8. [DOI] [Google Scholar]
- 62.Delgado Charro MB, Iglesias Vilas G, Blanco Mendez J, Lopez Quintela MA, Marty JP, Guy RH. Delivery of a hydrophilic solute through the skin from novel microemulsion systems. Eur J Pharm Biopharm. 1997;43:37–42. doi: 10.1016/S0939-6411(96)00016-1. [DOI] [Google Scholar]
- 63.Junyaprasert VB, Boonme P, Songkro S, Kravel K, Rades T. Transdermal delivery of hydrophobic and hydrophilic local anaesthetics from o/w and w/o Brij 97-based microemulsions. J Pharm Pharmaceut Sci. 2007;10:319–329. [PubMed] [Google Scholar]
- 64.Changez M, Varshney M, Chander J, Dinda AK. Effect of the composition of lecithin/n-propanol/isopropyl myristate/water microemulsions on barrier properties of mice skin for transdermal permeation of tetracaine hydrochloride: in vitro. Colloids Surf B: Biointerfaces. 2006;50:18–25. doi: 10.1016/j.colsurfb.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 65.Yun SR, Jung SP, San CC. Transdermal delivery of ketoprofen using microemulsions. Int J Pharm. 2001;228:161–170. doi: 10.1016/S0378-5173(01)00827-4. [DOI] [PubMed] [Google Scholar]
- 66.Rage BD, Kao JP, Polli JE. Effects of nonionic surfactants on membrane transporters in caco-2 cell monolayers. Eur J Pharm Sci. 2002;16:237–246. doi: 10.1016/S0928-0987(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 67.Hua L, Weisan P, Jiayu L, Ying Z. Preparation, evaluation and NMR characterization of vinpocetine microemulsion for transdermal delivery. Drug Dev Ind Pharm. 2004;30:657–666. doi: 10.1081/DDC-120039183. [DOI] [PubMed] [Google Scholar]
- 68.Mou D, Chen H, Du D, Mao C, Wan J, Xu H, Yang X. Hydrogel-thickened nanoemulsion system for topical delivery of lipophilic drugs. Int J Pharm. 2008;353:270–276. doi: 10.1016/j.ijpharm.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 69.Chen H, Chang X, Du D, Li J, Xu H, Yang X. Microemulsion based hydrogel formulation of ibuprofen for topical delivery. Int J Pharm. 2006;315:52–58. doi: 10.1016/j.ijpharm.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 70.Spiclin P, Homar M, Zupancic-Valant A. Sodium ascorbyl phosphate for topical microemulsions. Int J Pharm. 2003;256:65–73. doi: 10.1016/S0378-5173(03)00063-2. [DOI] [PubMed] [Google Scholar]
- 71.Valenta C, Schultz K. Influence of carrageenan on the rheology and skin permeation of microemulsion formulations. J Control Rel. 2004;95:257–267. doi: 10.1016/j.jconrel.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 72.Bonacucina G, Martelli S, Palmieri GF. Rheological, mucoadhesive and release properties of carbopol gels in hydrophilic cosolvents. Int J Pharm. 2004;282:115–130. doi: 10.1016/j.ijpharm.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Lapasin R, Grassi M, Coceani N. Effect of polymer addition on the rheology of o/w microemulsions. Rheol Acta. 2001;40:185–192. doi: 10.1007/s003970000151. [DOI] [Google Scholar]
- 74.Draize JH, Woodward G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82:377–390. [Google Scholar]